Abstract
Zone sedimentation through sucrose gradients was used for preparing Rhizobium bacteroids from lupin nodules and for separating them into slowly and rapidly sedimenting fractions.
The purity of the bacteroids was established by electron microscopy and by enzyme assays, and they were shown to contain a CO-insensitive cytochrome c oxidase.
Bacteroids sedimented more rapidly than broth-cultured Rhizobium bacteria, and bacteroids from old nodules sedimented more rapidly than bacteroids from young nodules.
There appear to be at least two forms of bacteroid in old nodules: slowly sedimenting bacteroids with moderate colony-forming ability resembling the bacteroids found in young nodules, and rapidly sedimenting bacteroids with much lower colony-forming ability.
Full text
PDF

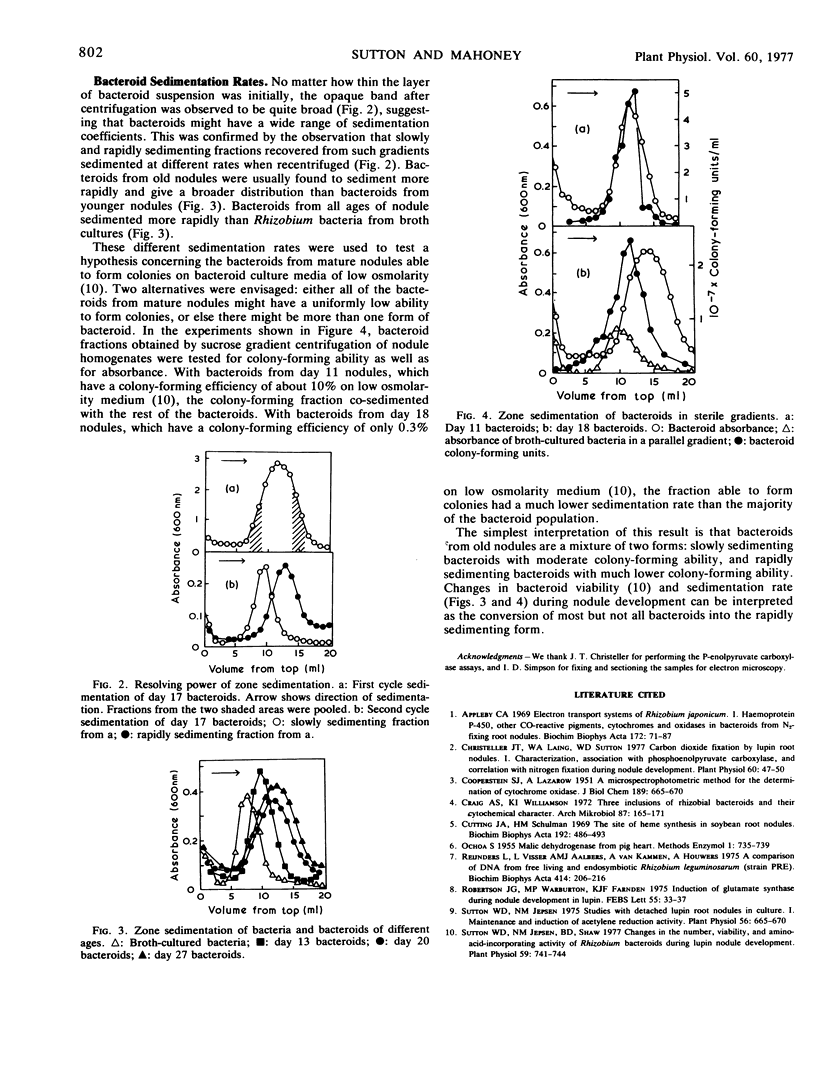
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Visser L., Aalbers A. M., Van Kammen A., Houwers A. A comparison of DNA from free living and endosymbiotic Rhizobium leguminosarum (strain PRE). Biochim Biophys Acta. 1975 Dec 4;414(2):206–216. doi: 10.1016/0005-2787(75)90224-5. [DOI] [PubMed] [Google Scholar]
- Robertson J. G., Warburton M. P., Farnden K. J. Induction of glutamate synthase during nodule development in lupin. FEBS Lett. 1975 Jul 15;55(1):33–37. doi: 10.1016/0014-5793(75)80950-1. [DOI] [PubMed] [Google Scholar]
- Sutton W. D., Jepsen N. M., Shaw B. D. Changes in the Number, Viability, and Amino-acid-incorporating Activity of Rhizobium Bacteroids during Lupin Nodule Development. Plant Physiol. 1977 Apr;59(4):741–744. doi: 10.1104/pp.59.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D., Jepsen N. M. Studies with detached lupin root nodules in culture: I. Maintenance and induction of acetylene reduction activity. Plant Physiol. 1975 Nov;56(5):665–670. doi: 10.1104/pp.56.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]



