Abstract
Purpose
The oxygen consumption rates (OCRs) in mice and cattle have been reported to change during preimplantation embryogenesis. On the other hand, mitochondrial DNA (mtDNA) copy number has been shown to be unchanged in mice and changed in cattle and pigs. The interactions between mitochondrial functions and mtDNA copy numbers in human embryos during preimplantation development remain obscure.
Methods
Sixteen oocytes and 100 embryos were used to assess mtDNA copy numbers and OCR. Three oocytes and 12 embryos were used to determine cytochrome c oxidase activity. All specimens were obtained between July 2004 and November 2014, and donated from couples after they had given informed consent. Mature oocytes and embryos at 2–14-cell, morula, and blastocyst stages were used to assess their OCR in the presence or absence of mitotoxins. The mtDNA copy number was determined using the samples after analysis of OCR. The relationships between developmental stages and OCR, and developmental stages and mtDNA copy number were analyzed. Furthermore, cytochrome c oxidase activity was determined in oocytes and 4-cell to blastocyst stage embryos.
Results
The structure of inner mitochondrial membranes and their respiratory function developed with embryonic growth and the mtDNA copy numbers decreased transiently compared with those of oocytes. The undifferentiated state of inner cell mass cells appears to be associated with a low OCR. On the other hand, the mtDNA copy numbers increased and aerobic metabolism of mitochondria increased in trophectoderm cells.
Conclusions
The mitochondrial respiratory function of human embryos developed along with embryonic growth although the copy numbers of mtDNA decreased transiently before blastulation. OCRs increased toward the morula stage ahead of an increase of mtDNA at the time of blastulation. Data regarding changes in mitochondrial function and mtDNA copy number during preimplantation development of human embryos will be useful for the development of ideal culture media.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0886-6) contains supplementary material, which is available to authorized users.
Keywords: Mitochondrial DNA, Mitochondrial function, Oxygen consumption
Introduction
Mitochondria-generated ATP plays important roles in both nuclear and cytoplasmic maturation of oocytes [1–6]. A study using mice lacking mitochondrial transcription factor A (TFAM) which is essential for the replication, transcription, and maintenance of mitochondrial DNA (mtDNA) has demonstrated that oocytes must contain threshold numbers of mtDNA to support the developmental competence of embryos to grow viable fetuses [7]. The amount of mtDNA copy numbers in whole embryos remained unchanged during successive cell divisions in mice [7, 8] leading to a progressive decrease in mtDNA in cleaving blastomeres. In contrast, the mtDNA copy numbers in cattle [9] and pigs [10, 11] have been reported to decrease temporarily during embryo development. Thus, quantitative changes in mitochondria in preimplantation embryos vary among animal species.
The numbers of mtDNA copy in oocytes or embryos have been shown to be involved in causes of infertility (ovarian insufficiency [12] and endometriosis [13]), mutations of mtDNA [14], female age [15], and aneuploidy of embryos [15, 16]. On the other hand, it has been shown that overall levels of mtDNA are largely equal between blastocysts stratified by ploidy, age, or implantation potential [17]. However, the changes in mtDNA copy numbers in human embryos during preimplantation development remain obscure.
In aerobic oxidation, fatty acids and sugars are metabolized using O2 to CO2 and H2O, and the released energy is converted to the chemical energy of phosphoanhydride bonds in ATP. In animal cells, ATP is generated mainly by this process. Thus, the measurement of oxygen consumption rate (OCR) of developing embryos would be a useful measurement to evaluate their normality and developmental competence. The OCR of mitochondria in mammalian embryos increases toward the blastocyst stage in mice and cattle [18–20] suggesting that the mitochondrial function also increases during embryogenesis. Once blastulation has occurred, trophectoderm (TE) cells rapidly increase the mtDNA copy numbers to match the energy demands of the cells [19]. Human embryos that consume more oxygen develop to blastocysts more quickly than those that consume less [21]. Blastocysts that consume more oxygen after vitrification also have higher developmental competence [22]. These findings are consistent with the fact that formation of the blastocoel cavity depends on cellular Na/K-ATPase activity [23]. However, the interactions between mitochondrial functions and mtDNA copy numbers in human embryos during preimplantation development have not been elucidated.
Here, to evaluate mitochondrial function during human embryonal development, we assessed the OCR of human oocytes and embryos during days 2 and 5 in the presence or absence of mitochondrial toxins. The mtDNA copy numbers in each oocyte or embryo were also measured.
Materials and methods
Ethical approval
This study was approved by the local ethics Institutional Review Board of IVF Namba Clinic and the Japan Society of Obstetrics and Gynecology (Registry nos. 135 and 138). Specimens were donated by 71 couples who gave informed consent. Vitrified–warmed pronuclear stage zygotes, cleavage stage embryos on days 2 and 3, and blastocysts on day 5 after insemination by intracytoplasmic sperm injection (ICSI) were used for the study after the couples had given informed consent. Mature oocytes were obtained after additional culture of immature cells donated for research at the time of ICSI 42 h after the administration of hCG. In Japan, the donation of oocytes which are mature 42 h after hCG injection is not generally admitted because of ethical reasons. Thus, in this study, we used oocytes with delayed cell cycle.
Ovarian stimulation and insemination
Patients were subjected to controlled ovarian stimulation according to their medical history as described [24]. For gonadotropin-releasing hormone (GnRH) agonist cycles, patients received oral contraceptive pills (1 mg norethisterone and 0.05 mg mestranol, Aska Pharmaceutical, Co., Ltd., Tokyo, Japan) on day 14 of the previous cycle and continued for 10 days, and a GnRH agonist (600 μg/day, Suprecur® nasal solution 0.15%; Mochida Pharmaceutical, Tokyo) on day 21 of the previous cycle until the induction of ovulation. On day 3 of the cycle, they received doses of follicle stimulating hormone (FSH) ranging from 150 to 300 IU for 4 days followed by administration of urinary human menopausal gonadotropin (hMG) at doses or 150–450 IU until ovulation induction. For GnRH antagonist cycles, a GnRH antagonist (2.5 mg, Ganirelix Acetate, MSD K.K., Tokyo) was administered daily after the leading follicles reached 13–14 mm in diameter as diagnosed by ultrasound. Ovulation was induced by human chorionic gonadotropin (hCG) administration when at least one leading follicle reached 18 mm in diameter. Transvaginal follicle aspiration was carried out 36 h after the hCG injection. Insemination was carried out by ICSI 40 h after hCG administration.
To avoid contamination from mtDNA from spermatozoa attached to the zona pellucida, all embryos in this study were generated by ICSI. Oocytes were collected between July 2004 and November 2014.
OCR
Normally fertilized zygotes were frozen by vitrification [25] at the pronuclear (PN) stage at 16–18 h, or on days 2, 3, and 5 after ICSI. After warming, embryos were cultured at 37 °C under 5% CO2, 5% O2, and 90% N2 with high humidity in potassium simplex optimized medium containing amino acids [26] and 5% (v/v) synthetic serum substitute (SSS, 99193; Irvine Scientific, St. Ana, CA, USA) for more than 19 h except for day 5 embryos. Day 5 blastocysts were cultured for 8 h and then their OCR was assessed. Embryos that showed cleavage within 19 h before the measurement were considered as developing, and those that did not were regarded as arrested. Blastocysts on day 5 that reached the expanded stage with a tightly packed inner cell mass (ICM) and TE consisting of cohesive epithelium were included in the analysis.
The OCR of samples was measured using scanning electrochemical microscopy (SCEM, CRAS-1.0; Clino Ltd., Miyagi, Japan) as previously described [22] at 37 °C under air. Briefly, each oocyte or embryo was transferred into a well filled with 5-mL human tubal fluid (HTF) medium buffered with 21 mM HEPES containing 2.7 mM glucose and 0.33 mM pyruvate (modified HTF medium; Irvine Scientific) containing 5% SSS, where it sank to the bottom of a cone-shaped microwell and remained at the lowest point. A platinum microdisk electrode was loaded with 5 ml of HEPES-buffered medium, and its tip potential was maintained at −0.6 V versus the Ag/AgCl electrode with a potentiostat to monitor the local oxygen concentration. The microelectrode scanned along the z-axis from the edge of the sample, and the OCR was calculated with custom software based on the spherical diffusion theory. OCR was measured at three points for each embryo and at the ICM and two TE sides of blastocysts. The OCR was also measured in the presence of mitochondrial toxins [27]. Stock solutions of 1 mM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; C2920, Merck Millipore Co., Darmstadt, Germany) and 1 M sodium cyanide (380970, Merck Millipore Co.) were prepared in ethanol and water, respectively. During the recording of oxygen consumption by embryos in the modified HTF medium, either FCCP or cyanide was added to give final concentrations of 1 μM and 1 mM, respectively. Cyanide at 1 mM completely inhibits mitochondrial cytochrome c oxidase activity. Characteristics of the donated oocytes and embryos are shown in the Supplementary Table.
mtDNA
All specimens after OCR measurement were sampled in 2 μl DNase-free water individually and cryopreserved at −65 °C until assay. Each sample was lysed in 4 μl lysis buffer (20 mM Tris (# 252859, Merck Millipore Co.), 0.4 mg/ml proteinase K (# P2308, Merck Millipore Co.), 0.9% Nonidet P-40 (# 21-3277-2, Merck Millipore Co.), and 0.9% Tween 20 (# P1379, Merck Millipore Co.)) at 55 °C for 30 min, followed by heating at 95 °C for 5 min; the lysate was diluted in DNase-free water to a final volume of 40 μl. A 4-μl aliquot of the lysate was added to 12.5 μl Quantifact SYBR Green master mix (Qiagen, Venlo, Limburg, the Netherlands), 8.5 μl DNase-free water and 1 μM of each primer (Table 1). Polymerase chain reaction (PCR) amplifications were run in duplicate in a Rotorgene Q thermal cycler (Qiagen) according to the following conditions: 95 °C for 5 min, 40 cycles of 95 °C for 5 s, and 60 °C for 10 s. SYBR green fluorescence was measured at the end of each cycle. The melting curve was analyzed to check the specificity of the PCR products. A standard curve was generated for each run using tenfold serial dilutions representing copies of the external standard (103 to 106 copies). The external standard used in the present study was the PCR product of the corresponding gene cloned into a vector using the Zero Blunt TOPO PCR cloning kit (Invitrogen Life Sciences, Carlsbad, CA, USA), and the product was confirmed by sequencing before use.
Table 1.
Primers used for qPCR
| Gene | Primer | Sequence (5′–3′) | Localization | Accession number |
|---|---|---|---|---|
| ND6 | Forward | GCCTGGTGATAGCTGGTTGT | 14,407–14,426 | NC_012920.1 |
| Reverse | GGTGGCTGCTTTTAGGCCTA | 14,568–14,549 | ||
| ND4 | Forward | TTCCCCAACCTTTTCCTCCG | 10,915–10,934 | NC_012920.1 |
| Reverse | TGGATAAGTGGCGTTGGCTT | 11,015–10,996 | ||
| b-Actin | Forward | AGCGGGAAATCGTGCGTGAC | 2133–2152 | NC_000007.14 |
| Reverse | AGGCAGCTCGTAGCTCTTCTC | 2248–2228 |
The primers were designed using Primer3Plus (http://sourceforge.net/projects/primer3/) and ND6 (525 bp region from 14,149 to 14,673) and ND4 sequences (1,378 bp region from 10,760 to 12,137) of human mitochondria (NC012920.1). To assess the mtDNA deletion [28, 29], the ratio of copy numbers of ND4 and ND6 regions was calculated. When the ratio was >0.75, the embryo was regarded as not having mtDNA deletions.
To standardize the copy number of mtDNA after DNA amplification, we used an internal genomic control (b-actin) for normalization. Quantitative RT-PCR was performed similarly to mtDNA amplification using 1 μM of each primer (Table 1). A standard curve was generated for each run using tenfold serial dilutions representing copies of the external standard (1 to 103 copies).
The copy number of mtDNA per cell was calculated by dividing the mtDNA copy number by the number of blastomeres before compaction or by nuclear number after compaction.
Analysis of cytochrome c oxidase (CCO)
Vitrified specimens were fixed at each stage (4 cells to blastocysts) after more than 24-h culture, and mature oocytes were immediately fixed to measure CCO activity as described [16]. Briefly, they were fixed in 0.05-M phosphate-buffered saline (PBS) with 2% glutaraldehyde (v/v, TAAB Laboratories, Berkshire, UK), 0.15-M sucrose (WAKO, Osaka, Japan), and for 15 min at 4 °C. After rinsing in the same buffer, they were incubated in 0.05-M PBS with 1.4-mM 3,3′-diaminobenzidine (DAB) tetrahydrochloride (Merck Millipore Co.), 0.1% (w/v) type II cytochrome c (Merck Millipore Co.), 0.23-M sucrose, and 0.0002% catalase (w/v; Merck Millipore Co.) for 3 h at 37 °C. Samples were then washed in PBS for 1 h. They were then postfixed in 1% (v/v) osmium tetroxide (TAAB Laboratories) for 2 h at 4 °C. They were dehydrated in increasing concentration of ethanol and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and examined using transmission electron microscopy (TEM; JEM-1011, JEOL, Tokyo, Japan). Mitochondria that showed well-developed crista structure and deeply stained membrane were categorized as having high CCO activity (High). Mitochondria with deeply stained membrane and few crista structures were categorized as having moderate CCO activity Mitochondria with poorly stained membranes and few crista structures were categorized as having low CCO activity.
Definitions
Mitochondrial OCR (mtOCR) was calculated by subtracting the value obtained in the presence of cyanide from those obtained without any mitochondrial toxins. The maximum (max mtOCR) was calculated by subtracting the values obtained in the presence of cyanide from those obtained in the presence of FCCP. The OCR/mtDNA was defined as the value of mtOCR divided by mtDNA copy numbers.
Statistics
Data for mtDNA and OCRs were compared by Steel-Dwass following Kruskal-Wallis analysis of variance (ANOVA). Effect of cause of infertility on the number of mtDNA copy was assessed using t test. Data for CCO activity were compared by Cochran-Armitage test among five groups and by Fisher’s exact test between two groups. P < 0.01 was considered statistically significant.
Results
mtDNA copy numbers
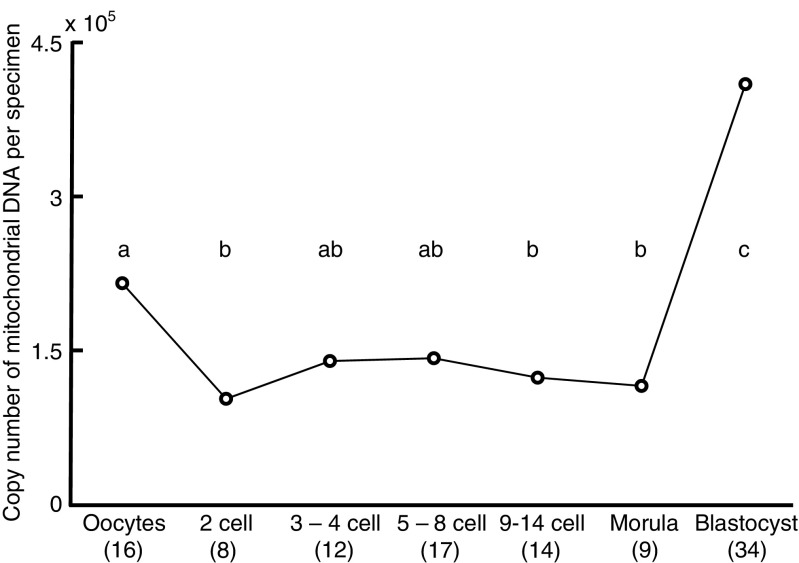
There were no significant differences in maternal age among the groups (Supplementary table). Deletions of mtDNA were not detected in this study. The number of mtDNA copy per specimen in embryos decreased transiently (P < 0.01; Fig. 1) at 2-cell (102,584), 9–14-cell (123,293), and morula stages (115,199) compared with oocyte (215,564). The number of mtDNA copy significantly increased (P < 0.01) on day 5 (expanded blastocyst stage, 410,212) compared with those in oocytes, 2–14-cell and morula stage embryos. Cause of infertility did not affect the number of mtDNA copy in 2-cell stage (P = 0.1549, endometriosis 113,795 vs. unknown (only male factor) 95,857) and in 3–4-cell stage embryos (P = 0.5654, endometriosis 200,702 vs. unknown (only male factor) 119,108).
Fig. 1.
Changes in mitochondrial DNA copy number per specimen (mean) during preimplantation development of human embryos. The numbers of oocytes or embryos examined are shown in parentheses. Different superscript letters (a–c) indicate significant differences (P < 0.01) by Steel-Dwass following Kruskal-Wallis following ANOVA
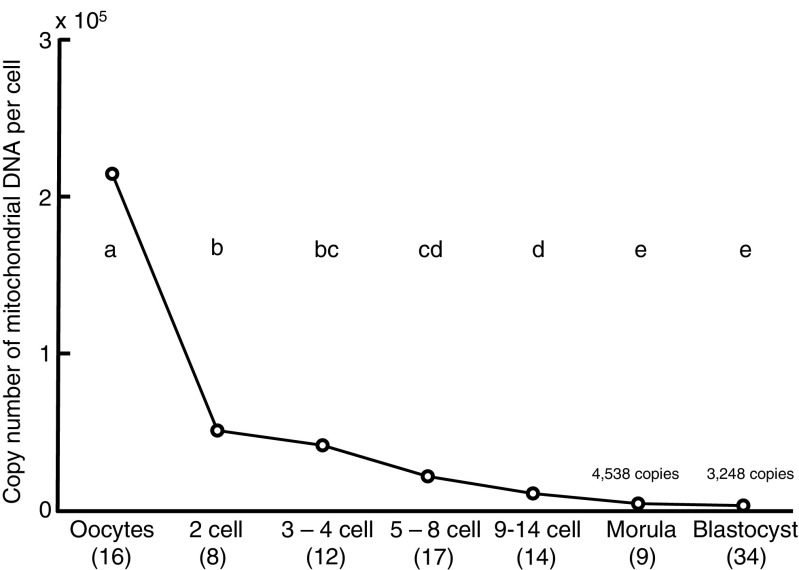
The numbers of mtDNA copy per cell in embryos decreased gradually (P < 0.01; Fig. 2) from oocytes (211,564) toward 2-cell (51,292), 3–4-cell (41,830), 5–8-cell (21,946), 9–14-cell (11,009), morula (4,538), and blastocyst stages (3,248).
Fig. 2.
Changes in mitochondrial DNA copy number per cell (mean) during preimplantation development of human embryos. The numbers of oocytes or embryos examined are shown in parentheses. Different superscript letters (a–e) indicate significant differences (P < 0.01) by Steel-Dwass following Kruskal-Wallis following ANOVA
OCR
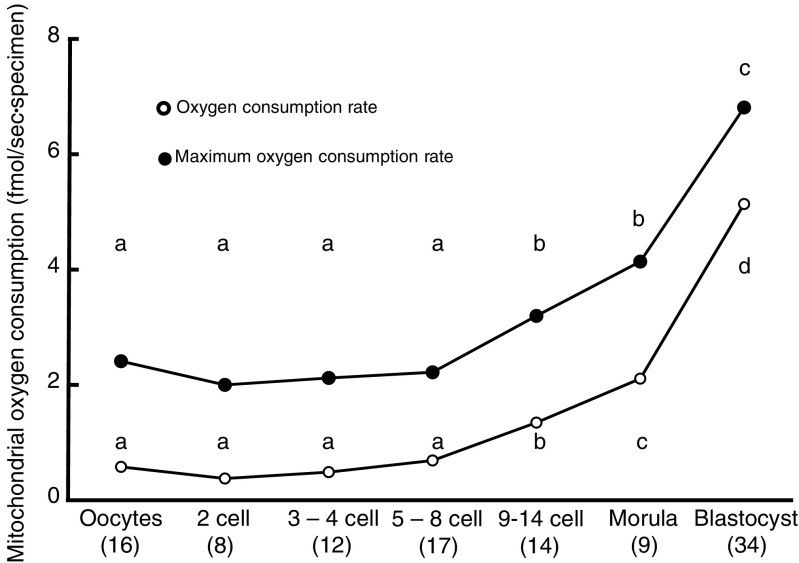
No difference was found between oocyte and 8-cell stages in mtOCR (0.37–0.68 fmol/s; Fig. 3) nor in the max mtOCR (1.99–2.41 fmol/s). However, mtOCR and max mtOCR increased rapidly at 9–14-cell stage (P < 0.01; mtOCR = 1.34; max mtOCR = 3.19 fmol/s) compared with those until 8-cell stage. Furthermore, the mtOCR (5.13 fmol/s) and max mtOCR at blastocyst stage (6.81 fmol/s at the expanded blastocyst stage) were significantly higher than those until 14-cell stage (P < 0.01). OCRs increased toward the morula stage ahead of an increase of mtDNA at blastulation.
Fig. 3.
Changes in mitochondrial oxygen consumption (mtOCR; mean) and maximum mtOCR (mean) during preimplantation development of human embryos. The mtOCR was calculated by subtracting the value obtained in the presence of cyanide from the value obtained without any mitotoxins. The maximum mtOCR was calculated by subtracting the value obtained in the presence of cyanide from the value obtained in the presence of FCCP. The numbers of oocytes or embryos examined are shown in parentheses. Different superscript letters (a–d) indicate significant differences (P < 0.01) by Steel-Dwass following Kruskal-Wallis following ANOVA
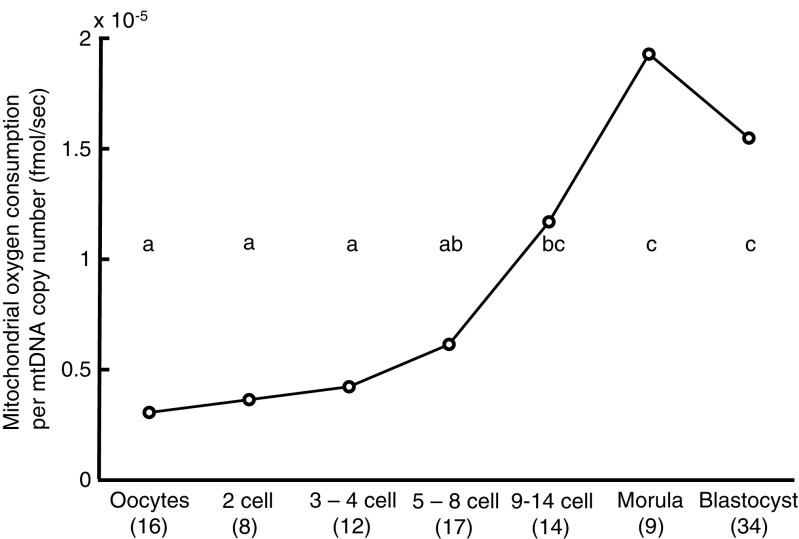
The mtOCR/mtDNA ratio increased toward blastocyst (P < 0.01, 1.55 × 10−5 fmol/s mtDNA, Fig. 4) and morula stage (P < 0.01; 1.93 × 10−5 fmol/s mtDNA) compared with those of oocyte to 8-cell stages (0.31–0.62 × 10−5). Taken together, mtOCR per mtDNA rose along with the development of embryos until morula stage.
Fig. 4.
Changes in mtOCR per mtDNA copy number (mean) during preimplantation development of human embryos. The OCR/mtDNA ratio was defined as the value of mtOCR divided by the mtDNA copy number. The numbers of oocytes or embryos examined are shown in parentheses. Different superscript letters (a–c) indicate significant differences (P < 0.01) by Steel-Dwass following Kruskal-Wallis following ANOVA
CCO activity
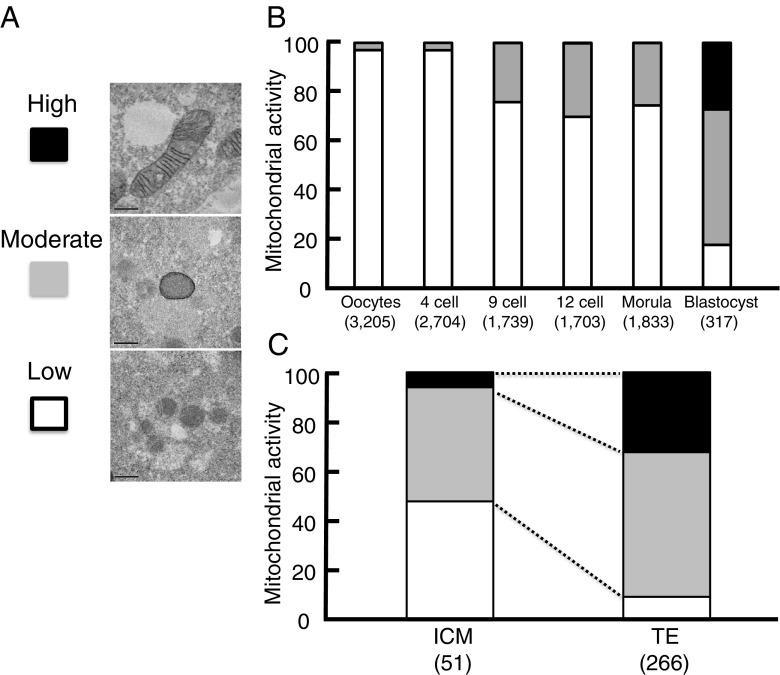
The proportion of mitochondria with high CCO activity increased in blastocysts (P < 0.0001; 27.1%; Fig. 5b) compared with those of mature oocytes (0%), and 4 cells (0%), 9 cells (0.1%), 12 cells (0.2%), and morulae (0.1%). The proportion of mitochondria with moderate CCO activity also increased in blastocysts (P < 0.01; 55.2%) compared with those of mature oocytes (3%), 4cells (3%), 9 cells (24.1%), 12 cells (30%), and morulae (25.4%). The proportion of mitochondria with low CCO activity decreased in blastocysts (P < 0.01; 17.7%) compared with those of mature oocytes (97.1%), 4 cells (97%), 9 cells (75.7%), 12 cells (69.9%), and morulae (74.5%). Taken together, mitochondrial CCO activity rose along with the development of embryos, especially in blastocysts.
Fig. 5.
Changes of cytochrome c oxidase (CCO) activity in human embryos during preimplantation development. DAB tetrahydrochloride is easily incorporated into mitochondria, and oxidative polymerization occurs by high-voltage potential. Accordingly, mitochondria with CCO activity were stained for transmission electron microscopy. a Mitochondria with high CCO activity (black) showed well-developed cristae structures and deeply stained membrane. Mitochondria with deeply stained membrane and without cristae structures were categorized as having moderate CCO activity (gray). Mitochondria with poorly stained membranes and few cristae structures were categorized as having low CCO activity (white). Original magnification ×50,000; bar = 0.5 μm. b The proportions of mitochondria with high, moderate, or low CCO activity in mature oocytes at meiosis II (MII) through to blastocysts (day 5) are shown. The proportions of mitochondria with these CCO activities in the inner cell mass (ICM) and trophectoderm (TE) are also shown (c). The numbers of mitochondria in oocytes or embryos examined are shown in parentheses. Data for CCO activity were compared by Cochran-Armitage test among five groups and by Fisher’s exact test between two groups
Comparison of mitochondrial competence between day 4 embryos
Fifteen embryos on day 4 showed compaction (compacted morula), 11 embryos had 10 or more than 10 blastomeres, and 10 embryos had fewer than 10 blastomeres. There were no differences in the mtDNA copy number among the three stages of day 4 embryos (from 103,773 to 175,044 copies; Table 2). However, the mtOCR, maximum mtOCR, and mtOCR/mtDNA ratio of embryos with <10 blastomeres were significantly lower (P < 0.01) than those of compacted morulae.
Table 2.
Mitochondrial competence of day 4 embryos
| Number of embryos | Maternal age (yo) | OCRs (fmol/s) | Maximum OCRs (fmol/s) | Copy number of mtDNA | OCR/mtDNA (fmol/s) | |
|---|---|---|---|---|---|---|
| Morula | 15 | 35.4 | 1.79a | 3.75a | 103,773 | 1.8 × 10−5a |
| ≥10 | 11 | 31.4 | 1.51ab | 3.43ab | 126,107 | 1.31 × 10−5a |
| <10 | 10 | 33.6 | 0.91b | 2.41b | 175,044 | 0.61 × 10−5b |
a,b p < 0.01 by Kruskal-Wallis test following ANOVA
Mitochondrial activity of ICM cells
mtOCR was measured at one point of the TE located near the ICM and at two points of the TE far from the ICM. The ICM was identified in all expanded blastocysts used in this study (N = 19). There was no difference in the mtOCR between the TE (5.3 fmol/s) and ICM sides 7 h after warming (5.2 fmol/s). In addition, the proportion of mitochondria with high CCO activity in ICM cells (6.1%) was lower than in TE cells (P < 0.01; 32.2%; Fig. 5c).
Discussion
Mitochondria in human oocytes and preimplantation embryos have minimum contents of matrix membranes and appear relatively inactive [30–32]. Here, we found that matrix membranes and mitochondrial functions developed in parallel with embryo growth while the mtDNA copy numbers decreased transiently. OCRs increased toward the morula stage ahead of an increase of mtDNA at the time of blastulation.
Although mtDNA copy numbers remained constant in preimplantation mouse embryos [7, 8], they decreased transiently in those of cattle [9] and pigs [10, 11]. The latter study was consistent with the findings that the expressions of DNA polymerase γ (POLG) and TFAM at 2-, 8-, and 16-cell stages decreased in porcine embryos [10]. The expression levels of transcription and replication factors of mtDNA before the morula stage were extremely low, even though expressions of these genes can be detected during cleavage stages in several mammalian species, such as the sheep [33] and pig [10]. Taken together, the expressions of these genes in human embryos might be downregulated until the morula stage, similarly to those of other mammals. The level of mtDNA in human embryos might also be transiently decreased by mechanisms similar to those involved in the low expression of embryonic genes before their activation [34]. In addition, it has been shown that mtDNA was detected in culture media [35]. Thus, transient decrease of mtDNA would be due to releasing of mtDNA into culture media.
Several recent studies describing an increase of the number of mtDNA copy would correlate with a stressed state [15, 16]. However, Victor et al. revealed that there were no differences after mathematical correction in the number of mtDNA copy in blastocyst between aged (38–46 years old) and young mothers (20–37), between euploid and aneuploidy, and between implanted and not implanted [17]. In this study, cause of infertility did not affect the number of mtDNA copy in 2–4-cell stage embryo in contrast to a previous report [13]. Moreover, we did not consider chromosome normality and further developmental competence after uterine transfer and used mature oocytes that were obtained after an additional culture of immature oocytes at the time of ICSI in accordance with guidelines of the Japan Society of Obstetrics and Gynecology and ethical reason. Time and further studies will tell whether mtDNA levels would be affected by stress state. The data was obtained from ICSI embryos and may not be indicative of what happens with IVF nor natural fertilization.
Estimates of the contribution of mitochondrial respiration to energetic requirements during embryonic development suggest that about 10% of glucose available is metabolized through aerobic respiration in the early stages of development, while it increases to 85% in the blastocyst stage [30], with concomitant increases in OCR in mice [19, 20, 36] and cattle [18]. The present work shows that mtOCR, max mtOCR, and CCO activity in human preimplantation embryos increased along with their development while the mtDNA copy numbers decreased transiently, indicating the activation of mitochondrial metabolism. The mtOCR of well-developed embryos on day 4 was higher than that of delayed embryos with a concomitant increase in the mtOCR/mtDNA ratio, suggesting that the increase in OCR parallels the morphological changes in embryos and might serve as a marker for viable embryos after day 4 because the formation of blastocoel cavity depends on cellular Na/K-ATPase activity [23]. In addition, OCRs increased drastically from morula to blastocyst stage in accordance with previous data [22, 27] earlier than an increase of mtDNA at the time of blastulation.
There was no difference in the OCR between the ICM and TE sides of blastocysts, suggesting a low OCR in the ICM. Cells in the ICM retain a pluripotent status as shown by the expressions of Oct4, Tra-1–60, Tra-1–81, and alkaline phosphatase [37], indicating a relatively dormant state with persistently low levels of POLG and TFAM [4], and depending on anaerobic metabolism [38]. The TE cells contained elongated mitochondria with high CCO activity whereas the mitochondria in ICM cells were spherical with low CCO activity (Fig. 5). Taken together, the undifferentiated state of ICM cells appears to be associated with low levels of expression of mtDNA transcription and replication factors. On the other hand, the mtDNA copy number increases and energy production shifts from anaerobic glycolysis to mitochondrial aerobic metabolism in TE cells during development.
Viable human blastocysts can be grown in defined culture systems in the absence of serum and somatic cells [39]. The development and use of sequential culture media, designed to cater for the changing requirements of embryos during their development and differentiation, have been shown to produce blastocysts with high viability [40–42]. Modifying the energy sources required for mitochondrial function should provide a less stressful environment for embryos. Thus, the present work showing changes in mitochondrial structure, function, and mtDNA copy numbers during preimplantation development will be useful in optimizing culture media for the development of human embryos.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PPTX 73 kb)
Acknowledgments
Author’s contributions
S.H. was involved in the literature review, experimental design, data acquisition, interpretation and analysis, and manuscript preparation. N.M., T.Y., H.G., Y.M., and Y.N. prepared oocytes and embryos. M.Y. and H.M. performed TEM. M.Y. measured OCR. H.M., M.I., and H.S. wrote the manuscript.
Compliance with ethical standards
Funding
Part of this work was supported by a grant (16gk0110014h0001) from the Japan Agency for Medical Research and Development to S.H. and Y.M.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0886-6) contains supplementary material, which is available to authorized users.
References
- 1.Krisher RL, Bavister BD. Responses of oocytes and embryos to the culture environment. Theriogenology. 1998;59:103–14. doi: 10.1016/S0093-691X(97)00405-6. [DOI] [PubMed] [Google Scholar]
- 2.Van Blerkom J, Davis P, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 3.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–80. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 4.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–9. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- 5.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229:353–61. doi: 10.1002/jcp.24457. [DOI] [PubMed] [Google Scholar]
- 7.Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cree LM, Samuels DC, de Sousa Lopes SC, Rajasimha HK, Wonnapinij P, Mann JR, et al. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet. 2008;40:249–54. doi: 10.1038/ng.2007.63. [DOI] [PubMed] [Google Scholar]
- 9.May-Panloup P, Vignon X, Chrétien MF, Heyman Y, Tamassia M, Malthièry Y, et al. Increase of mitochondrial DNA content and transcripts in early bovine embryogenesis associated with upregulation of mtTFA and NRF1 transcription factors. Reprod Biol Endocrinol. 2005;3:65. doi: 10.1186/1477-7827-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod. 2007;76:327–35. doi: 10.1095/biolreprod.106.054536. [DOI] [PubMed] [Google Scholar]
- 11.Cagnone GL, Tsai TS, Makanji Y, Matthews P, Gould J, Bonkowski MS, et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Sci Rep. 2016;6:23229. doi: 10.1038/srep23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May-Panloup P, Chretien MF, Malthiery Y, Reynier P. Mitochondrial DNA in the oocyte and the developing embryo. Curr Top Dev Biol. 2007;77:51–83. doi: 10.1016/S0070-2153(06)77003-X. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Guo N, Zhang XM, Shi W, Tong XH, Iqbal F, et al. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep. 2015;5:10779. doi: 10.1038/srep10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monnot S, Samuels DC, Hesters L, Frydman N, Gigarel N, Burlet P, et al. Mutation dependance of the mitochondrial DNA copy number in the first stages of human embryogenesis. Hum Mol Genet. 2013;22:1867–72. doi: 10.1093/hmg/ddt040. [DOI] [PubMed] [Google Scholar]
- 15.Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241. doi: 10.1371/journal.pgen.1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril. 2015;104:534–41. doi: 10.1016/j.fertnstert.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2016 doi: 10.1016/j.fertnstert.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil. 1996;106:299–306. doi: 10.1530/jrf.0.1060299. [DOI] [PubMed] [Google Scholar]
- 19.Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62:1866–74. doi: 10.1095/biolreprod62.6.1866. [DOI] [PubMed] [Google Scholar]
- 20.Ottosen LD, Hindkjaer J, Lindenberg S, Ingerslev HJ. Murine pre-embryo oxygen consumption and developmental competence. J Assist Reprod Genet. 2007;24:359–65. doi: 10.1007/s10815-007-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnusson C, Hillensjo T, Hamberger L, Nilsson L. Oxygen consumption by human oocytes and blastocysts grown in vitro. Hum Reprod. 1986;1:183–4. doi: 10.1093/oxfordjournals.humrep.a136377. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka M, Hashimoto S, Amo A, Ito-Sasaki T, Abe H, Morimoto Y. Developmental assessment of human vitrified-warmed blastocysts based on oxygen consumption. Hum Reprod. 2011;26:3366–71. doi: 10.1093/humrep/der324. [DOI] [PubMed] [Google Scholar]
- 23.Watson AJ. The cell biology of blastocyst development. Mol Reprod Dev. 1992;33:492–504. doi: 10.1002/mrd.1080330417. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto S, Nakano T, Yamagata K, Inoue M, Morimoto Y, Nakaoka Y. Multinucleation per se is not always sufficient as a marker of abnormality to decide against transferring human embryos. Fertil Steril. 2016;106:133–9. doi: 10.1016/j.fertnstert.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Kuwayama M, Vajta G, Leda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–14. doi: 10.1016/S1472-6483(10)61169-8. [DOI] [PubMed] [Google Scholar]
- 26.Biggers JD, Racowsky C. The development of fertilized human ova to the blastocyst stage in KSOMAA medium: is a two-step protocol necessary? Reprod Biomed Online. 2002;5:133–40. doi: 10.1016/S1472-6483(10)61615-X. [DOI] [PubMed] [Google Scholar]
- 27.Maezawa T, Yamanaka M, Hashimoto S, Amo A, Ohgaki A, Nakaoka Y, et al. Possible selection of viable human blastocysts after vitrification by monitoring morphological changes. J Assist Reprod Genet. 2014;31:1099–104. doi: 10.1007/s10815-014-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner CA, Wolny YM, Barritt JA, Matt DW, Munné S, Cohen J. Mitochondrial DNA deletion in human oocytes and embryos. Mol Hum Reprod. 1998;4:887–92. doi: 10.1093/molehr/4.9.887. [DOI] [PubMed] [Google Scholar]
- 29.Gibson TC, Kubisch HM, Brenner CA. Mitochondrial DNA deletions in rhesus macaque oocytes and embryos. Mol Hum Reprod. 2005;11:785–9. doi: 10.1093/molehr/gah227. [DOI] [PubMed] [Google Scholar]
- 30.Bavister BD, Squirrell JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod. 2000;15(Suppl 2):189–98. doi: 10.1093/humrep/15.suppl_2.189. [DOI] [PubMed] [Google Scholar]
- 31.Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15((Supplt 2)):129–47. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- 32.Sathananthan AH, Selvaraj K, Girijashankar ML, Ganesh V, Selvaraj P, Trounson AO. From oogonia to mature oocytes: inactivation of the maternal centrosome in humans. Microsc Res Tech. 2006;69:396–407. doi: 10.1002/jemt.20299. [DOI] [PubMed] [Google Scholar]
- 33.Bowles EJ, Lee JH, Alberio R, Lloyd RE, Stekel D, Campbell KH, et al. Contrasting effects of in vitro fertilization and nuclear transfer on the expression of mtDNA replication factors. Genetics. 2007;176:1511–26. doi: 10.1534/genetics.106.070177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, Svoboda P, Schultz RM, Stein P. Regulation of zygotic gene activation in the preimplantation mouse embryo: global activation and repression of gene expression. Biol Reprod. 2001;64:1713–21. doi: 10.1095/biolreprod64.6.1713. [DOI] [PubMed] [Google Scholar]
- 35.Stigliani S, Anserini P, Venturini PL, Scaruffi P. Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Hum Reprod. 2013;28:2652–60. doi: 10.1093/humrep/det314. [DOI] [PubMed] [Google Scholar]
- 36.Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996;44:476–85. doi: 10.1002/(SICI)1098-2795(199608)44:4<476::AID-MRD7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.Guest DJ, Allen WR. Expression of cell-surface antigens and embryonic stem cell pluripotency genes in equine blastocysts. Stem Cells Dev. 2007;16:789–96. doi: 10.1089/scd.2007.0032. [DOI] [PubMed] [Google Scholar]
- 38.Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74:11–8. doi: 10.1111/j.1432-0436.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 39.Scholtes MC, Zeilmaker GH. A prospective, randomized study of embryo transfer results after 3 or 5 days of embryo culture in in vitro fertilization. Fertil Steril. 1996;65:1245–8. doi: 10.1016/S0015-0282(16)58349-6. [DOI] [PubMed] [Google Scholar]
- 40.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–82. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 41.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–40. doi: 10.1093/humrep/13.12.3434. [DOI] [PubMed] [Google Scholar]
- 42.Jones GM, Trounson AO, Gardner DK, Kausche A, Lolatgis N, Wood C. Evolution of a culture protocol for successful blastocyst development and pregnancy. Hum Reprod. 1998;13:169–77. doi: 10.1093/humrep/13.1.169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX 73 kb)