Abstract
Rationale: The receptor for advanced glycation end products (RAGE) is underexpressed in idiopathic pulmonary fibrosis (IPF) lung, but the role of RAGE in human lung fibrosis remains uncertain.
Objectives: To examine (1) the association between IPF risk and variation at rs2070600, a functional missense variant in AGER (the gene that codes for RAGE), and (2) the associations between plasma-soluble RAGE (sRAGE) levels with disease severity and time to death or lung transplant in IPF.
Methods: We genotyped the rs2070600 single-nucleotide polymorphism in 108 adults with IPF and 324 race-/ethnicity-matched control subjects. We measured plasma sRAGE by ELISA in 103 adults with IPF. We used generalized linear and additive models as well as Cox models to control for potential confounders. We repeated our analyses in 168 (genetic analyses) and 177 (sRAGE analyses) adults with other forms of interstitial lung disease (ILD).
Results: There was no association between rs2070600 variation among adults with IPF (P = 0.31). Plasma sRAGE levels were lower among adults with IPF and other forms of ILD than in control subjects (P < 0.001). The rs2070600 allele A was associated with a 49% lower sRAGE level (95% confidence interval [CI], 11 to 71%; P = 0.02) among adults with IPF. In adjusted analyses, lower sRAGE levels were associated with greater disease severity (14% sRAGE decrement per 10% FVC decrement; 95% CI, 5 to 22%) and a higher rate of death or lung transplant at 1 year (adjusted hazard ratio, 1.9 per logarithmic unit of sRAGE decrement; 95% CI, 1.2–3.3) in IPF. Similar findings were observed in a heterogeneous group of adults with other forms of ILD.
Conclusions: Lower plasma sRAGE levels may be a biological measure of disease severity in IPF. Variation at the rs2070600 single-nucleotide polymorphism was not associated with IPF risk.
Keywords: pulmonary fibrosis, biomarker, interstitial lung disease
The receptor for advanced glycosylated end products (RAGE) is a cell surface protein that has been implicated in a wide number of disease processes, including atherosclerosis, Alzheimer’s disease, and diabetic nephropathy (1–4). RAGE ligand binding promotes nuclear factor-κB–mediated inflammation and generation of reactive oxidative species. RAGE can also exist in a soluble form (sRAGE) lacking the transmembrane and cytoplasmic domain (5), which acts as a decoy receptor that binds RAGE ligands, thereby attenuating RAGE-mediated inflammation.
RAGE is expressed primarily on alveolar epithelial cells (AECs), prompting great interest in its role in pulmonary diseases. For example, circulating sRAGE levels are lower and correlate with disease severity in chronic obstructive pulmonary disease (COPD) (6), and RAGE stimulation in the lung contributes to primary graft dysfunction after lung transplant (7). Because RAGE ligand binding promotes inflammation and injury, RAGE has also been studied in interstitial lung disease (ILD), with contradictory findings. RAGE-knockout mice are more susceptible to spontaneous and asbestos-induced pulmonary fibrosis (8), but other studies have suggested that RAGE knockouts are protected from bleomycin-induced lung injury (9, 10). In humans, a number of studies have shown that RAGE expression is decreased in the alveolar and bronchial epithelia in idiopathic pulmonary fibrosis (IPF) (8, 11).
A nonsynonymous single-nucleotide polymorphism (SNP) (rs2070600) located in exon 3 of AGER, the gene that codes for RAGE, has a missense allele (Gly82Ser, allele A) that promotes inflammation by both increasing signal transduction following ligand binding due to altered RAGE glycosylation (12–14) and lowering circulating levels of sRAGE in both healthy adults and individuals with COPD (6, 15). We have reported nearby SNPs in association with reduced percentage of emphysema on computed tomographic scans (16), but no studies have examined AGER variation or sRAGE in IPF.
In light of the potential role of RAGE in promoting inflammation and injury as well as the proinflammatory phenotype of the AGER SNP rs2070600 A allele, we hypothesized that the AGER SNP rs2070600 A allele would be associated with both IPF risk and lower plasma sRAGE levels in IPF. We also hypothesized that lower sRAGE levels would be associated with more severe disease and a higher mortality rate in IPF.
Methods
All participants provided informed consent, and the study was approved by the Columbia University Medical Center Institutional Review Board. Additional methods are presented in the online supplement.
Study Participants and Data Sources
We prospectively enrolled 364 adults with ILD at Columbia University Medical Center who agreed to participate in genetic studies of ILD between 2007 and 2011 (17). We excluded 15 with poor genotype quality control, 27 outliers in race-/ethnicity-specific principal components of ancestry, and an additional 6 for whom a matching control subject was not available, leaving 316 cases for genetic analyses: 108 with IPF and 208 with other ILDs. Of these, 291 had available plasma. We further excluded 6 with unreliable sRAGE measurements and 5 with missing covariate data, leaving 280 with sRAGE measurements. Twenty-one healthy control subjects were also recruited.
Control subjects for genetic analyses were sampled from the Multi-Ethnic Study of Atherosclerosis (MESA), a National Heart, Lung, and Blood Institute–funded prospective cohort study of 6,814 community-dwelling adults aged 45–84 years old at enrollment in 2000–2002 and from the MESA Air and MESA Family studies (see online supplement) (18–20). We restricted selection of control subjects to those MESA participants free of self-reported chronic lung diseases other than asthma. Each adult with ILD was then matched to three eligible MESA control subjects of the same race/ethnicity.
We also examined non-Hispanic white participants with fibrotic idiopathic interstitial pneumonia (IIP) (n = 1,616) in a previously published Columbia University genome-wide association study and a group of control subjects (n = 4,683) (21). Fibrotic IIPs included IPF (77%; sporadic and familial), unclassified fibrosis, and some nonspecific interstitial pneumonia.
Genotyping
rs2070600 was among the genotyped SNPs passing quality control in patients with ILD (Axiom Biobank chip; Affymetrix, Santa Clara, CA), MESA (HumanExome BeadChip version 1.0; Illumina, San Diego, CA), and the Colorado-based genome-wide association study (Illumina 600 Quad BeadChip).
sRAGE Measurement and AGER Expression in Lung Tissue
Using commercially available ELISA kits (R&D Systems, Minneapolis, MN), we measured plasma levels of sRAGE in participants with ILD. The intraassay coefficient of variation for this assay is less than or equal to 6.2%, and the interassay coefficient of variation is less than or equal to 8.2%. We also measured sRAGE in 21 healthy control subjects. mRNA expression of AGER was measured by quantitative reverse transcription–polymerase chain reaction in anonymized optimal cutting temperature compound–embedded lung tissue from 15 adults with IPF (and a confirmed usual interstitial pneumonia pattern on biopsy) and from 15 adults without lung disease undergoing lung resection for reasons other than ILD obtained from the Columbia University Pathology Shared Resource Tissue Bank.
Statistical Analyses
We performed a case–control study to examine the association of the rs2070600 A allele with IPF in 108 adults with IPF and 324 race-/ethnicity-matched control subjects using logistic regression. We repeated this analysis in 168 adults with other forms of ILD and 504 control subjects. We used Spearman correlation coefficients, Wilcoxon rank-sum tests, Kruskal-Wallis tests, and Dunn’s post hoc comparisons with Hochberg’s method with a two-sided α of 0.05 when appropriate. We used linear regression to test the association between sRAGE levels and AGER rs2070600 genotype with adjustment for age, sex, body mass index (BMI), and FVC percent predicted (FVC%), and we used logistic regression to generate receiver operating characteristic curves. We used generalized additive models with locally weighted smoothing terms for FVC% to examine associations between FVC% and sRAGE, adjusting for age, sex, BMI, and AGER genotype. We used Cox proportional hazards models to examine associations between sRAGE level and time to the combined event of death or lung transplant, adjusted for age, sex, smoking status, and BMI.
Results
Baseline Characteristics
Study participants with IPF had a mean age of 65 years, 28% were women, 86% were of non-Hispanic white race, 5% were of non-Hispanic black race, and 5% were Hispanic (Table 1; see also Table E1 in the online supplement). The mean FVC% was 62%, and the mean diffusing capacity of the lung for carbon monoxide (DlCO) was 36% of the predicted value. Sixty-seven percent were former smokers, 19% had coronary artery disease, 40% had gastroesophageal disease, 43% had gastroesophageal reflux disease, 37% were prescribed corticosteroids, 10% were prescribed an immunosuppressive or immunomodulatory therapy (in combination with a corticosteroid in all but one case), and 30% were prescribed oral N-acetylcysteine. The characteristics of participants with other ILDs are in shown in Table 1.
Table 1.
Baseline characteristics of participants with plasma soluble receptor for advanced glycation end products measurements
| Characteristic | IPF | Other ILD |
|---|---|---|
| Age, yr | 64.8 ± 8.2 | 56.7 ± 11.2 |
| Female sex | 28% | 91% |
| Race/ethnicity | ||
| White | 86% | 72% |
| Black | 5% | 27% |
| Hispanic | 5% | 7% |
| Other | 7% | 6% |
| Diagnosis | ||
| Connective tissue disease/ILD | — | 27% |
| Other idiopathic interstitial pneumonia* | — | 42% |
| Chronic hypersensitivity pneumonitis | — | 15% |
| Combined pulmonary fibrosis and emphysema | — | 7% |
| Other† | — | 9% |
| FVC, % predicted | 62.4 ± 18.7 | 53.9 ± 18.4 |
| Diffusing capacity of the lung for carbon monoxide, % predicted | 35.5 ± 11.1 | 32.5 ± 11.3 |
| Body mass index, kg/m2 | 27.8 ± 4.6 | 28.9 ± 5.8 |
Definition of abbreviations: ILD = interstitital lung disease; IPF = idiopathic pulmonary fibrosis.
Data are mean ± SD and percentage. Diffusing capacity of the lung for carbon monoxide data were available for 175 participants. FVC and body mass index data were available for 270 participants.
Idiopathic nonspecific interstitial pneumonia (n = 32), desquamative interstitial pneumonia (n = 1), cryptogenic organizing pneumonia (n = 5), idiopathic pleuroparenchymal fibroelastosis (n = 2), and unclassifiable idiopathic interstitial pneumonia (n = 34).
Two cases each of pulmonary asbestosis, pulmonary berylliosis, and pulmonary Langerhans cell histiocytosis; one case each of drug-induced ILD, radiation-induced ILD, pulmonary sarcoidosis, pulmonary hyalinizing granulomatosis, idiopathic pulmonary hemosiderosis, microscopic polyangiitis with pulmonary fibrosis, Hermansky-Pudlak syndrome, and ILD attributed to Cogan’s syndrome.
Associations between AGER Genotype and IPF Risk
The prevalence of the rs2070600 A allele was 4.2% among IPF cases and 2.8% among control subjects. We did not detect an association between the A allele and IPF risk (conditional odds ratio, 1.51; 95% confidence interval [CI], 0.68–3.36; P = 0.31) (Table E2). Findings were similar among those with other forms of ILD (conditional odds ratio, 1.71; 95% CI, 0.90–3.29; P = 0.10). In an analysis restricted to non-Hispanic white subjects, the rs2070600 A allele was associated with non-IPF forms of ILD (conditional odds ratio, 2.18; 95% CI, 1.07–4.43; P = 0.03) but not with IPF (P = 0.34). We did not identify a statistically significant association of rs2070600 with fibrotic IIP in the Colorado genome-wide association study (P = 0.54).
sRAGE Levels in IPF and Other Forms of ILD
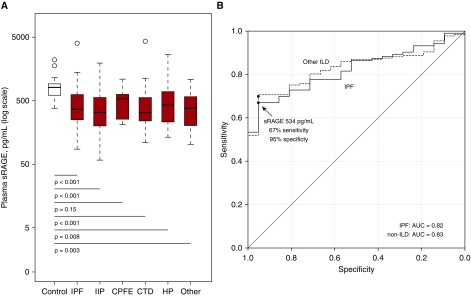
The mean (SD) sRAGE level was 487.1 pg/ml (451.3 pg/ml) among those with IPF and 855.9 pg/ml (410.5 pg/ml) among 21 healthy control subjects (P < 0.001) (Figure 1A). Plasma sRAGE levels were lower among those receiving immunosuppressive therapy (P = 0.001), but they did not appear to differ by smoking status, presence of gastroesophageal reflux, or between those with IPF versus combined pulmonary fibrosis and emphysema (Figure E1). Compared with control subjects, mean plasma sRAGE levels were consistently lower among those with other IIPs, connective tissue disease–related ILD, hypersensitivity pneumonitis, and a heterogeneous group of other ILDs (P < 0.01 compared with control subjects for each comparison) (Figure 1A). The range of sRAGE levels was qualitatively similar across ILD categories (Figure 1A).
Figure 1.
Plasma sRAGE levels in 280 adults with interstitial lung disease and 21 healthy adult control subjects. (A) Results are presented as box plots of natural log-transformed sRAGE levels. Within each box plot, the thick horizontal line represents the median fold difference, the ends of the box plots are placed at the 25th and 75th percentiles (interquartile range), the whiskers extend to 1.5 times the interquartile range, and outliers are represented by open circles. The white color denotes healthy control subjects, and the red color denotes adults with ILD. P < 0.001 by Kruskal-Wallis test for comparisons between the seven ILD groups. P values for comparison of sRAGE values between each case group and control subjects are derived from Dunn’s post hoc test with Hochberg’s adjustment. (B) Receiver operating characteristic curves for plasma sRAGE to discriminate between adults with IPF and control subjects (solid line; AUC, 0.82) and between adults with other ILDs and control subjects (dashed line; AUC, 0.83). A plasma sRAGE level less than 534 pg/ml had a 67% sensitivity and 95% specificity for a diagnosis of IPF, denoted by the arrow and the black dot. The black dot on the dashed line represents the sensitivity and specificity of 534 pg/ml for adults with other ILDs compared with control subjects (see text). AUC = area under the receiver operating characteristic curve; CPFE = combined pulmonary fibrosis and emphysema; CTD = connective tissue disease-related interstitial lung disease; HP = hypersensitivity pneumonitis; IIP = other idiopathic interstitial pneumonias; ILD = interstitital lung disease; IPF = idiopathic pulmonary fibrosis; sRAGE = soluble receptor for advanced glycation end products.
Lower plasma sRAGE levels moderately discriminated IPF cases from control subjects (area under the receiver operating characteristic curve [AUC], 0.82; 95% CI, 0.75–0.87) (Figure 1B). Plasma sRAGE levels below 534.3 pg/ml (the value maximizing sensitivity and specificity) had a 67% sensitivity and a 95% specificity for IPF. The AUC was 0.83 for non-IPF ILD cases compared with control subjects (95% CI, 0.77–0.89). The cutoff of 534.3 pg/ml had a 75% sensitivity and a 95% specificity for non-IPF ILD compared with control subjects.
Associations between Plasma RAGE Levels and AGER Genotype
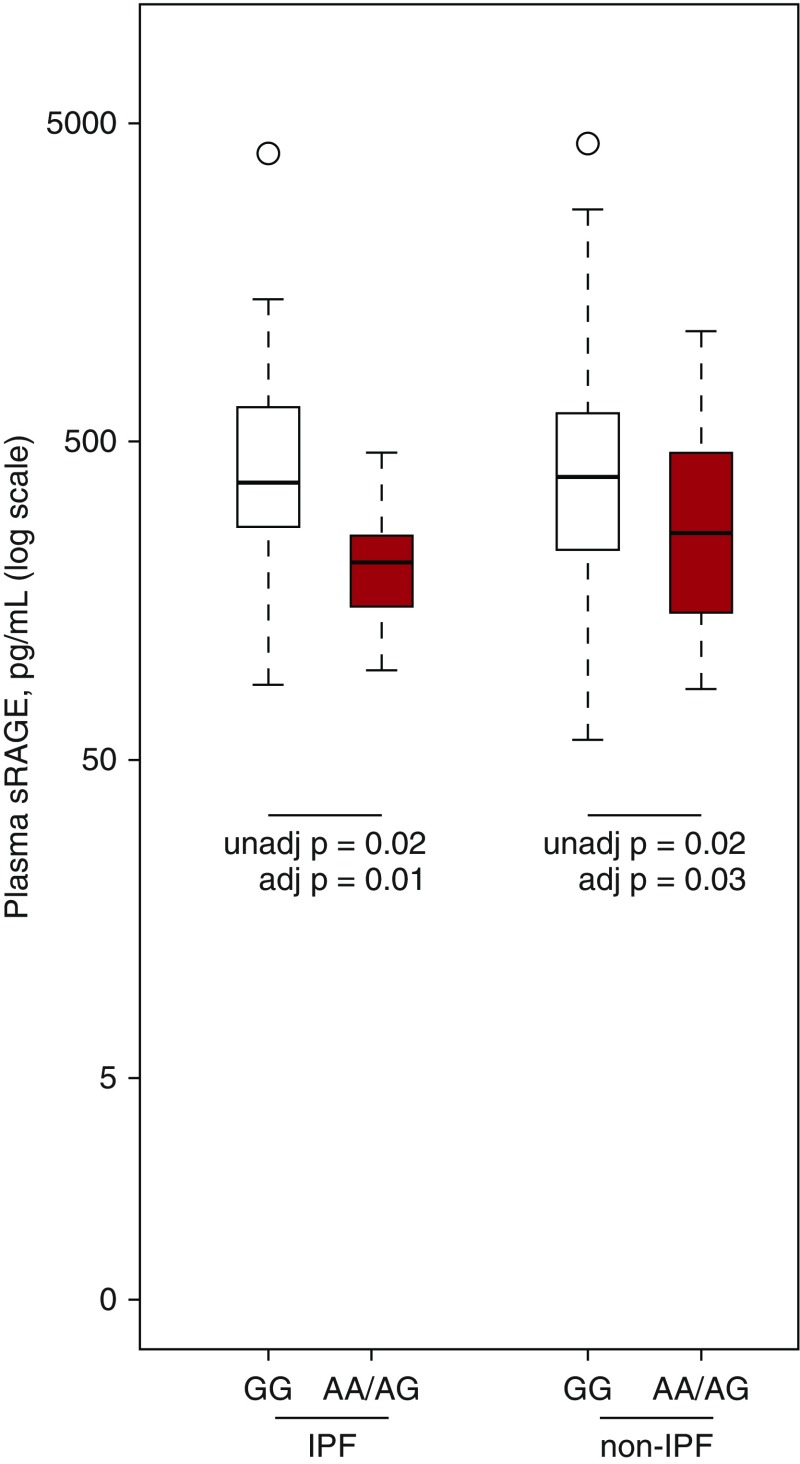
Among those with IPF, plasma sRAGE levels were 49% lower among those with the rs2070600 A allele (95% CI, 11 to 71%; P = 0.02) (Figure 2). After adjustment for age, sex, BMI, diagnosis, and FVC%, this difference persisted (49% reduction; 95% CI, 15 to 69%; P = 0.01). Among those with other forms of ILD, sRAGE levels were also lower in those with the A allele in unadjusted (33% reduction; 95% CI, 6 to 53%; P = 0.02) (Figure 2) and adjusted (29% reduction; 95% CI, 4 to 47%; P = 0.03) analyses.
Figure 2.
Variation in plasma sRAGE levels by rs2070600 genotype in adults with IPF and other interstitial lung diseases. Red color denotes those with the AA or AG genotype, and white color denotes those with the GG genotype. Unadjusted P values for the comparisons between rs2070600 genotypes are derived from Wilcoxon rank-sum tests. Adjusted P values are derived from Wald tests for rs2070600 genotype in a linear model where log-transformed sRAGE is the dependent variable. Covariates in all models were age, sex, body mass index, and FVC percent predicted. IPF = idiopathic pulmonary fibrosis; sRAGE = soluble receptor for advanced glycation end products. Please see legend to Figure 1 for definitions of boxes and whiskers in this plot.
Associations between Plasma RAGE Levels, FVC, and Time to Death or Lung Transplant
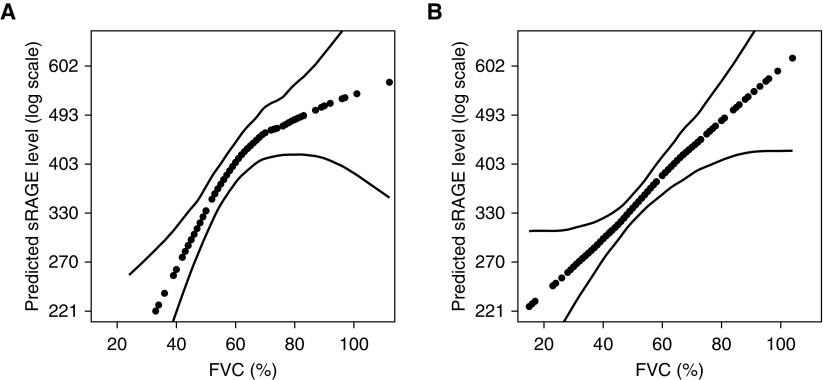
The Spearman correlation coefficient for plasma sRAGE and FVC% was 0.46 among those with IPF (P < 0.001) and 0.27 (P < 0.001) among those with non-IPF forms of ILD, respectively. Correlations between plasma sRAGE and other measurements are shown in Table E3. After adjustment for age, sex, BMI, and AGER rs2070600 genotype, each absolute 10% decrement in FVC% was associated with a 14% decrement in sRAGE among those with IPF (95% CI, 5 to 22%; P = 0.001) and a 12% decrement in sRAGE among those with non-IPF forms of ILD (95% CI, 6 to 20%; P = 0.001) (Figure 3).
Figure 3.
Continuous association between FVC percent predicted (FVC%) and plasma soluble receptor for advanced glycation end products (sRAGE) level among (A) adults with idiopathic pulmonary fibrosis (IPF) and (B) adults with other forms of interstitial lung disease (ILD). The dotted lines are smoothed regression lines adjusted for age, sex, AGER genotype, and body mass index. The thin dashed lines are 95% confidence intervals. The P values for the associations between FVC% and sRAGE were 0.001 for IPF and less than 0.001 for other ILDs.
The median follow-up time was 1.6 years (interquartile range, 6.2 mo to 3.8 yr) among those with IPF, and it was 1.3 years (interquartile range, 4.8 mo to 3.6 yr) among those with other ILDs. In an unadjusted analysis, there was a twofold increased rate of death or lung transplant at 1 year per natural logarithmic decrement in sRAGE (hazard ratio [HR], 2.0 per doubling; 95% CI, 1.2–3.4; P = 0.005) (Table 2). After adjustment for age, sex, BMI, and smoking status, this association persisted (HR, 1.9 per doubling; 95% CI, 1.2–3.3; P = 0.01). Among those with other forms of ILD, this adjusted association was similar (HR, 1.9 per doubling; 95% CI, 1.2–3.0; P = 0.009). Additional adjustment for FVC% greatly attenuated these associations (data not shown), suggesting that the association between sRAGE level and mortality is explained largely by the ability of sRAGE to capture disease severity. The associations between sRAGE levels and the time to death or lung transplant at 3 years were somewhat attenuated compared with 1-year outcomes, but the adjusted associations remained significant (Table 2).
Table 2.
Association between plasma soluble receptor for advanced glycation end products level and time to death or lung transplant at 1 year
| IPF | Other ILDs | |
|---|---|---|
| 1-yr outcomes | ||
| Number of decedents | 14 | 27 |
| Number of transplant events | 26 | 23 |
| Person-years | 75.3 | 135.7 |
| Event rate (95% CI)* | 53.1 (38.5–71.6) | 36.9 (27.6–48.1) |
| Hazard ratio (95% CI) per natural logarithmic decrement in sRAGE | ||
| Unadjusted | 2.0 (1.2–3.4) | 1.5 (0.98–2.3) |
| Adjusted† | 1.9 (1.2–3.3) | 1.9 (1.2–3.0) |
| 3-yr outcomes | ||
| Number of decedents | 35 | 54 |
| Number of transplant events | 26 | 32 |
| Person-years | 157.9 | 298.5 |
| Event rate (95% CI)* | 38.6 (29.8–49.3) | 28.8 (23.2–35.4) |
| Hazard ratio (95% CI) per natural logarithmic decrement in sRAGE | ||
| Unadjusted | 1.6 (1.1–2.5) | 1.2 (0.9–1.7) |
| Adjusted† | 1.6 (1.03–2.4) | 1.5 (1.04–2.1) |
Definition of abbreviations: CI = confidence interval; ILD = interstitital lung disease; IPF = idiopathic pulmonary fibrosis; sRAGE = soluble receptor for advanced glycation end products.
Rate of death or lung transplant per 100 person-years.
Adjusted for age, sex, body mass index, and smoking status.
AGER Expression in IPF Lung Tissue
AGER mRNA expression in lung tissue was lower among IPF cases than among control subjects (mean fold change in expression among cases compared with control subjects, 0.16; 95% CI, 0.07–0.39; P = 0.001) (Figure E2). There were no appreciable qualitative differences in the intensity or distribution of RAGE immunostaining between IPF and control lung: sRAGE protein was uniformly present to a mild to moderate degree in bronchiolar epithelium, club cells, type 1 AECs, type 2 AECs, endothelial cells, smooth muscle cells, and alveolar macrophages, and there was little to no expression in fibroblasts.
Discussion
We found that plasma sRAGE levels are reduced in adults with IPF and other forms of ILD, and that lower plasma sRAGE levels are strongly associated with greater disease severity in IPF. Among adults with IPF, circulating sRAGE levels were lower among those with the rs2070600 missense A allele, a finding also reported in COPD and in healthy adults (6, 15). Consistent with a previous report (11), we also found reduced expression of AGER mRNA in usual interstitial pneumonia lung. There was no association between rs2070600 variation and IPF risk. We found similar results among a heterogeneous group of patients with ILD.
Our findings as a whole indicate that lower sRAGE levels track closely with disease severity in IPF. Indeed, lower sRAGE levels were associated with a higher rate of death or lung transplant in our study, a finding confounded by FVC%. Although this result may at first appear to negate the importance of sRAGE in IPF, we believe a more nuanced relationship underlies this observation, namely that reductions in circulating sRAGE levels may be a biological measure of abnormal lung structure (through loss of RAGE-expressing AECs) and therefore may be a biomarker of FVC%, reflecting the loss AECs (the source of sRAGE) that accompanies lung fibrosis. The consistency of the association between disease severity and sRAGE levels in IPF, in various forms of ILD, and in COPD suggest that this relationship may hold true across disease states and be specific to AECs rather than to a particular disease. For example, lower sRAGE levels are associated with lower FEV1/FVC ratio, lower DlCO, increased emphysema, and higher Global Initiative for Chronic Obstructive Lung Disease stage in COPD (6). Although sRAGE levels should not replace FVC and FEV1 as the gold standard measures of disease severity in IPF and COPD, respectively, the availability of a biological measure of the burden of pathological changes in the lung may be of use in the research setting as a measure of response to treatments aimed at ameliorating (or even reversing) attrition of healthy alveolar tissue in these disease states.
Although our data do not support a role for the RAGE pathway in promoting lung injury and inflammation in ILD, it is intriguing to postulate mechanisms by which sRAGE might play a protective role in IPF or other ILDs by sequestering RAGE ligands. In contrast to membrane-bound RAGE, sRAGE exerts an antiinflammatory effect by acting as a decoy receptor that sequesters RAGE ligands, thereby attenuating membrane-bound RAGE stimulation (15). sRAGE expression is decreased in IPF lung (8, 22, 23), and stimulation of RAGE by its ligands leads to proinflammatory signaling via the mitogen-activated protein kinase pathway, which has been implicated in IPF pathogenesis (24, 25). In addition, lysophosphatidic acid, a candidate therapeutic target in IPF (26), has been shown to bind to RAGE (27). Nevertheless, targeting the RAGE pathway in IPF should be approached cautiously. A recent phase II clinical trial failed to identify a beneficial effect of a RAGE ligand–binding inhibitor in Alzheimer’s disease (28), despite strong preclinical evidence (29). Furthermore, sRAGE administration has been shown not to attenuate lung fibrosis in the bleomycin model (9). Nevertheless, sRAGE analogues, RAGE antagonists, and interventions that block RAGE signal transduction may hold promise to attenuate disease progression in IPF.
Allelic variation at the rs2070600 SNP has previously been shown to have functional significance, which may be relevant in IPF. The A allele enhances downstream signaling following RAGE ligand binding, perhaps due to increased N-glycosylation of the RAGE protein (4, 13), which could facilitate lung inflammation and fibrosis. RAGE signaling through this pathway has been shown to promote fibroblast activation (30). Data from the Encyclopedia of DNA Elements (ENCODE) project predict enhancer histone marks, DNase hypersensitivity sites, and multiple regulatory motifs altered at the site of rs2070600 (31). Although we were unable to identify an association between rs2070600 SNP variation and IPF risk, the rs2070600 A allele was associated with reduced sRAGE levels, which could attenuate lung injury, perhaps by binding RAGE ligands, such as S100A12 (32), that might contribute to lung inflammation or injury.
Our study was subject to a number of limitations. First, our results should not be interpreted to indicate that sRAGE levels are a clinically useful diagnostic or prognostic biomarker in IPF, particularly because sRAGE is not associated with survival after accounting for disease severity. The high AUC and specificity for a low plasma sRAGE level to discriminate individuals with IPF from control subjects should not be applied clinically, because these results likely overestimate the true ability of sRAGE to identify IPF or ILD in a more heterogeneous clinical population. Second, the observational nature of our study limits the inferences we can make about the role of sRAGE in IPF progression. Interventional studies designed to attenuate RAGE signaling in the lung (or to increase sRAGE levels) might shed light on whether the RAGE pathway is involved in IPF progression. Finally, we lacked longitudinal measurement of sRAGE, which might provide additional prognostic information.
In summary, adults with IPF have reduced sRAGE levels compared with healthy control subjects, and lower sRAGE levels are associated with greater disease severity and higher mortality in IPF. Our data suggest that sRAGE may be a biological measure of AEC mass in IPF. Future studies should investigate whether circulating sRAGE may be responsive to therapies aimed at preserving the alveolar epithelium in IPF. In addition, consideration should be given to determining whether interventions that specifically raise sRAGE levels help slow IPF progression.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the participants, investigators, and study staff of MESA, the Columbia ILD Study, and the Colorado GWAS.
Footnotes
This work was primarily supported by the National Institutes of Health (NIH) and the Pulmonary Fibrosis Foundation. The Multi-Ethnic Study of Atherosclerosis (MESA) and the MESA SNP Health Association Resource (SHARe) project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-001079, UL1-TR-000040, and DK063491. MESA Family is conducted and supported by the NHLBI in collaboration with MESA investigators. Support is provided by grants and contracts R01 HL071051, R01 HL071205, R01 HL071250, R01 HL071251, R01 HL071258, and R01 HL071259; by National Center for Research Resources grant UL1RR033176; and by National Center for Advancing Translational Sciences grant UL1TR000124. MESA Air is conducted and supported by the U.S. Environmental Protection Agency (EPA) in collaboration with MESA Air investigators, with support provided by grant RD83169701. Funding for MESA genotyping was provided by NHLBI contract N02‐HL‐6‐4278. MESA SHARe genotyping was performed at Affymetrix (Santa Clara, CA) and the Broad Institute of Harvard University and the Massachusetts Institute of Technology (Boston, MA) using the Affymetrix Genome-Wide Human SNP Array 6.0. The MESA Lung and MESA COPD studies are funded by NIH grants R01 HL077612 and R01 HL093081. The MESA Lung/SHARe Study was funded by NIH grant RC1HL100543. The MESA Lung Fibrosis Study was funded by NIH grant R01 HL103676 and the Pulmonary Fibrosis Foundation. The present study was supported by NIH grants K23 HL086714, KL2 TR000081, UL1 TR000040, R01 HL106041, K24 HL103844, and K24 HL131937. The Colorado-based idiopathic interstitial pneumonia genome-wide association study was supported by the NHLBI (grants R01 HL095393, R01 HL097163, P01 HL092870, RC2 HL101715, U01 HL089897, U01 HL089856, U01 HL108642, and P50 HL0894932), the Veterans Administration (grant 1I01BX001534), the Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease at UPMC, and InterMune.
The views expressed in this document are solely those of the authors. The EPA and the Pulmonary Fibrosis Foundation do not endorse any products or commercial services mentioned in this publication.
Author Contributions: Study design: A.M., G.R., S.M.K., T.E.F., D.A.S., S.S.R., R.G.B., and D.J.L.; phenotype data acquisition and quality control: A.M., E.A.F., J.D.K., K.D.H.-S., S.M.K., S.J., D.A.S., R.G.B., and D.J.L.; genotype data acquisition and quality control: A.M., L.S., S.O.-G., E.A.F., T.E.F., D.A.S., and S.S.R.; tissue acquisition and staining, biomarker measurement, pathology review and interpretation, and gene expression analysis: L.S., A.C.B., and W.L.; data analysis: A.M., L.S., S.K.M., W.Z., T.E.F., J.L.S., S.S.R., R.G.B., and D.J.L.; and critical revision of the manuscript: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schmidt AM, Mora R, Cao R, Yan SD, Brett J, Ramakrishnan R, Tsang TC, Simionescu M, Stern D. The endothelial cell binding site for advanced glycation end products consists of a complex: an integral membrane protein and a lactoferrin-like polypeptide. J Biol Chem. 1994;269:9882–9888. [PubMed] [Google Scholar]
- 2.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice: a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 5.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng DT, Kim DK, Cockayne DA, Belousov A, Bitter H, Cho MH, Duvoix A, Edwards LD, Lomas DA, Miller BE, et al. TESRA and ECLIPSE Investigators. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:948–957. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 7.Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, Vittal R, Capitano ML, Kim Y, Allette YM, et al. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med. 2014;6:252ra124. doi: 10.1126/scitranslmed.3009443. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, et al. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008;172:583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englert JM, Kliment CR, Ramsgaard L, Milutinovic PS, Crum L, Tobolewski JM, Oury TD. Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. Int J Clin Exp Pathol. 2011;4:241–254. [PMC free article] [PubMed] [Google Scholar]
- 10.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, Yamaya M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1427–L1436. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 11.Queisser MA, Kouri FM, Königshoff M, Wygrecka M, Schubert U, Eickelberg O, Preissner KT. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am J Respir Cell Mol Biol. 2008;39:337–345. doi: 10.1165/rcmb.2007-0244OC. [DOI] [PubMed] [Google Scholar]
- 12.Park SJ, Kleffmann T, Hessian PA. The G82S polymorphism promotes glycosylation of the receptor for advanced glycation end products (RAGE) at asparagine 81: comparison of wild-type rage with the G82S polymorphic variant. J Biol Chem. 2011;286:21384–21392. doi: 10.1074/jbc.M111.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osawa M, Yamamoto Y, Munesue S, Murakami N, Sakurai S, Watanabe T, Yonekura H, Uchigata Y, Iwamoto Y, Yamamoto H. De-N-glycosylation or G82S mutation of RAGE sensitizes its interaction with advanced glycation endproducts. Biochim Biophys Acta. 2007;1770:1468–1474. doi: 10.1016/j.bbagen.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, Lalla E, Chitnis S, Monteiro J, Stickland MH, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–135. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 15.Jang Y, Kim JY, Kang SM, Kim JS, Chae JS, Kim OY, Koh SJ, Lee HC, Ahn CW, Song YD, et al. Association of the Gly82Ser polymorphism in the receptor for advanced glycation end products (RAGE) gene with circulating levels of soluble RAGE and inflammatory markers in nondiabetic and nonobese Koreans. Metabolism. 2007;56:199–205. doi: 10.1016/j.metabol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Manichaikul A, Hoffman EA, Smolonska J, Gao W, Cho MH, Baumhauer H, Budoff M, Austin JH, Washko GR, Carr JJ, et al. Genome-wide study of percent emphysema on computed tomography in the general population: the Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189:408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184:842–847. doi: 10.1164/rccm.201104-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, Loyd JE, Cosgrove GP, Lynch D, Groshong S, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlmeier S, Mazur W, Salmenkivi K, Myllärniemi M, Bergmann U, Kinnula VL. Proteomic studies on receptor for advanced glycation end product variants in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Proteomics Clin Appl. 2010;4:97–105. doi: 10.1002/prca.200900128. [DOI] [PubMed] [Google Scholar]
- 23.Bargagli E, Penza F, Bianchi N, Olivieri C, Bennett D, Prasse A, Rottoli P. Controversial role of RAGE in the pathogenesis of idiopathic pulmonary fibrosis. Respir Physiol Neurbiol. 2009;165:119–122. doi: 10.1016/j.resp.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Vittal R, Fisher A, Gu H, Mickler EA, Panitch A, Lander C, Cummings OW, Sandusky GE, Wilkes DS. Peptide-mediated inhibition of mitogen-activated protein kinase-activated protein kinase-2 ameliorates bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2013;49:47–57. doi: 10.1165/rcmb.2012-0389OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro-Partida J, Martinez-Rizo AB, Gonzalez-Cuevas J, Arrevillaga-Boni G, Ortiz-Navarrete V, Armendariz-Borunda J. Pirfenidone restricts Th2 differentiation in vitro and limits Th2 response in experimental liver fibrosis. Eur J Pharmacol. 2012;678:71–77. doi: 10.1016/j.ejphar.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 27.Rai V, Touré F, Chitayat S, Pei R, Song F, Li Q, Zhang J, Rosario R, Ramasamy R, Chazin WJ, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209:2339–2350. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, Thomas RG, Aisen PS Alzheimer’s Disease Cooperative Study. Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology. 2014;82:1536–1542. doi: 10.1212/WNL.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Chen H, Zhu X, Ma Y, Liu Q, Xue Y, Chu H, Wu W, Wang J, Zou H. S100A9 promotes human lung fibroblast cells activation through receptor for advanced glycation end-product-mediated extracellular-regulated kinase 1/2, mitogen-activated protein-kinase and nuclear factor-κB-dependent pathways. Clin Exp Immunol. 2013;173:523–535. doi: 10.1111/cei.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.