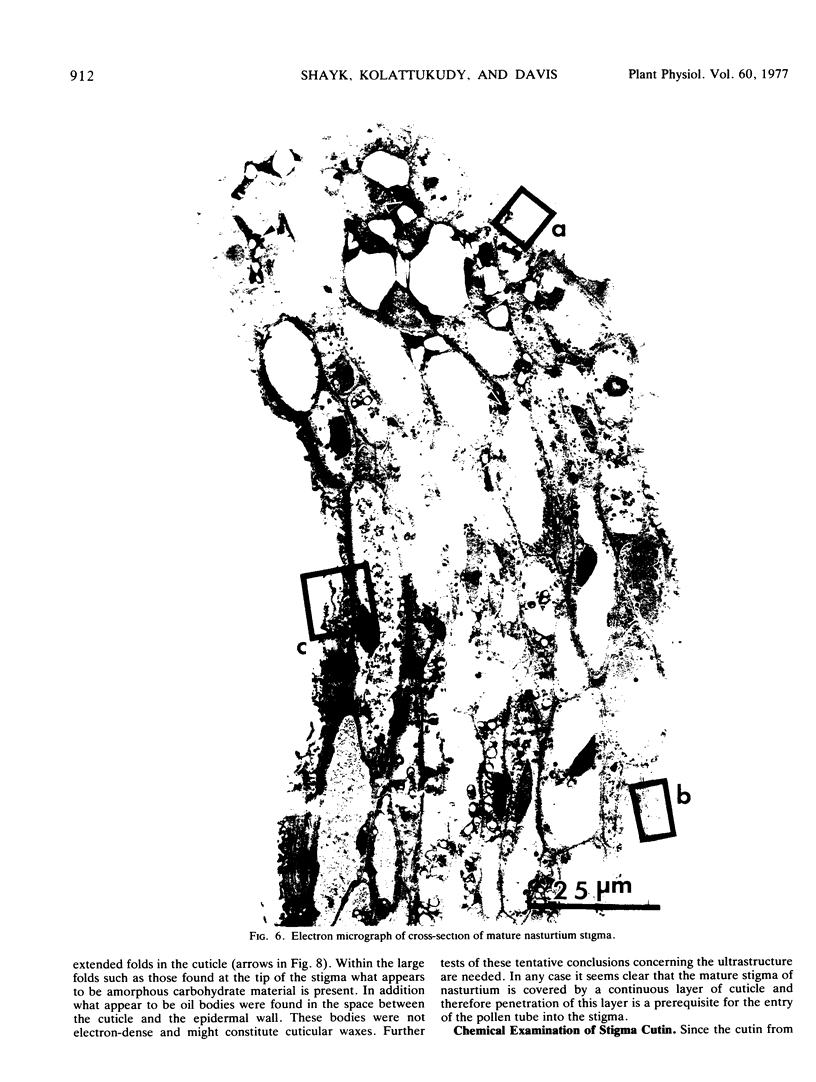
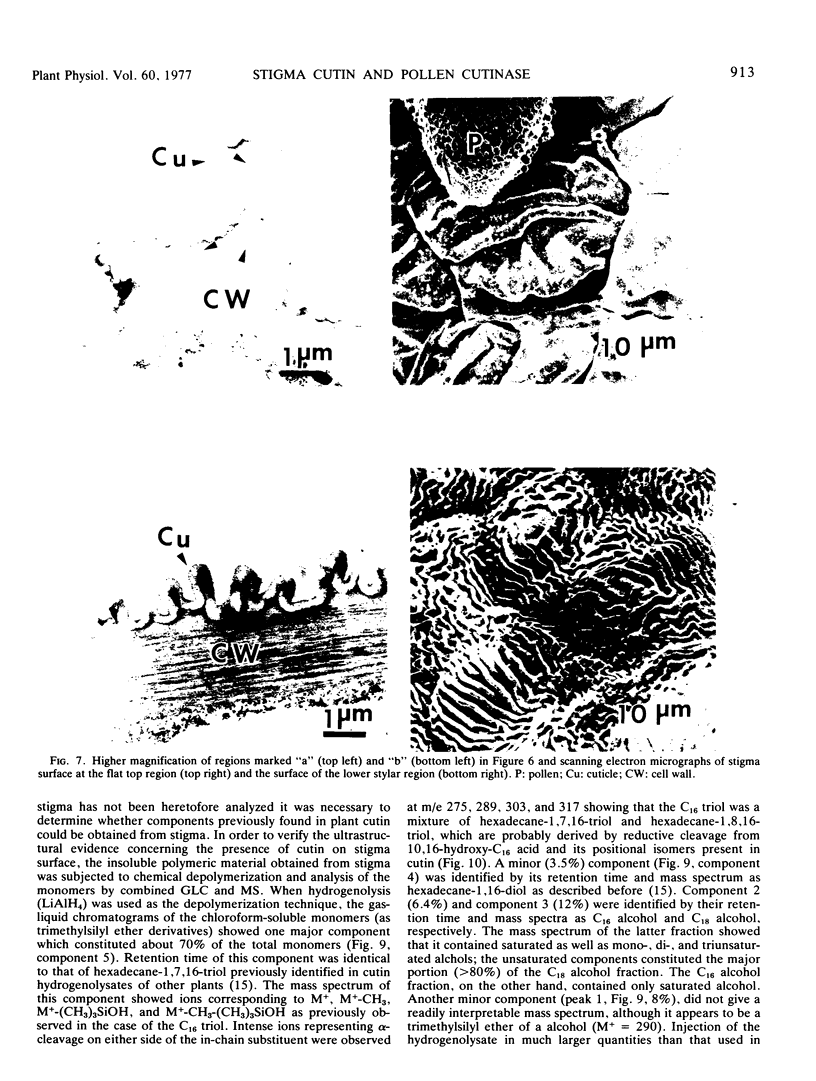
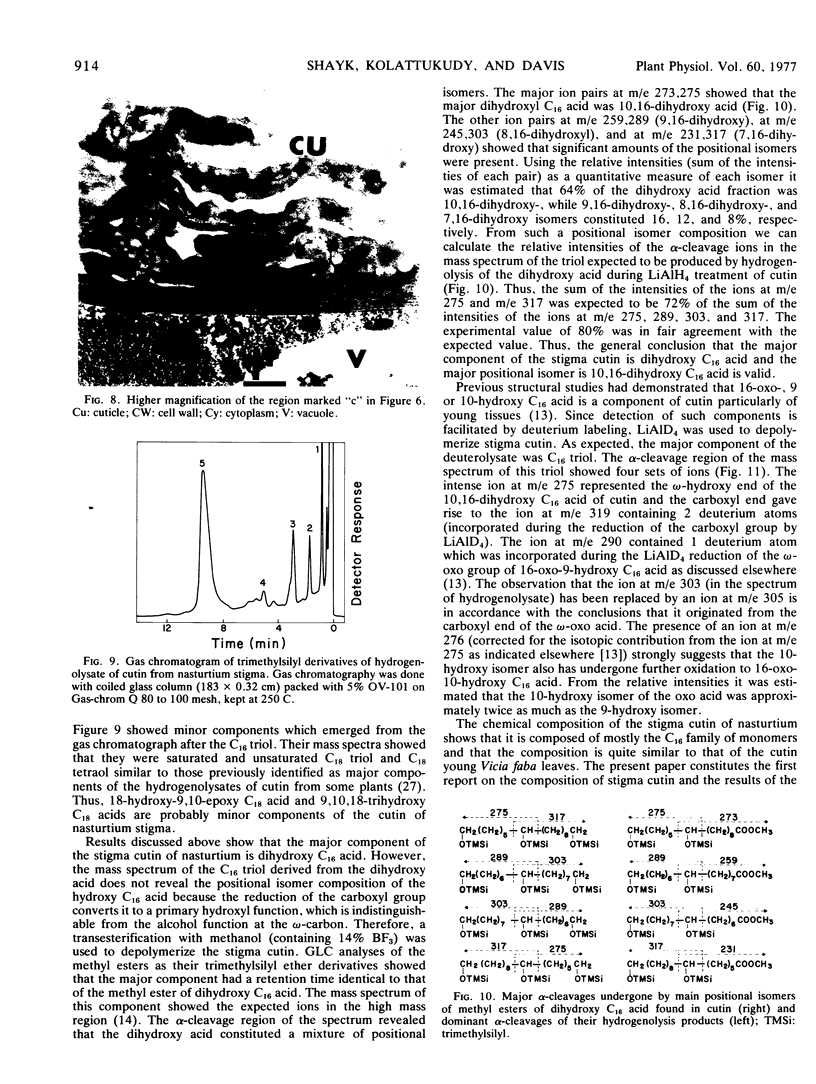
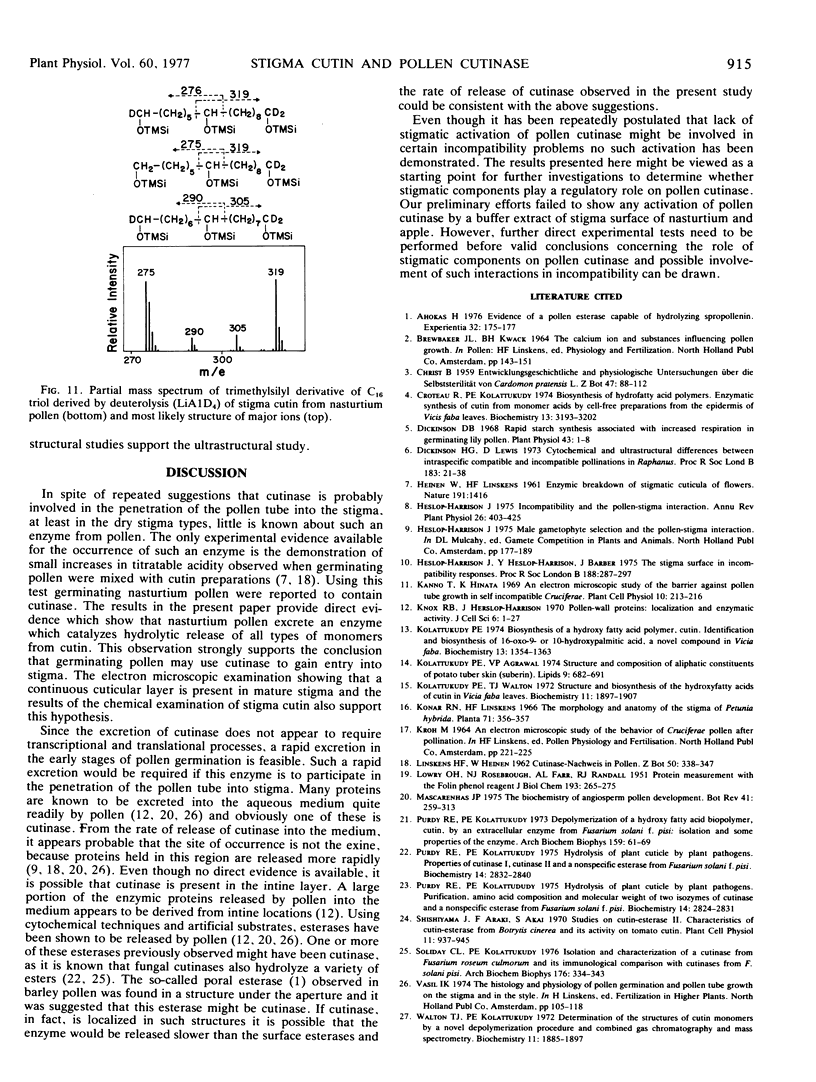
Abstract
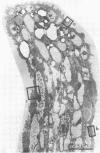
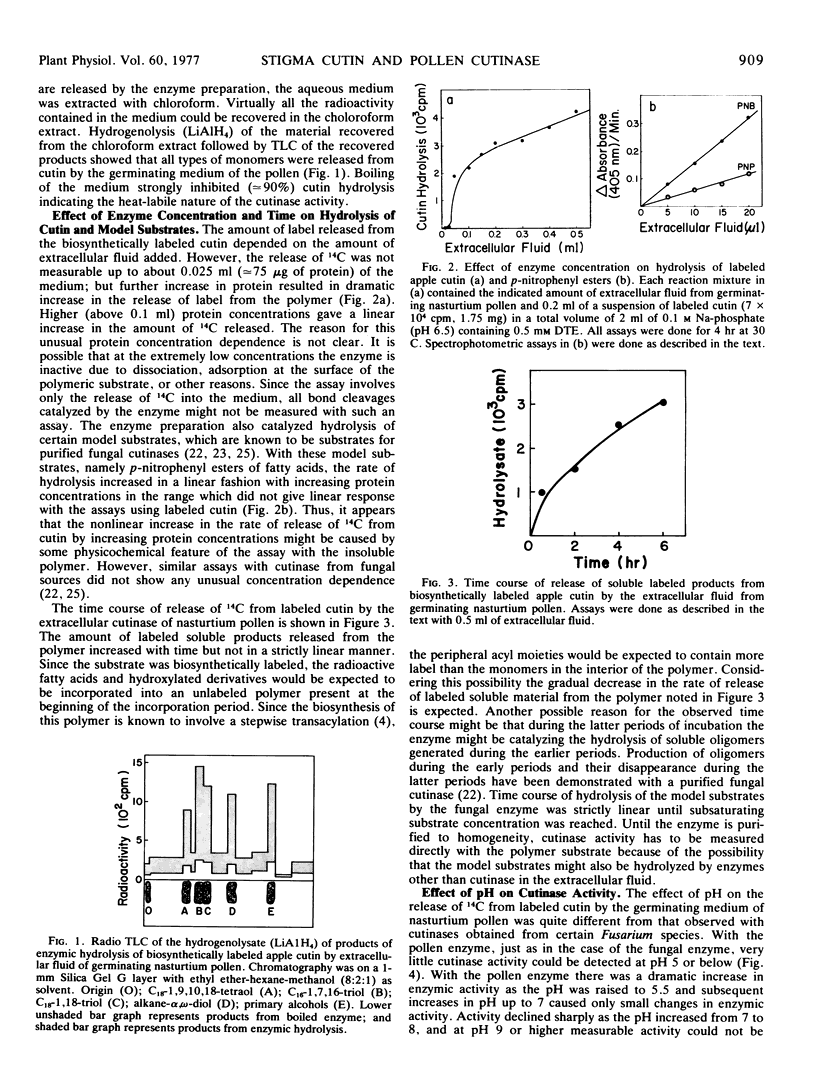
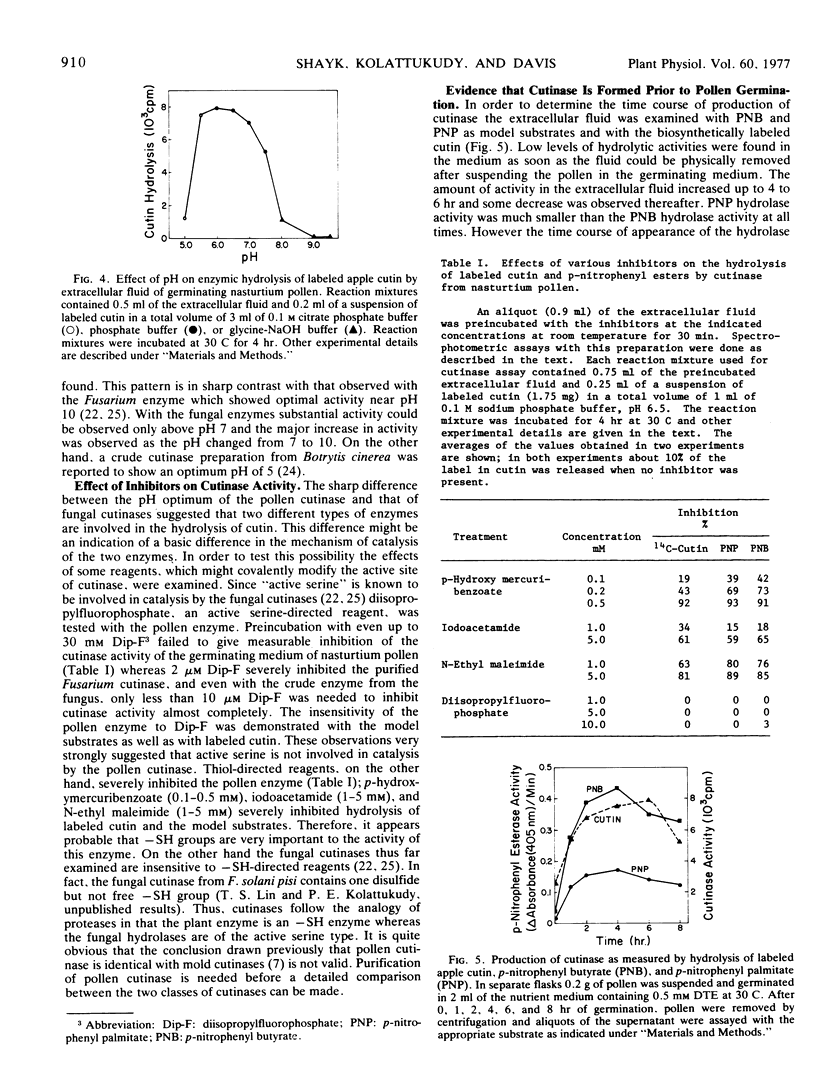
Germinating nasturtium pollen (Tropaeolum majus) is shown to excrete an enzyme(s) which hydrolyzes all types of monomers from biosynthetically labeled cutin and p-nitrophenyl esters, which are model substrates for fungal cutinases. The pollen cutinase showed an optimum pH near 6.5 and was inhibited by thiol-directed reagents such as p-hydroxymercuribenzoate and N-ethyl maleimide but not by diisopropyl-fluorophosphate, an “active serine”-directed reagent indicating that the pollen enzyme is an “-SH cutinase” unlike the fungal enzyme which is a serine cutinase. Excretion of the pollen cutinase into the extracellular fluid was complete within 4 to 6 hours at 30 C. Since actinomycin D and cycloheximide showed little effect on the level of cutinase excreted, it appears that cutinase is an enzyme synthesized prior to germination. Release of cutinase into the medium did not require germination. Electron microscopy revealed the presence of a continuous cutin layer on mature stigma with extensive folds, which are proposed to play a role similar to that played by the cellular papillae found in the stigma of other plants. Chemical analysis of stigma cutin by depolymerization and combined gas-liquid chromatography and mass spectrometry showed that this cutin consists of mainly the C16 family of acids. The major (70%) components were dihydroxy C16 acids which consisted of 10,16- (64%), 9,16- (16%), 8,16- (12%), and 7,16- (8%) dihydroxy plamitic acid. Deuterium-labeling studies showed the presence of 16-oxo-9-hydroxy C16 acid and 16-oxo-10-hydroxy C16 acid in this cutin. The biochemical and ultrastructural studies indicate that the pollen tube may gain entry into stigma using cutinase excreted by the pollen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Croteau R., Kolattukudy P. E. Biosynthesis of hydroxyfatty acid polymers. Enzymatic synthesis of cutin from monomer acids by cell-free preparations from the epidermis of Vicia faba leaves. Biochemistry. 1974 Jul 16;13(15):3193–3202. doi: 10.1021/bi00712a030. [DOI] [PubMed] [Google Scholar]
- Knox R. B., Heslop-Harrison J. Pollen-wall proteins: localization and enzymic activity. J Cell Sci. 1970 Jan;6(1):1–27. doi: 10.1242/jcs.6.1.1a. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthesis of a hydroxy fatty acid polymer, cutin. Identification and biosynthesis of 16-oxo-9- or 10-hydroxypalmitic acid, a novel compound in Vicia faba. Biochemistry. 1974 Mar 26;13(7):1354–1363. doi: 10.1021/bi00704a008. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J. Structure and biosynthesis of the hydroxy fatty acids of cutin in Vicia faba leaves. Biochemistry. 1972 May 9;11(10):1897–1907. doi: 10.1021/bi00760a026. [DOI] [PubMed] [Google Scholar]
- MANKIEWICZ E. Mycobacteriophages isolated from persons with tuberculous and non-tuberculous conditions. Nature. 1961 Sep 30;191:1416–1417. doi: 10.1038/1911416b0. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Depolymerization of a hydroxy fatty acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani f. pisi: isolation and some properties of the enzyme. Arch Biochem Biophys. 1973 Nov;159(1):61–69. doi: 10.1016/0003-9861(73)90429-3. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Hydrolysis of plant cuticle by plant pathogens. Properties of cutinase I, cutinase II, and a nonspecific esterase isolated from Fusarium solani pisi. Biochemistry. 1975 Jul;14(13):2832–2840. doi: 10.1021/bi00684a007. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Hydrolysis of plant cuticle by plant pathogens. Purification, amino acid composition, and molecular weight of two isozymes of cutinase and a nonspecific esterase from Fusarium solani f. pisi. Biochemistry. 1975 Jul;14(13):2824–2831. doi: 10.1021/bi00684a006. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Isolation and characterization of a cutinase from Fusarium roseum culmorum and its immunological comparison with cutinases from F. solani pisi. Arch Biochem Biophys. 1976 Sep;176(1):334–343. doi: 10.1016/0003-9861(76)90172-7. [DOI] [PubMed] [Google Scholar]
- Walton T. J., Kolattukudy P. E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas chromatography and mass spectrometry. Biochemistry. 1972 May 9;11(10):1885–1896. doi: 10.1021/bi00760a025. [DOI] [PubMed] [Google Scholar]