Abstract
In Spinacia oleracea the kinetics of CO2 fixation, of starch formation, and of changes in the levels of metabolites in chloroplasts and the surrounding medium has been investigated during light-dark and dark-light transitions with isolated intact chloroplasts.
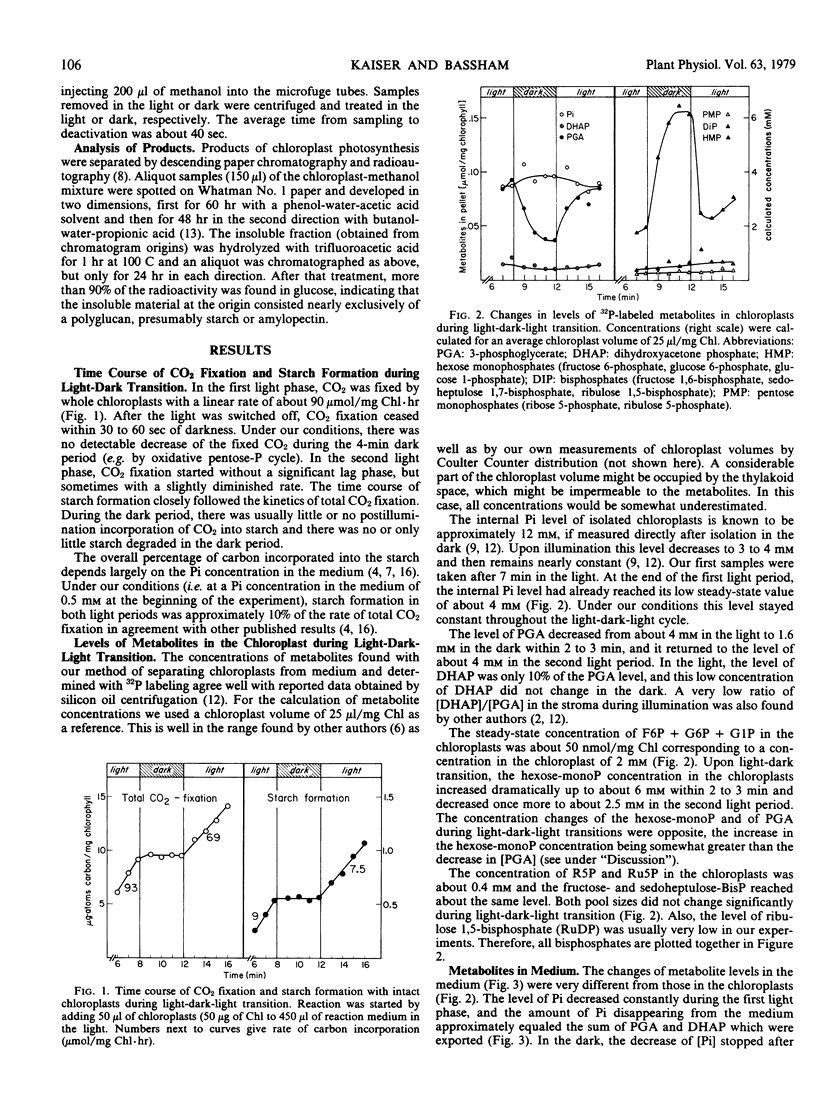
The internal level of orthophosphate stays constant throughout a light-dark-light cycle. The concentration of 3-phosphoglycerate in the chloroplasts is about 4 millimolar in the light and decreases in the dark within 3 minutes to about 1.6 millimolar. The level of the hexose monophosphates shows a reverse trend, increasing from about 2.2 millimolar in the light to 6 millimolar in darkness. In the subsequent light period both compounds reach their original levels within 2 minutes. The chloroplastic concentrations of dihydroxyacetone phosphate, of the pentose monophosphates, and of the hexose- and heptose bisphosphates remain constant at about 0.4 millimolar throughout the light-dark-light cycle.
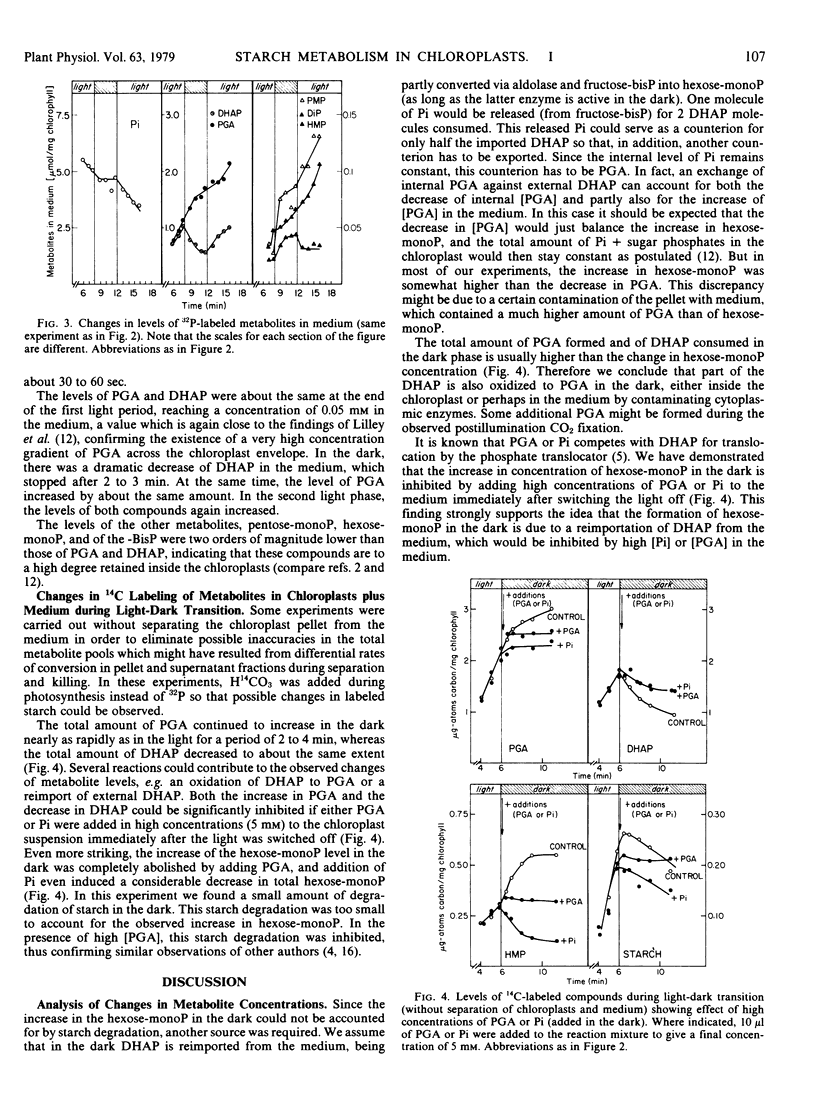
In the medium, the concentration of 3-phosphoglycerate increases and dihydroxyacetone phosphate decreases in the dark phase: this is due to an exchange of internal 3-phosphoglycerate for external dihydroxyacetone phosphate. Part of the reimported dihydroxyacetone phosphate is converted into hexose monophosphates via aldolase and fructose bisphosphatase during the first minutes of darkness. Due to the observed exchange transport reactions, the large difference between the transenvelope concentration gradients of 3-phosphoglycerate, dihydroxyacetone phosphate, and orthophosphate which exist in the light, is completely abolished after 2 to 3 minutes in the dark.
The kinetics and the magnitudes of the changes of metabolite concentrations during the light-dark-light cycle are compared to the kinetics of starch formation, and their relevance for a possible light-dark regulation of starch synthesis is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A., Kirk M., Jensen R. G. Photosynthesis by isolated chloroplasts. I. Diffusion of labeled photosynthetic intermediates between isolated chloroplasts and suspending medium. Biochim Biophys Acta. 1968 Jan 15;153(1):211–218. doi: 10.1016/0005-2728(68)90162-x. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W., Urbach W. The effect of dihydroxyacetone phosphate and 3-phosphoglycerate on O2 evolution and on the levels of ATP, ADP and Pi in isolated intact chloroplasts. Biochim Biophys Acta. 1977 Mar 11;459(3):337–346. doi: 10.1016/0005-2728(77)90035-4. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Kanazawa K., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in Chlorella pyrenoidosa during photosynthesis and respiration. Biochim Biophys Acta. 1972 Mar 16;256(3):656–669. doi: 10.1016/0005-2728(72)90201-0. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Chon C. J., Mosbach A., Heldt H. W. The distribution of metabolites between spinach chloroplasts and medium during photosynthesis in vitro. Biochim Biophys Acta. 1977 May 11;460(2):259–272. doi: 10.1016/0005-2728(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Sanwal G. G., Greenberg E., Hardie J., Cameron E. C., Preiss J. Regulation of starch biosynthesis in plant leaves: activation and inhibition of ADPglucose pyrophosphorylase. Plant Physiol. 1968 Mar;43(3):417–427. doi: 10.1104/pp.43.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M., Peavey D. G., Gibbs M. The regulation of starch metabolism by inorganic phosphate. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1554–1561. doi: 10.1016/s0006-291x(76)80191-x. [DOI] [PubMed] [Google Scholar]


