Abstract
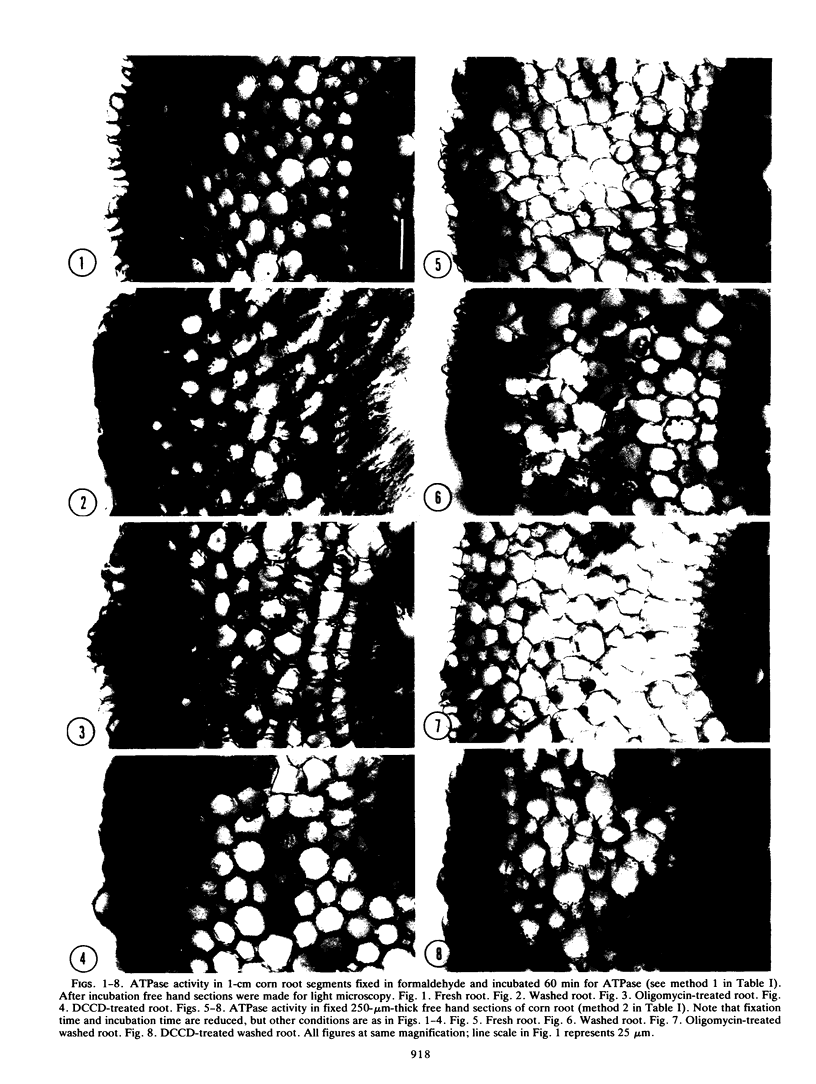
A cytochemical study has been made on the localization of ATPase activity in corn (Zea mays L.) roots. Light microscopy shows washing for 4 hours to increase the general ATPase activity in the peripheral layers of the root cortex; oligomycin and N,N-dicyclohexylcarbodiimide inhibit this activity, oligomycin being more effective. Ultrastructural studies of ATPase location show oligomycin treatment to inhibit both mitochondrial and plasmalemma ATPase, but only in the epidermis and outer cortex. Studies with lipid-soluble dyes indicate that oligomycin might not penetrate very deeply into root tissue in the time span of these experiments. It is suggested that the strong inhibition of ion absorption by oligomycin without a corresponding decline in ATP content is probably due to inhibition of ion absorption in the peripheral cell layers, thus limiting the supply of ion for symplastic transport to the uninhibited tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Eckermann G., Grant M., Robertson R. N. Salt accumulation and adenosine triphosphate in carrot xylem tissue. Proc Natl Acad Sci U S A. 1966 Mar;55(3):560–564. doi: 10.1073/pnas.55.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe C., Cole C. V., Ross C. Oligomycin inhibition of phosphate uptake and ATP labeling in excised maize roots. Plant Physiol. 1969 Jul;44(7):1040–1044. doi: 10.1104/pp.44.7.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. W., Bandurski R. S. Exocellular Enzymes of Corn Roots. Plant Physiol. 1964 Jan;39(1):60–64. doi: 10.1104/pp.39.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder J., Cronshaw J. A biochemical and cytochemical study of adenosine triphosphatase activity in the phloem of Nicotiana tabacum. J Cell Biol. 1974 Jan;60(1):221–235. doi: 10.1083/jcb.60.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder J., Cronshaw J. The distribution of adenosine triphosphatase activity in differentiating and mature phloem cells of Nicotiana tabacum and its relationship to phloem transport. J Ultrastruct Res. 1973 Sep;44(5):388–404. doi: 10.1016/s0022-5320(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Grossman I. W., Heitkamp D. H. Electron microscopic localization of mitochondrial adenosine triphosphatase activity. J Histochem Cytochem. 1968 Oct;16(10):645–653. doi: 10.1177/16.10.645. [DOI] [PubMed] [Google Scholar]
- Hall J. L. Cytochemical localization of ATP-ase activity in plant root cells. J Microsc. 1971 Jun;93(3):219–225. doi: 10.1111/j.1365-2818.1971.tb02284.x. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S., BARDEN H. ULTRAMICROSCOPIC LOCALIZATION OF MITOCHONDRIAL ADENOSINETRIPHOSPHATASE. J Ultrastruct Res. 1964 Apr;10:189–193. doi: 10.1016/s0022-5320(64)80003-4. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Increased Membrane-bound Adenosine Triphosphatase Activity Accompanying Development of Enhanced Solute Uptake in Washed Corn Root Tissue. Plant Physiol. 1972 Mar;49(3):436–440. doi: 10.1104/pp.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Induction and development of increased ion absorption in corn root tissue. Plant Physiol. 1972 Mar;49(3):430–435. doi: 10.1104/pp.49.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Phosphate absorption rates and adenosine 5'-triphosphate concentrations in corn root tissue. Plant Physiol. 1974 Sep;54(3):250–256. doi: 10.1104/pp.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurkin I. T., McClurkin D. C. Cytochemical demonstration of a sodium-activated and a potassium-activated adenosine triphosphatase in loblolly pine seedling root tips. Plant Physiol. 1967 Aug;42(8):1103–1110. doi: 10.1104/pp.42.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. Factors affecting the activity of adenosine triphosphatase and other phosphatases as measured by histochemical techniques. J Histochem Cytochem. 1955 May;3(3):161–169. doi: 10.1177/3.3.161. [DOI] [PubMed] [Google Scholar]
- Van Rossum G. D. The effects of oligomycin on energy metabolism and cation transport in slices of rat liver. Inhibition of oxidative phosphorylation as the primary action. Biochim Biophys Acta. 1976 Jan 15;423(1):111–121. doi: 10.1016/0005-2728(76)90105-5. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WHEELER K. P., BLAKE A. OLIGOMYCIN AND ACTIVE TRANSPORT REACTIONS IN CELL MEMBRANES. Nature. 1964 Aug 15;203:720–724. doi: 10.1038/203720a0. [DOI] [PubMed] [Google Scholar]

























