Abstract
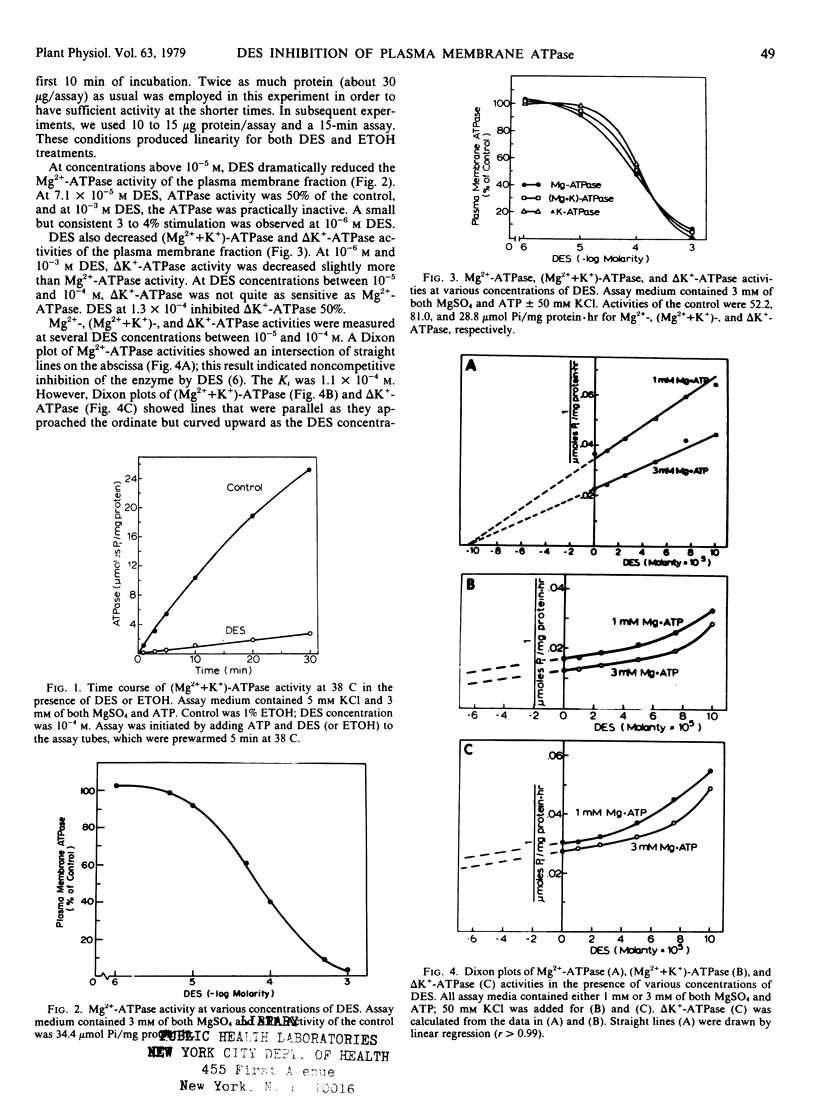
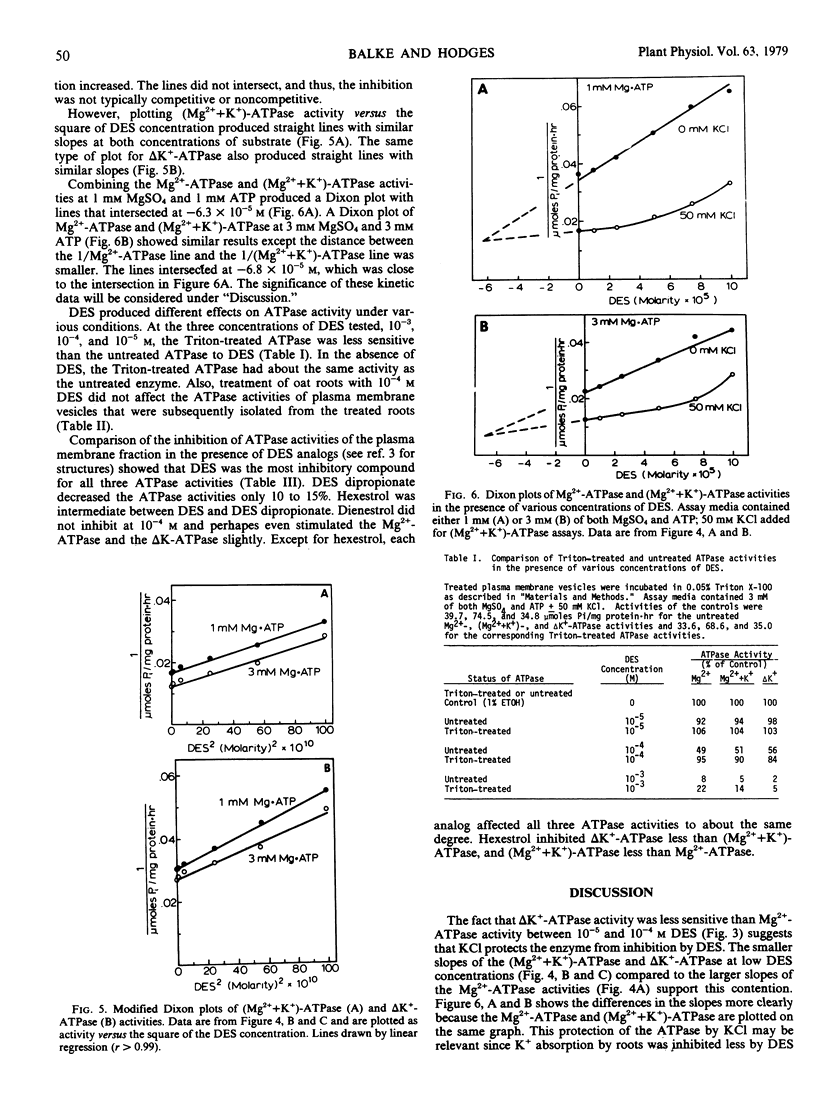
Diethylstibestrol (DES) inhibited noncompetitively the ATPase in the plasma membrane fraction from Avena sativa L. cv. Goodfield roots when assayed in the presence of MgSO4 or MgSO4 plus KCl. In the presence of MgSO4, 7.1×10−5 molar DES inhibited the enzyme 50%; whereas in the presence of MgSO4 and KCl, 1.3×10−4 molar DES was required for the same inhibition. Dixon plots indicated that in the presence of MgSO4, one molecule of DES bound to one molecule of ATPase; however, in the presence of MgSO4 and KCl, two or more molecules bound to one ATPase molecule. These results suggested that KCl causes a conformational change in the enzyme which exposes additional binding sites for DES, but that these sites are not as inhibitory as the first binding site.
In addition to KCl, other factors also affected the DES inhibition of the ATPase. Plasma membrane vesicles warmed to 38 C were inhibited more than vesicles kept on ice prior to assay. DES inhibited the Triton X-100-treated ATPase less than the ATPase which was not detergent-treated. Finally, studies with DES analogs showed that the hydroxyl groups of DES were essential for inhibition and that steric configurations of the molecule were important.
DES inhibition of the ATPase suggests that DES inhibits K+ absorption in oat roots by inhibiting the ATPase. Inhibition of K+ absorption was greater than inhibition of the ATPase, and thus DES may also inhibit other aspects of metabolism that are involved with ion absorption.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Effect of diethylstilbestrol on ion fluxes in oat roots. Plant Physiol. 1979 Jan;63(1):42–47. doi: 10.1104/pp.63.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Dulley J. R. Determination of inorganic phosphate in the presence of detergents or protein. Anal Biochem. 1975 Jul;67(1):91–96. doi: 10.1016/0003-2697(75)90275-4. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Durham N. N., Adams L. S. The structural specificity involved in the inhibition of a microbial D-glucose-6-phosphate dehydrogenase by synthetic estrogens. Biochim Biophys Acta. 1966 May 26;121(1):90–101. doi: 10.1016/0304-4165(66)90351-5. [DOI] [PubMed] [Google Scholar]
- Heikel T. A., Lathe G. H. The effect of 17-alpha-ethinyl-substituted steroids on adenosine triphosphatases of rat liver plasma membrane. Biochem J. 1970 Jun;118(1):187–189. doi: 10.1042/bj1180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- KIMBERG D. V., YIELDING K. L. Pyruvate kinase. Structural and functional changes induced by diethylstilbestrol and certain steroid hormones. J Biol Chem. 1962 Oct;237:3233–3239. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martonosi A., Donley J., Halpin R. A. Sarcoplasmic reticulum. 3. The role of phospholipids in the adenosine triphosphatase activity and Ca++ transport. J Biol Chem. 1968 Jan 10;243(1):61–70. [PubMed] [Google Scholar]
- Martonosi A. Steroid effects on the calcium ion transport of rabbit skeletal muscle microsomes. Arch Biochem Biophys. 1968 Apr;125(1):295–302. doi: 10.1016/0003-9861(68)90664-4. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Diethyl stilbestrol--mode of inhibition of the (Na + +K + )-dependent adenosine triphosphatase. Biochem Pharmacol. 1970 May;19(5):1852–1855. doi: 10.1016/0006-2952(70)90186-3. [DOI] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Characterization of passive ion transport in plasma membrane vesicles of oat roots. Plant Physiol. 1976 Sep;58(3):304–308. doi: 10.1104/pp.58.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Selectivity of alkali cation influx across the plasma membrane of oat roots: cation specificity of the plasma membrane ATPase. Plant Physiol. 1977 Apr;59(4):641–646. doi: 10.1104/pp.59.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yielding K. L., Tomkins G. M. STRUCTURAL ALTERATIONS IN CRYSTALLINE GLUTAMIC DEHYDROGENASE INDUCED BY STEROID HORMONES. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1483–1488. doi: 10.1073/pnas.46.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]


