Abstract
The mechanisms underlying chemotherapy-induced acceleration of ovarian insufficiency are not fully understood, particularly for ovarian granulosa cells (GCs). We used two widely used cancer chemotherapeutic reagents, bleomycin and VP-16, and an in vivo GC-specific DNA topoisomerase II-β (TOP2β) (Top2b) knockout mouse model to investigate the effects of chemotherapy-induced DNA damage on growing mouse follicles. Bleomycin and VP-16 caused massive double-strand DNA breaks in the GCs of growing follicles in a time-dependent manner as shown by DNA-damage checkpoint activation. This damage was associated with apoptotic GC death and resulted in follicle atresia and ovulation failure. However, FSH-regulated ovarian functions, including estrogen biosynthesis and estrogen target gene expression, were not significantly affected by these genotoxins. TOP2β, a target of several chemotherapeutic drugs including VP-16, was abundantly expressed in the GCs of growing follicles. GC-specific deletion of Top2b using Cyp19-Cre caused DNA damage accumulations in these cells, follicle atresia, and decreased ovulation in response to exogenous gonadotropins. The ovaries of Top2b conditional knockout mice were also more sensitive to low-dose genotoxin treatment than wild-type mice ovaries. Thus, our results indicate that GCs are hypersensitive to genotoxic chemotherapeutic drugs and can activate the canonical DNA-damage checkpoint and the p53-dependent apoptotic pathway in response to insults that damage DNA. We also newly identified TOP2β as a factor involved in regulating GC genomic integrity and follicle atresia. This study has clinical implications for ovarian functional defects both for premenopausal cancer survivors and healthy women.
In women, ovarian failure and infertility are common consequences of the genotoxic effects of chemotherapy. Chemotherapy-induced ovarian failure can result in infertility in young cancer survivors and can also increase the risk for early menopause-related complications (1, 2). However, the mechanisms underlying chemotherapy-induced ovarian aging have not been adequately characterized in humans or in experimental animals.
In response to genotoxic stress, eukaryotic cells activate a complex signaling network called the DNA-damage checkpoint, which results in cell cycle arrest or apoptosis. Recent studies indicated that oocytes were sensitive to genotoxins, but their DNA damage responses were different from those of somatic cells in several respects. For example, the tumor suppressor protein p53 is a key mediator of DNA damage-induced responses in somatic cells but is not required for oocyte degeneration induced by ionizing radiation. In contrast, the p53 homolog p63 in conjunction with the protein tyrosine kinase c-ABL activated by chemotherapeutic drugs are responsible for DNA damage-induced oocyte death in mouse ovaries (3, 4). In addition, fully grown G2-phase arrested oocytes fail to effectively activate the master regulator of the DNA damage response pathway, ataxia telangiectasia mutated (ATM) kinase and thus lack an efficient DNA-damage checkpoint (5).
These recent studies focused on genome protection mechanisms in oocytes (6). However, little is known regarding chemotherapeutic drug-induced DNA damage responses in ovarian granulosa cells (GCs), which are the predominant somatic cell type in the mammalian ovary that surround and nurse developing oocytes.
GC apoptosis is the cellular basis of ovarian follicular atresia (7). Whether a developing follicle will continue to grow or will undergo atresia is determined by the relative expression and actions of cell death and survival regulators at the ovarian cell level (8, 9). The pituitary gonadotropin FSH is a well-established cell survival factor, and a limited FSH supply during estrous cycles causes physiological follicle atresia in both humans and experimental rodents (9, 10). Our recent studies showed that FSH-stimulated phosphatidylinositol-4,5-bisphosphate 3-kinase/phosphatase and tensin homolog/AKT and WNT/β-catenin signaling pathways in GCs promoted follicle growth and prevented their atresia (11, 12). However, there is little information on how genotoxic factors, such as chemotherapeutic drugs, are involved in inducing follicular cell apoptosis and atresia.
DNA topoisomerase II (TOP2) is an enzyme that alters DNA topology through a double-strand break (DSB) and its subsequent religation and is the target of several important chemotherapeutic drugs (13). As examples, etoposide (VP-16) and doxorubicin are widely used to kill tumor cells in cancer patients (14). These agents inhibit the DNA ligase activity of TOP2 and, thus, create DNA DSBs in the genome. However, these drugs are highly toxic and have notable side effects, such as female infertility (1, 15). Two closely related TOP2 isoforms have been identified in mammalian cells: TOP2α (170 kDa) and TOP2β (180 kDa). Although they share high homology in their primary sequences, these two isoforms have different roles and are differentially regulated during the cell cycle (16, 17).
Targeted disruption of the mouse Top2b gene showed that TOP2β had a critical role in neural and neuromuscular development (18). Top2b-null pups died of breathing impairments at birth, most likely due to neural and neuromuscular defects (19). Furthermore, a recent study showed that cardiomyocyte-specific deletion of Top2b protected cardiomyocytes from doxorubicin-induced DNA DSBs (20). However, it remains unknown whether these TOP2-inhibiting chemotherapeutic drugs are highly genotoxic to ovarian GCs and whether TOP2β is a factor involved in regulating GC apoptosis and follicle atresia.
To address these questions on ovarian biology, we investigated the acute effects of chemotherapeutic drugs on the ovaries of female mice at the pubertal stage and the ovarian function of TOP2β using in vivo models. We found that two commonly used chemotherapeutic drugs, bleomycin (BLM) and VP-16, had remarkable effects by inducing a DNA damage response and apoptosis in GCs. GCs were even more sensitive to DNA damage insults than other types of somatic cells. We also found that TOP2β was abundantly expressed in GCs of growing follicles and appeared to be necessary for developmentally regulated follicle atresia. TOP2β function is not limited to normal follicle development and may also be important for protecting ovarian follicles from genotoxic environmental factors.
Materials and Methods
Mice
Wild-type (WT) C57/BL6 mice were obtained from the Center of Experimental Animals, Zhejiang University. Mice with GC-specific knockout of Top2b (Top2bgc−/−) were generated by crossing Cyp19-Cre mice (11, 21) with previously reported Top2bfl/fl mice (18, 19). Animals were housed under a 14-hour light, 10-hour dark schedule, provided food and water ad libitum, and treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
To study ovarian responses to exogenous gonadotropins, postnatal day (PD) 21 to PD23 female mice were analyzed to avoid the complexity of ovarian functions associated with estrous cycles and endogenous surges of gonadotropins. Immature mice were injected ip with 5 IU pregnant mare serum gonadotropin (PMSG) (Ningbo Sansheng Pharmaceutical Co, Ltd) to stimulate preovulatory follicle development followed 48 hours later with 5 IU human chorionic gonadotropin (hCG) (Ningbo Sansheng Pharmaceutical) to stimulate ovulation and luteinization.
In vivo chemotherapeutic drug treatment
PD21 mice were injected ip with BLM or VP-16 (5 mg/kg body weight for both drugs) dissolved in 0.9% saline. Mice were killed at 3, 6, 12, and 24 hours after drug injections. In superovulation experiments, BLM or VP-16 was injected at 24 hours after PMSG treatment.
GC culture
GCs were harvested from PMSG-primed (24 hours) PD21 mice as described previously (22, 23). Briefly, undifferentiated GCs were released from antral follicles by puncturing with a 26.5-gauge needle. Cells were cultured at a density of 1 × 106 cells in DMEM/F12 medium (Invitrogen) containing 5% fetal bovine serum (Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin in 24-well culture dishes. After overnight culture, cells were washed and cultured in serum-free medium before any additional treatments.
RNA isolation and real-time RT-PCR
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer's instructions. Real-time PCR analysis used Q Tag SYBR Green Master Mix (Becton Dickinson Medical Devices Co, Ltd) and an Applied Biosystems 7500 real-time PCR system. Relative mRNA levels were calculated by normalizing to the levels of endogenous β-actin mRNA (used as an internal control) using Microsoft Excel. For each indicated gene, the relative transcript level of the control sample (left-hand bar of each graph) was set as 1. The relative transcript levels of other samples were compared with the control, and fold changes are shown in the graph. For each experiment, quantitative PCR were done in triplicate. Primer sequences are available upon request to the authors.
TUNEL assay
Terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assays were performed on 10% formalin-fixed paraffin-embedded sections using the ApopTag Plus peroxidase in situ apoptosis detection kit (Serologicals Corporation) according to manufacturer's instructions.
Histological analysis and immunohistochemistry
Ovaries were fixed overnight in 10% PBS buffered formalin and then embedded in paraffin. Ovary samples were serially sectioned at 5 μm thickness and were stained with hematoxylin and eosin (H&E). For immunohistochemistry (IHC), sections were deparaffinized and rehydrated and were incubated with primary antibodies for 1 hour at room temperature, followed by biotin-labeled secondary antibodies for 30 minutes. Staining procedure was performed using the Vectastain ABC kit and diaminobenzidine peroxidase substrate kit (Vector Laboratories).
Immunofluorescence
Ovarian tissues were fixed in 4% paraformaldehyde, embedded in O.C.T. compound (Sakura Finetek USA Inc), and stored at −80°C before preparing 7-μm sections using a Leica CM1950 cryomicrotome (Leica Microsystems). Cultured GCs were seeded on coverslips, and 24 hours later, cells were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.3% Triton X-100, and incubated with blocking buffer (PBS containing 0.3% Triton X-100 and 5% BSA). Sections or cells were sequentially probed with primary antibodies as indicated in Results and Alexa Fluor 594- or 488-conjugated secondary antibodies (Molecular Probes). Slides were mounted using VectaShield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Digital images were acquired using an epifluorescence microscope (Nikon Eclipse 80i) with ×4 to ×100 objectives.
Western blot analysis
Protein extracts were dissolved in sodium dodecyl sulfate sample buffer. Protein lysates (30 μg total protein per lane) were separated by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore Corp). After probing with primary antibodies, the membranes were washed in Tris-buffered saline with Tween-20 and incubated with a horseradish peroxidase-linked secondary antibody. Finally, bands on the membranes were detected using an enhanced chemiluminescence detection kit (Amersham).
Hormone level assays
Mice were anesthetized, and blood was collected by cardiac puncture. Serum and blood cells were separated by centrifugation. Serum hormone levels were determined by Di'an Medical Diagnostics Limited Corporation.
Statistical analysis
Results are given as means ± SDs; each experiment included at least 3 independent samples and was repeated at least 3 times. Group comparisons were made by unpaired Student's 2-tailed t tests. P values < .05 were considered significant.
Results
Ovarian GCs are hypersensitive to chemotherapeutic drug-induced DNA damage
We first assessed the effects of BLM, a widely used chemotherapeutic drug, on the ovaries of pubertal mice. Female mice were ip injected with BLM at a concentration comparable to the dosage used for human patients (5 mg/kg body weight) at PD21 to PD23. Within 6 hours after BLM injection, significant apoptotic signals were detected in GCs of growing follicles by TUNEL assay and immunofluorescent staining of cleaved caspase 3 (CC3) (Figure 1, A and C, left panels). VP-16 (etoposide, 5 mg/kg body weight), another commonly used chemotherapeutic drug, had similar in vivo apoptosis-inducing effects on ovarian GCs (Figure 1B).
Figure 1.
Ovarian GCs are hypersensitive to chemotherapeutic drug-induced DNA damage. A and B, TUNEL assay results showing increased GC apoptosis and follicle atresia in the ovaries of BLM- and VP-16–injected mice. All scale bars, 200 μm. C, Immunofluorescent staining results for cleaved caspase 3 (green) and pH2AX (red) in the ovaries of BLM-injected mice. DNA was counterstained with DAPI. D, TUNEL assay results showing cellular apoptosis in the uterus and intestine of the same BLM-injected mice as in A. E, TUNEL assay results showing the absence of luteal cell apoptosis with or without BLM injection.
In addition, numerous DNA DSBs were generated in these apoptotic GCs, as demonstrated by immunofluorescent staining for the DSB marker phosphorylated histone H2AX (pH2AX) (Figure 1C, right panels). In the same animals, increased cellular apoptosis was also detected in other actively self-renewing tissues, including uterine endometrium and small intestine, although there were far fewer apoptotic cells than what was observed in growing ovarian follicles (Figure 1D).
Interestingly, when mice were pretreated with PMSG and hCG to induce GC luteinization before BLM injection, BLM had little apoptosis-inducing effect on terminally differentiated GCs or luteal cells (Figure 1E). These results indicated that ovarian GCs were more sensitive to chemotherapeutic drug-induced DNA damage than other types of somatic cells and that this might be related to their highly dynamic proliferation and apoptosis during estrous cycles.
Bleomycin and VP-16 induce DNA-damage checkpoint activation in ovarian GCs
The in vivo effects of BLM and VP-16 on inducing DNA DSBs and apoptosis in GCs were further confirmed by Western blotting results for pH2AX and CC3, respectively (Figure 2, A and B). In addition, checkpoint kinase (CHK) 1 and CHK2, key kinases of the DNA-damage checkpoint, were significantly phosphorylated/activated after BLM or VP-16 treatment as shown by Western blotting (Figure 2, A and B) and IHC (Figure 2D), respectively. To determine more acute and synchronized effects of BLM and VP-16 on GCs, we used these drugs to treat cultured primary GCs isolated from growing follicles of PMSG-primed PD21 to PD23 mice. The background levels of H2AX, CHK1, and CHK2 phosphorylation were very low in cultured GCs. In contrast, remarkable phosphorylation of these proteins was detected at 1 hour after BLM or VP-16 (10μM for each drug) treatment (Figure 2C).
Figure 2.
BLM induces DNA-damage checkpoint activation in ovarian GCs. A and B, Western blot results for CC3, pH2AX, and pCHK1 in lysates of ovaries. Lysates were prepared using ovaries from mice injected with BLM (A) or VP-16 (B) (5 mg/kg body weight). Each lane was loaded with 30 μg protein. C, Immunofluorescent staining results for pH2AX (red), pCHK1 (green), and pCHK2 (yellow) in cultured mouse GCs, with or without BLM and VP-16 treatment. D and E, IHC results for pCHK2 (D) and p53 (E) in mouse ovaries before and after BLM treatment. Scale bar, 200 μm. F, Quantitative RT-PCR results using GCs isolated from ovaries with or without BLM treatment for the expressions of the p53 target genes Cdkn1a, Cdkn1b, Puma, and Noxa. For each indicated gene, the relative transcript level of the control sample (left-hand bar in each graph) was set to 1.
Previous studies with cultured cell lines showed that numerous DNA DSBs and hyperactivation of DNA-damage checkpoints also resulted in the accumulation of the key tumor repressor p53 and transcriptional activation of apoptosis-related genes. In our in vivo model, p53 protein levels were low in the GCs in healthy follicles before BLM treatment. However, at 6 hours after BLM injection, there was a significant increase in p53 levels as detected by IHC (Figure 2E). The p53 target genes involved in cell cycle arrest (Cdkn1a and Cdkn1b) and apoptosis (Puma and Noxa) were also markedly induced in GCs after BLM treatment, as shown by real-time RT-PCR analysis (Figure 2F).
Taken together, these results indicated that these chemotherapeutic drugs resulted in strong activation of the DNA-damage checkpoint and apoptotic pathways in GCs of pubertal mice in vivo.
Acute BLM treatment effects on ovulation and ovarian endocrine functions
The chemotherapeutic drugs BLM and VP-16 induced numerous DNA DSBs and activated apoptosis pathways in the GCs of growing follicles. We also investigated their in vivo effects on gonadotropin-triggered ovulation and ovarian endocrine functions. As shown in Figure 3A, BLM and VP-16 treatments strongly blocked PMSG/hCG-induced ovulation. At 16 hours after hCG injection, a time point at which most antral follicles in control ovaries had already ovulated, some unruptured antral follicles remained in the ovaries of BLM-treated mice (Figure 3B). In addition, multiple, fully developed corpora lutea (CL) were found in control ovaries at 48 hours after hCG injection. However, CL were poorly developed in the ovaries of BLM-treated mice. In addition, unovulated oocytes were trapped in the centers of these poorly formed CL (Figure 3B). Consistent with the CL development defects we observed on ovarian sections, BLM-treated mice had significantly lower serum progesterone levels than control mice at 48 hours after hCG injection (Figure 3C).
Figure 3.
Effects of acute BLM treatment on ovulation and ovarian endocrine functions. A, Superovulation assay results showing decreased ovulation in mice injected with BLM or VP-16 (n = 6 for each treatment group). B, H&E staining showing follicle rupture and CL formation in ovaries at 16 and 48 hours after hCG treatment in control and BLM-treated mice, respectively. Scale bar, 200 μm. C, Serum estrogen (E2) and progesterone (P4) concentrations in mice with or without BLM treatment. E2 and P4 levels were determined at 44 hours after PMSG injection (when follicles were fully grown in control mice) and 48 hours after hCG injection (when CL were well developed in control mice), respectively. D, IHC results for 3β-HSD in the ovaries of control and BLM-injected mice after hormonal treatments. Scale bar, 200 μm. E, Western blotting results for 3β-HSD in the lysates of ovaries from control and BLM-injected mice at PD23. F, Quantitative RT-PCR results using GCs isolated from control and BLM-treated ovaries (with or without PMSG injections) for the expression of the FSH target genes Cyp19, Fshr, and Lhcgr.
Surprisingly, however, BLM-treated mice had serum estradiol levels comparable to those of controls (Figure 3C). Consistently, 3β-hydroxysteroid dehydrogenase (3β-HSD), a key enzyme involved in steroidogenesis in the ovary, was normally expressed by granulosa and luteal cells with or without BLM treatment (Figure 3D). Western blotting results also showed that 3β-HSD protein levels were comparable before and after BLM injection (Figure 3E). In addition to 3β-HSD, the expression of other well-established FSH target genes, including Fshr (encodes FSH receptor), Lhcgr (encodes LH receptor), and Cyp19a1 (encodes aromatase, a key enzyme of estrogen biosynthesis), were not remarkably affected by BLM treatment, as shown by real-time RT-PCR (Figure 3F).
Taken together, these results indicated that although these chemotherapeutic drugs induced GC DNA damage and apoptosis, they did not affect the endocrine functions and FSH-regulated gene expression profiles in growing follicles.
DNA TOP2β, a target of VP-16, is expressed in ovarian GCs
VP-16 (etoposide) is used in chemotherapy as a half-inhibitor of TOP2, because it selectively inhibits the DNA ligase activity of TOP2 but not its endonuclease activity. Thus, this drug causes the accumulation of DNA DSBs and thereby activates DNA-damage checkpoints in rapidly proliferating cancer cells. In cultured GCs, both VP-16 (2 μg/ml) and BLM (20μM) induced DNA damage responses including H2AX and CHK1/2 phosphorylation (Figure 4, A and B). In addition, ICRF193 is an inhibitor of TOP2 endonuclease activity. It does not induce DSB accumulation but activates the G2-phase decatenation checkpoint and CHK1/2 in HeLa cells (17). Similarly, ICRF193 treatment also caused increased phosphorylation of CHK1/2 but not H2AX in GCs (Figure 4B). These results of VP-16 and ICRF193 treatment suggested that TOP2 was functionally active in this noncancer somatic cell type. A recent report indicated that the pharmacological effects of TOP2 half-inhibitors in the heart were primarily mediated by TOP2β, which is encoded by the Top2b gene (20). Therefore, we investigated TOP2β expression in mouse ovaries.
Figure 4.
DNA TOP2 is expressed in ovarian GCs, and its inhibition causes DNA damage and ovulation defects. A and B, Western blot results for changes induced by VP-16 (2 μg/ml), ICRF-193 (10μM), and BLM (20μM) in TOP2β and DNA-damage checkpoint proteins in cultured GCs. Primary GCs were isolated from the antral follicles of PMSG-primed WT mice and treated with VP-16, ICRF-193, and/or BLM for the indicated times. C, Western blot results for TOP2β protein levels in tissue lysates made from the indicated mouse organs. D, Western blot results for the TOP2β protein levels in lysates of ovaries prepared at the indicated times of hormonal treatment. E, IHC results for TOP2β expression in developing mouse ovaries at PD5, PD10, and PD23. F, IHC results for TOP2β expression in WT ovaries after hormonal treatment. G, Immunofluorescent staining results for the expression and subcellular localization of TOP2β (red) in cultured GCs. Cell shape is reflected by α-tubulin staining (green). DNA was stained with DAPI (blue).
Western blotting results showed that TOP2β protein could be detected in the lysates of ovaries (Figure 4C), although more abundant TOP2β expression was found in the testis. Furthermore, TOP2β expression in the ovary was constitutive and was not markedly affected by gonadotropin treatment (Figure 4D). We also investigated the expression pattern of TOP2β in developing ovaries by IHC.
TOP2β expression was detected in GCs as early as PD5 when primary follicles had just formed (Figure 4E, left panel). At PD10, TOP2β expression was more pronounced in the GCs of primary and secondary follicles. However, TOP2β expression was low in the GCs of primordial follicles and in oocytes at all developmental stages (Figure 4E, middle panel). At PD21, TOP2β was extensively expressed by all GCs in preantral follicles (Figure 4E, right panel).
We also investigated the TOP2β expression patterns in ovaries after exogenous gonadotropin treatment. In the ovaries of PMSG-treated mice at 44 hours, TOP2β was abundantly expressed in the GCs of preovulatory follicles (Figure 4F). At 16 hours after hCG treatment, TOP2β expression was maintained in the luteinizing GCs of ruptured follicles but not in the cumulus cells that surrounded the ovulated eggs within the oviduct. However, in fully developed CL, TOP2β expression in luteal cells was lower than that in GCs of adjacent growing follicles (Figure 4F). In follicles at all developmental stages, TOP2β signals were detected only in GC nuclei but not in the cytoplasm. Immunofluorescent staining results for TOP2β in cultured GCs confirmed its nuclear localization (Figure 4G).
Selective Top2b deletion in GCs causes increased follicle atresia
To investigate Top2b's ovarian functions, we generated a GC-specific Top2b-knockout mouse strain in which Cre recombinase was exclusively expressed in ovarian GCs by crossing Top2bfl/fl mice with Cyp19-Cre transgenic mice. IHC results for ovarian sections of Top2bgc−/− mice showed that ∼70% of GCs in large antral follicles were TOP2β-negative (Figure 5A). Real-time RT-PCR analysis showed that the Top2b mRNA level in GCs isolated from Top2bfl/−;Cyp19-Cre mice was only 20% of that in WT GCs (Figure 5B). Decreased TOP2β protein levels were also found by Western blotting of Top2b-deleted GC lysates (Figure 6E).
Figure 5.
Top2b deletion in GCs results in follicle atresia. A, IHC results for TOP2β expression in Top2bfl/fl;Cyp19-Cre (Top2bgc−/−) ovaries at 44 hours after PMSG treatment. B, Quantitative RT-PCR results for the expressions of Top2b mRNA in GCs isolated from WT and Top2bgc−/− ovaries (23 days old, 44 hours after PMSG treatment; n = 3). C, IHC and TUNEL staining results showing TOP2β deletion and follicle atresia in 21-day-old hormonally untreated WT and Top2bgc−/− mice. D, IHC and TUNEL staining results showing TOP2β deletion and follicle atresia in PD21 to PD23 WT and Top2bgc−/− mice treated with PMSG for 44 hours. E, Quantification of TUNEL-positive atretic follicles shown in D. Average numbers of atretic follicles on each ovarian section (n = 6) were counted. Scale bar, 200 μm. F, Images of GCs isolated from ovaries of PMSG-primed WT and Top2bgc−/− mice at 48 hours after overnight culture. The average numbers of cells from 6 different microscopic fields are given.
Figure 6.
Top2b deletion in GCs results in DNA-damage checkpoint activation. A, Immunofluorescent staining results for pH2AX (green) and CC3 (red) in WT and Top2bgc−/− mouse ovaries at PD23. DNA was counterstained with DAPI (blue). Scale bar, 100 μm. B and C, IHC results for pCHK2 (C) and p53 (D) in WT and Top2bgc−/− mouse ovaries at PD23. Scale bar, 200 μm. D, IHC results for p27 in WT ovaries with or without BLM treatment for 3 hours and in Top2bgc−/− mouse ovaries at PD23. Scale bar, 200 μm. E, Western blot results for TOP2β and DNA-damage checkpoint proteins in lysates prepared using ovaries from WT and Top2bgc−/− mice at PD23.
In the ovaries of 21-day-old, hormonally untreated pubertal Top2bgc−/− mice, TOP2β deletion was detected by IHC in some of the GCs in preantral follicles (Figure 5C). Those follicles that contained TOP2β-negative GCs were atretic, as determined by a TUNEL assay for adjacent ovarian sections (Figure 5D). Furthermore, PMSG injection induced widespread TOP2β depletion in antral follicles and also promoted significant follicle atresia (Figure 5, D and E). GCs became apoptotic immediately after TOP2β deletion and were disappearing from ovarian sections (Figure 5D). In addition, when we attempted to isolate GCs from WT and Top2bgc−/− mice and establish primary cell cultures, we consistently obtained fewer surviving GCs from Top2bgc−/− mice after overnight culture (Figure 5F). Therefore, we postulated that TOP2β was indispensable for GC survival and that Top2b-knockout GCs had died soon after TOP2β protein deletion. As a result, we observed more TOP2β-positive GCs remaining on ovarian sections than was observed in vivo.
Selective Top2b deletion in GCs results in DNA-damage checkpoint activation
Consistent with the results we obtained with VP-16 treatment, deleting endogenous TOP2β caused an accumulation of DNA DSBs in GCs, as shown by both immunofluorescent staining and Western blotting for pH2AX (Figure 6, A and E). DSB accumulation further activated the DNA-damage checkpoint and the apoptosis pathway, as indicated by increased CHK2 phosphorylation (Figure 6, B and E) and p53 protein accumulation (Figure 6C), respectively. In addition, p27 expression (Figure 6D) and cleavage activation of caspase 3 (Figure 6, A and E), downstream events induced by p53, were also detected in GCs of Top2bgc−/− ovaries. These results suggested that Top2b was a housekeeping gene in GCs that prevented DNA damage accumulation and apoptosis.
Selective Top2b deletion in GCs causes ovulation defects
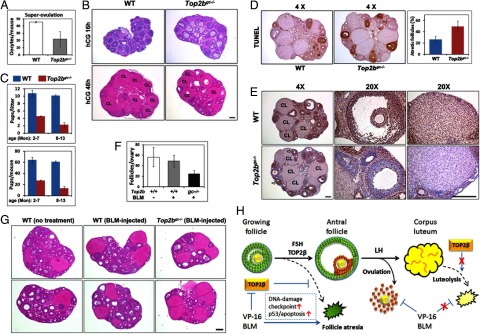
In a superovulation assay, Top2bgc−/− mice ovulated less mature oocytes than did control mice (Figure 7A). At 16 hours after hCG injection, a time point at which most antral follicles in control ovaries had already ovulated, some unruptured antral follicles remained in the ovaries of Top2bgc−/− mice (Figure 7B). In addition, multiple fully developed CL were found in control ovaries at 48 hours after hCG injection. However, there were fewer CL in the ovaries of Top2bgc−/− mice (Figure 7B).
Figure 7.
Effects of Top2b conditional knockout on ovarian reproductive functions. A, Superovulation assay results showing decreased ovulation in Top2bgc−/− mice (n = 6 for each genotype). B, H&E staining showing follicle rupture and CL formation at 16 and 48 hours after hCG treatment in WT and Top2bgc−/− ovaries, respectively. Scale bar, 200 μm. C, Fertility test results. WT and Top2bgc−/− female mice were continuously mated with fertile males for longer than 1 year. Average litter sizes and total pup numbers before and after 7 months of age are shown. D, TUNEL assay results showing atretic follicles in ovaries of adult WT and Top2bgc−/− mice (∼6 months old). The average percentages of atretic follicles on 4 different sections are given. E, IHC results for TOP2β expression in ovaries of adult WT and Top2bgc−/− mice. Arrows indicate TOP2β-positive healthy growing follicles and asterisks indicate TOP2β-negative atretic follicles. Scale bar, 200 μm. F and G, Quantitation of growing follicles (secondary, preantral, and antral) (F) and H&E staining results for mice with or without BLM injections (G). WT and Top2bgc−/− mice were injected with BLM (2.5 μg/kg body weight) for 3 consecutive days from PD21 to PD23 and were humanely killed for ovarian histological examination at 8 weeks of age (n = 4 for each treatment group). H, A schematic diagram of TOP2β functions and chemotherapeutic drug effects in GCs during follicle development. TOP2β depletion in growing follicles causes GC DNA damage and follicle atresia. With normally expressed TOP2β, FSH stimulates follicles to grow beyond the early antral stage and establishes specific gene expression patterns in mural GCs and cumulus cells. GCs of growing follicles were hypersensitive to chemotherapeutic drugs that cause massive DNA damage in GCs followed by DNA-damage checkpoint activation, p53-mediated apoptosis, and ovulation failure. However, chemotherapeutic drug treatment for longer times or TOP2β depletion does not cause DNA damage accumulation and apoptosis in nondividing luteal cells.
Because of these defects during exogenous gonadotropin-induced follicle growth and ovulation, naturally mated adult Top2bgc−/− female mice showed reduced fertility; both the litter sizes and the accumulated pup numbers were significantly smaller than WT control females (Figure 7C). Interestingly, relatively aged Top2bgc−/− females (7–13 months old) showed a more remarkable reduction in fertility than young adults (2–6 months old). The ovaries of adult Top2bgc−/− mice had increased numbers of atretic follicles as compared with WT ovaries (Figure 7D), although this was not as pronounced as that promoted by PMSG treatment.
IHC results indicated that, in Top2bgc−/− adult ovaries, TOP2β protein was mostly depleted in the GCs in atretic follicles (Figure 7E, asterisks), but not as efficiently in healthy growing follicles (Figure 7E, arrows). This may account for why these mice were not completely infertile. However, TOP2β deletion was nearly complete in the CL of Top2bgc−/− mice. Interestingly, these TOP2β-negative CL were well developed and exhibited few apoptotic signals in a TUNEL assay (Figure 7D). This was consistent with our previous results that luteal cells were insensitive to DNA damage insults, such as by BLM treatment (Figure 1D).
To test whether the ovaries of Top2bgc−/− mice were more sensitive to therapeutic drugs, we injected WT and Top2bgc−/− mice with BLM (2.5 mg/kg body weight) for 3 consecutive days from PD21 to PD23 and examined their ovarian histology at 8 weeks of age. This low-dosage BLM treatment had little effect on WT ovaries (Figure 7, F and G). The ovaries of 8-week-old Top2bgc−/− mice without BLM treatment were histologically indistinguishable from WT ovaries (data not shown). However, after low-dosage BLM treatment, the numbers of growing follicles in Top2bgc−/− ovaries were markedly decreased (Figure 7, F and G).
Discussion
Based on previous reports and our current results, we suggest that there are 3 possible reasons for GC hypersensitivity to DNA damage insults, including chemotherapeutic drug treatment and Top2b deletion. First, the GC is a unique cell type that is prepared to die at any time. Follicle growth and ovulation are exquisitely regulated by gonadotropins, intra-ovarian signaling pathways, and environmental inputs (9, 24). To ensure that only the healthiest follicles are selected for ovulation, the DNA-damage checkpoint and apoptosis pathways are functionally present and probably set at low thresholds in GCs, as demonstrated in Figures 1 and 2.
Second, GCs within a follicle are physiologically assigned to live or die together. In each follicle, GCs are interconnected by gap junctions, which are essential for normal follicle development (25). They exchange small nutrient or signaling molecules through these gap junctions (26). Therefore, the apoptotic signals of a small number of GCs can be rapidly propagated to the entire follicle. This phenomenon is a reflection of natural adaptation; the ovarian estrous cycles will be disturbed if some GCs are still proliferating and secreting hormones, whereas most others are apoptotic in an atretic follicle.
Third, like cancer cells, GCs are hyperproliferative. Previous reports showed that GCs were among the most proliferative of cell types in the body (27, 28). Almost all GCs in growing follicles are positive for proliferating cell nuclear antigen (29). In bromodeoxyuridine incorporation assays, more than 50% of GCs in growing follicles are labeled by bromodeoxyuridine within 1 to 2 hours (21, 23). This proliferation rate is much higher than those for rapidly proliferating intestine and uterine epithelial cells. To ensure this high proliferation rate, a special subset of genes related to proliferation, including Top2b investigated in this study, are required by GCs. For example, knocking out the genes encoding cyclin D2 (Ccnd2) (30), estrogen receptors (Esr1/2) (31), and IGF-1 (Igf1) caused more severe proliferation defects in GCs than in other cell types (32).
Chemotherapeutic drugs were designed to kill cancer cells rather than normal body cells. Their effects are primarily because cancer cells are generally more proliferative than normal cells. The GC is an exception. Many cancer cells acquire drug clearance capability via exocytosis or a certain degree of resistance to DNA damage-induced apoptotic signals, whereas normal GCs do not (33).
It is generally acknowledged that TOP2α is widely expressed in all cell types and plays a housekeeping role during the cell cycle, particularly at G2 (34). In contrast, TOP2β is expressed in certain differentiated (in most cases nonproliferating) cell types and has specialized functions, such as transcription regulation and the DNA damage response (13, 16). Interestingly, TOP2β protein was highly expressed in both the testis and the ovary, which suggested that it had reproduction-related functions in both males and females.
In the mouse testis, TOP2B is expressed in a stage-specific manner throughout spermatogenesis. In particular, high levels of TOP2β are found in nuclear foci of elongated and condensing spermatids, but TOP2α is not found in the germ cells at these stages (35). Therefore, it has been suggested that TOP2β rather than TOP2α might produce transient DNA strand breaks during sperm DNA condensation to support the overall changes in DNA topology and relieve torsional stress (36).
Our IHC staining results indicated that TOP2β was highly expressed in GCs, but its expression was low in oocytes. We also generated oocyte-specific Top2b-knockout mice, but these did not show any reproduction or oocyte development defects (unpublished results). These results suggested that TOP2β played an indispensable role in GCs, although it either may not be required or may have overlapping functions with TOP2α in oocytes. It would have been interesting to compare the localization patterns of TOP2α and TOP2β in the ovary. Unfortunately, the TOP2α-specific antibody available to us did not work well for IHC or Western blotting.
The chemotherapeutic drug VP-16 inhibits the DNA ligase activity of TOP2α/β. It has been reported that TOP2β was the major in vivo target of these TOP2 half-inhibitors in the heart (20). Similar to VP-16 treatment, selective TOP2β deletion in GCs caused follicle atresia and ovulation defects. These results suggested that the genotoxic effect of VP-16 in GCs was primarily mediated by TOP2β. As discussed earlier, GCs are very rapidly proliferating cells and are concomitantly hypersensitive to DNA damage. Thus, it is not surprising that the DNA decatenation and repairing capabilities of TOP2 are essential for GC survival and follicle growth.
In this study, we also provided clear evidence that active proliferation was a prerequisite for the genotoxic effects of chemotherapeutic drugs or TOP2β deletion, because the nonproliferating, terminally differentiated luteal cells were nearly completely insensitive to these genotoxic insults as compared with the proliferating GCs within the same ovaries (Figures 1E and 7E). In addition, Top2b conditional knockout female mice exhibited more severe reproductive defects with increased age. This suggested that GCs had decreased DNA damage repair capability during the aging process and became more dependent on TOP2β for genome maintenance. Indeed, Top2bgc−/− mice were more sensitive to low-dose genotoxin treatment, as shown in Figure 7, F and G, which provided further evidence for a role for TOP2β in GC genome protection.
In summary, in this study, we investigated the chemotherapeutic drug-induced DNA damage response in ovarian GCs and its effects on ovarian functions. We also identified the chemotherapeutic drug target TOP2β as a key enzyme for GC survival and follicle growth. These findings are summarized in Figure 7H. These results shed new light on fertility protection of young female cancer patients as well as preventative effects from unidentified environmental genotoxic insults.
Acknowledgments
This work was supported by the National Basic Research Program of China (2011CB944504 and 2012CB944403), the National Natural Science Foundation of China (81172473), and Basic Scientific Research Funding of Zhejiang University (2011QN81001). The project was also supported by the Open Research Fund of the State Key Laboratory of Cellular Stress Biology, Xiamen University (SKLCSB2013KF001).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the National Basic Research Program of China (2011CB944504 and 2012CB944403), the National Natural Science Foundation of China (81172473), and Basic Scientific Research Funding of Zhejiang University (2011QN81001). The project was also supported by the Open Research Fund of the State Key Laboratory of Cellular Stress Biology, Xiamen University (SKLCSB2013KF001).
Footnotes
- BLM
- bleomycin
- CC3
- cleaved caspase 3
- CHK
- checkpoint kinase
- CL
- corpora lutea
- DAPI
- 4′,6-diamidino-2-phenylindole
- DSB
- double-strand break
- GC
- granulosa cell
- hCG
- human chorionic gonadotropin
- H&E
- hematoxylin and eosin
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- IHC
- immunohistochemistry
- PD
- postnatal day
- pH2AX
- phosphorylated histone H2AX
- PMSG
- pregnant mare serum gonadotropin
- TOP2
- topoisomerase II
- TUNEL
- terminal deoxynucleotidyl transferase nick end labeling
- WT
- wild-type.
References
- 1. Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tilly JL, Kolesnick RN. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochim Biophys Acta. 2002;1585:135–138. [DOI] [PubMed] [Google Scholar]
- 3. Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. [DOI] [PubMed] [Google Scholar]
- 4. Suh EK, Yang A, Kettenbach A, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. [DOI] [PubMed] [Google Scholar]
- 5. Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol. 2012;22:989–994. [DOI] [PubMed] [Google Scholar]
- 6. Jurisicova A, Lee HJ, D'Estaing SG, Tilly J, Perez GI. Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ. 2006;13:1466–1474. [DOI] [PubMed] [Google Scholar]
- 7. Edson MA, Nagaraja AK, Matzuk MM. The Mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11:162–177. [DOI] [PubMed] [Google Scholar]
- 9. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. [DOI] [PubMed] [Google Scholar]
- 10. Dierich A, Sairam MR, Monaco L, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. β-Catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol. 2010;24:1529–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burden DA, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:139–154. [DOI] [PubMed] [Google Scholar]
- 15. Ben-Aharon I, Bar-Joseph H, Tzarfaty G, et al. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakaguchi A, Kikuchi A. Functional compatibility between isoform α and β of type II DNA topoisomerase. J Cell Sci. 2004;117:1047–1054. [DOI] [PubMed] [Google Scholar]
- 17. Bower JJ, Karaca GF, Zhou Y, Simpson DA, Cordeiro-Stone M, Kaufmann WK. Topoisomerase IIα maintains genomic stability through decatenation G2 checkpoint signaling. Oncogene. 2010;29:4787–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lyu YL, Wang JC. Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIbeta. Proc Natl Acad Sci U S A. 2003;100:7123–7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang X, Li W, Prescott ED, Burden SJ, Wang JC. DNA topoisomerase IIβ and neural development. Science. 2000;287:131–134. [DOI] [PubMed] [Google Scholar]
- 20. Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 21. Fan HY, Shimada M, Liu Z, et al. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan HY, Liu Z, Johnson PF, Richards JS. CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol. 2011;25:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan HY, Liu Z, Shimada M, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pru JK, Kaneko-Tarui T, Jurisicova A, Kashiwagi A, Selesniemi K, Tilly JL. Induction of proapoptotic gene expression and recruitment of p53 herald ovarian follicle loss caused by polycyclic aromatic hydrocarbons. Reprod Sci. 2009;16:347–356. [DOI] [PubMed] [Google Scholar]
- 25. Park JY, Richard F, Chun SY, et al. Phosphodiesterase regulation is critical for the differentiation and pattern of gene expression in granulosa cells of the ovarian follicle. Mol Endocrinol. 2003;17:1117–1130. [DOI] [PubMed] [Google Scholar]
- 26. Norris RP, Freudzon M, Mehlmann LM, et al. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–940. [DOI] [PubMed] [Google Scholar]
- 28. Robker RL, Richards JS. Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod. 1998;59:476–482. [DOI] [PubMed] [Google Scholar]
- 29. Fan HY, Liu Z, Paquet M, et al. Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009;69:6463–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sicinski P, Donaher JL, Geng Y, et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. [DOI] [PubMed] [Google Scholar]
- 31. Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–3262. [DOI] [PubMed] [Google Scholar]
- 32. Baker J, Hardy MP, Zhou J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. [DOI] [PubMed] [Google Scholar]
- 33. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo K, Yuan J, Chen J, Lou Z. Topoisomerase IIα controls the decatenation checkpoint. Nat Cell Biol. 2009;11:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamauchi Y, Shaman JA, Ward WS. Topoisomerase II-mediated breaks in spermatozoa cause the specific degradation of paternal DNA in fertilized oocytes. Biol Reprod. 2007;76:666–672. [DOI] [PubMed] [Google Scholar]
- 36. Leduc F, Maquennehan V, Nkoma GB, Boissonneault G. DNA damage response during chromatin remodeling in elongating spermatids of mice. Biol Reprod. 2008;78:324–332. [DOI] [PubMed] [Google Scholar]