Abstract
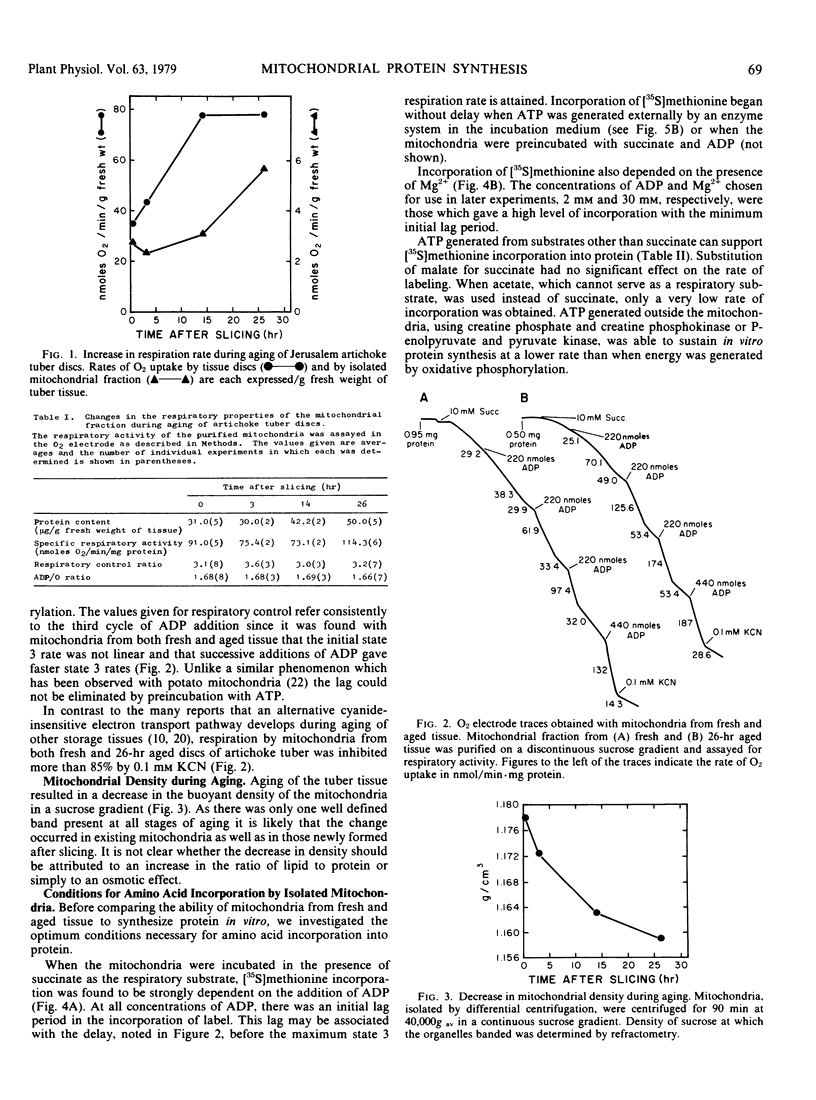
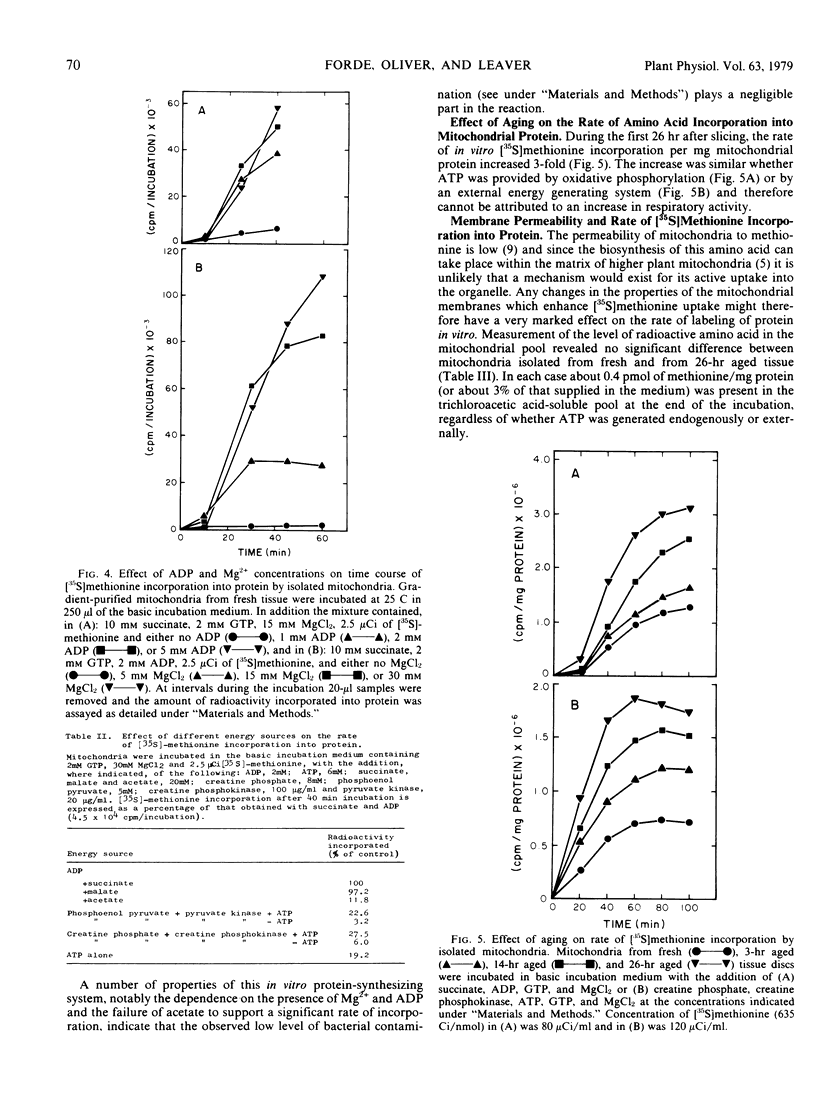
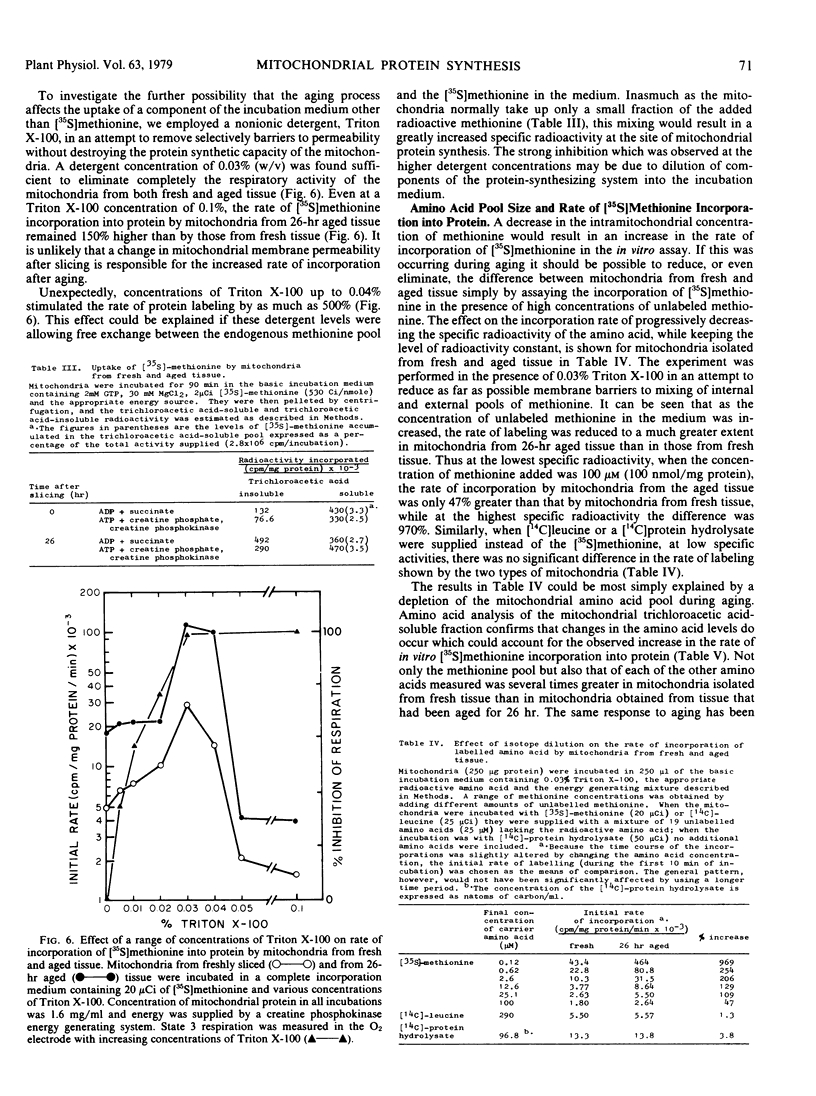
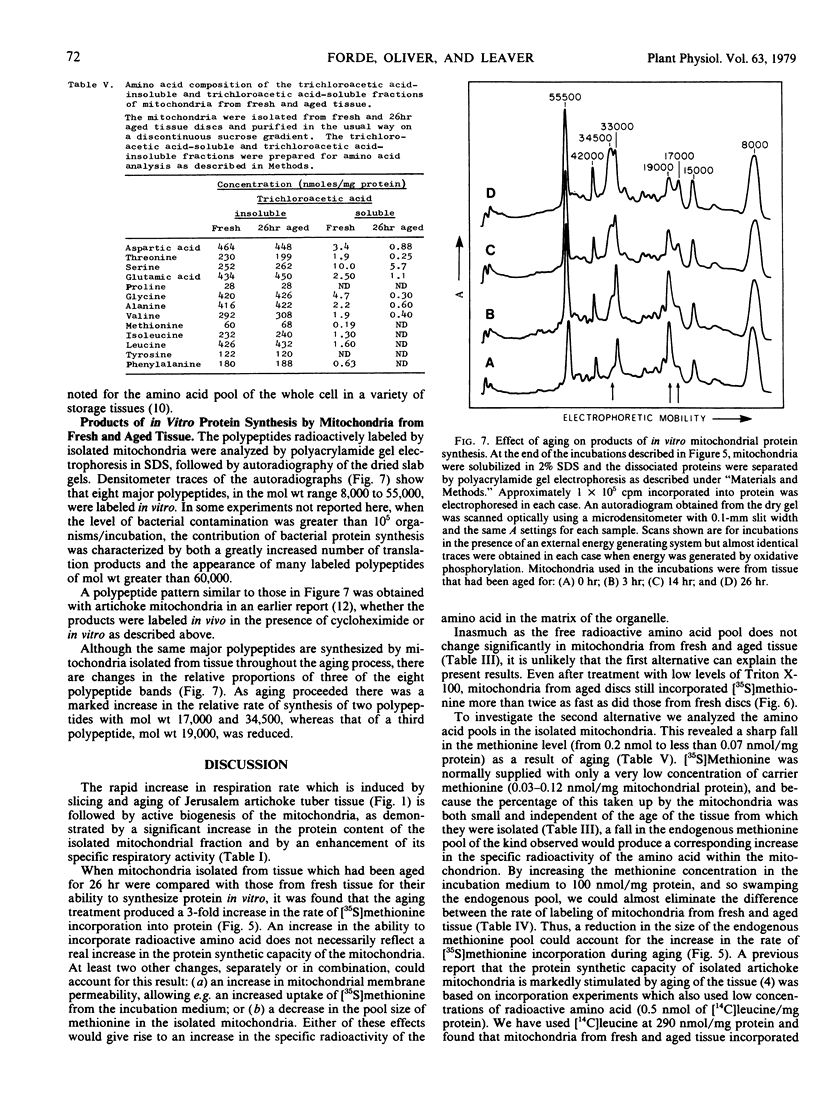
Mitochondrial biogenesis was induced in Jerusalem artichoke (Helianthus tuberosus) tuber by aging tissue discs in distilled water for up to 26 hours. Changes in the purified mitochondrial fraction during aging included an increase in both protein content and specific respiratory activity. Using intact isolated mitochondria, conditions were optimized for incorporation of radioactive amino acid into protein. Incorporation was dependent upon the supply of an oxidizable substrate or an external ATP-generating system and showed characteristic sensitivity to inhibitors of protein synthesis. Aging of the tissue resulted in a 3-fold increase in the rate of in vitro incorporation of [35S]methionine into mitochondrial protein. An analysis of the free amino acid pool in the mitochondrial fraction showed that the decrease in methionine level during aging of intact tissue was sufficient to account for the increased rate of protein labeling. The activation of mitochondrial biogenesis which occurs after slicing is not dependent on an increase in the capacity of mitochondria to synthesize protein as assayed in vitro.
Analysis, by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography, showed that isolated mitochondria synthesized about 15 polypeptides in the molecular weight range 8,000 to 55,000. As aging proceeded, significant changes were observed in the relative rates of labeling of three out of the eight major polypeptides synthesized by mitochondria in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. B., Rowan K. S. Glycolytic control of respiration during aging of carrot root tissue. Plant Physiol. 1970 Apr;45(4):490–494. doi: 10.1104/pp.45.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON J. V., Jr, PATTERSON J. A. ACCELERATED AUTOMATIC CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS ON A SPHERICAL RESIN. Anal Chem. 1965 Aug;37:1108–1110. doi: 10.1021/ac60228a008. [DOI] [PubMed] [Google Scholar]
- CLICK R. E., HACKETT D. P. THE ROLE OF PROTEIN AND NUCLEIC ACID SYNTHESIS IN THE DEVELOPMENT OF RESPIRATION IN POTATO TUBER SLICES. Proc Natl Acad Sci U S A. 1963 Aug;50:243–250. doi: 10.1073/pnas.50.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. M., Edelman J. Relationship between Protein Synthesis in Tuber Discs and the Protein Synthetic Activity of a Cell-Free Preparation. Plant Physiol. 1967 Aug;42(8):1140–1146. doi: 10.1104/pp.42.8.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizengremel P., Lance C. Control of Changes in Mitochondrial Activities during Aging of Potato Slices. Plant Physiol. 1976 Aug;58(2):147–151. doi: 10.1104/pp.58.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Halling P. J., Brand M. D., Chappell J. B. Permeability of mitochondria to neutral amino acids. FEBS Lett. 1973 Aug 15;34(2):169–171. doi: 10.1016/0014-5793(73)80785-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., Kavanagh J. Regulation of mitochondrial protein synthesis by cytoplasmic proteins. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3947–3951. doi: 10.1073/pnas.73.11.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]


