Abstract
Chlorophyll-deficient barley (Hordeum vulgare) mutants were studied that had chlorophyll a/b ratios either higher or lower than the wild type. Mutants with high ratios (>5.2) had a reduced proportion of their photosynthetic lamellae appressed into grana (“grana-deficient” mutants) compared with wild type (chlorophyll a/b = 3.2), while the majority of lamellae in the chloroplasts with low chlorophyll a/b ratios (2.0-2.4) were organized into grana (“grana-rich” mutants).
All mutants catalyzed photosystem I and photosystem II electron transport, were tightly coupled as evidenced by increased rates of electron transport in the presence of methylamine, and were able to generate a light-dependent transmembrane proton gradient. Differences were evident in rates of electron transport per mole of chlorophyll. The mutants having high chlorophyll a/b ratios catalyzed 15- to 50-fold higher rates of ferricyanide photoreduction than the mutants having low chlorophyll a/b ratios, and 5- to 7-fold higher than the wild type.
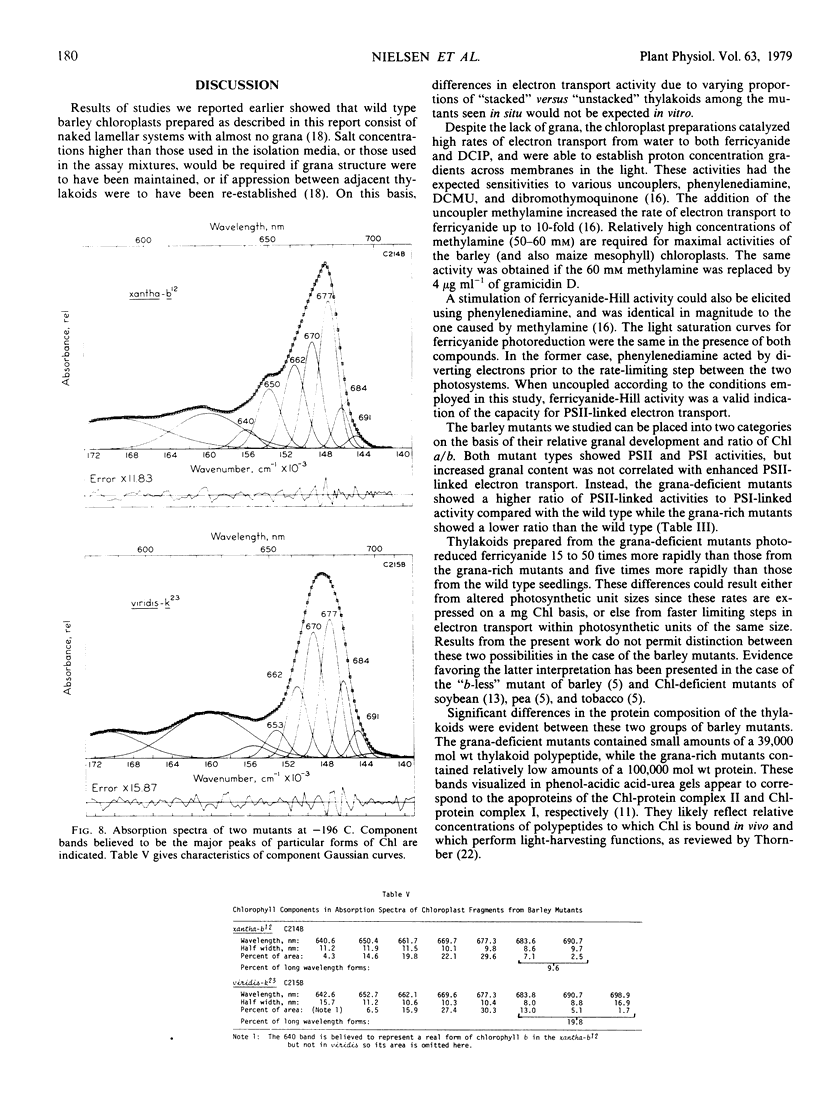
Low temperature absorption spectra of chloroplast fragments showed that the grana-deficient mutant with a high a/b ratio had a chlorophyll spectrum characteristic of a PSI preparation while mutants with the low ratio had a spectrum typical of a PSII preparation.
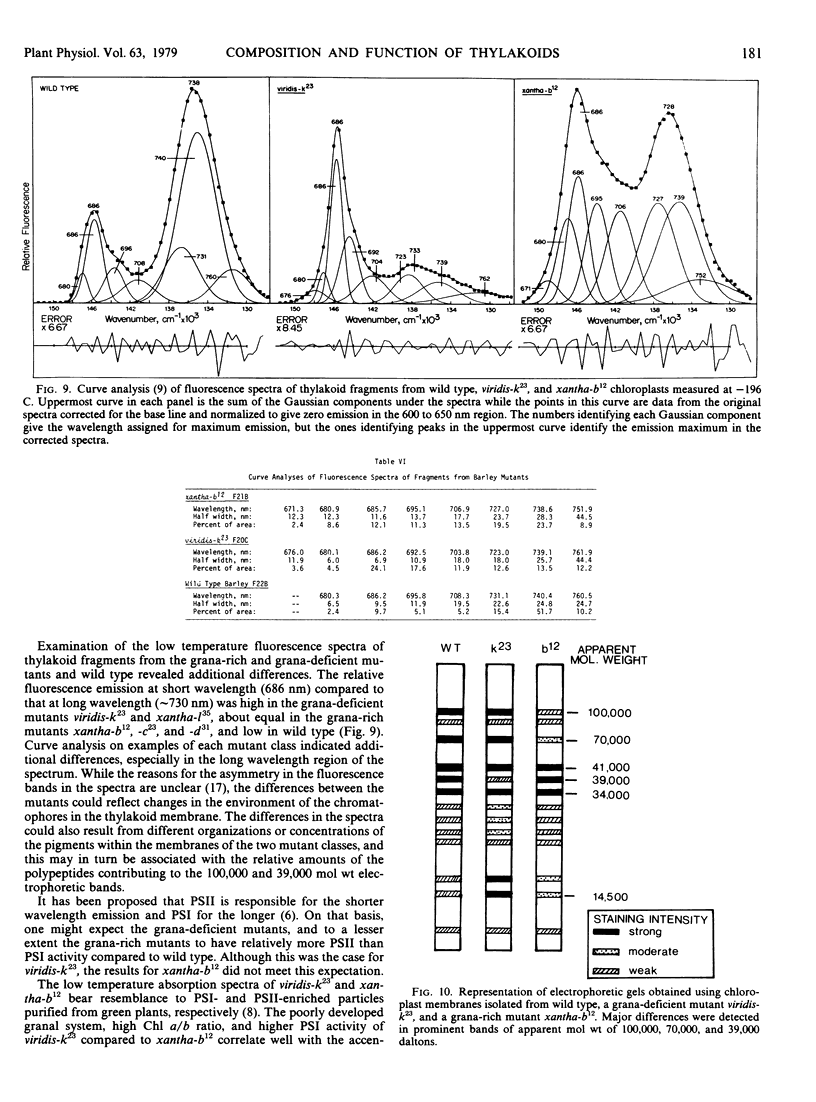
The temperature fluorescence emission spectra of thylakoid membrane fragments from the two types of mutants were also strikingly different from one another, as were the electrophoretic patterns of the thylakoid polypeptides.
Full text
PDF


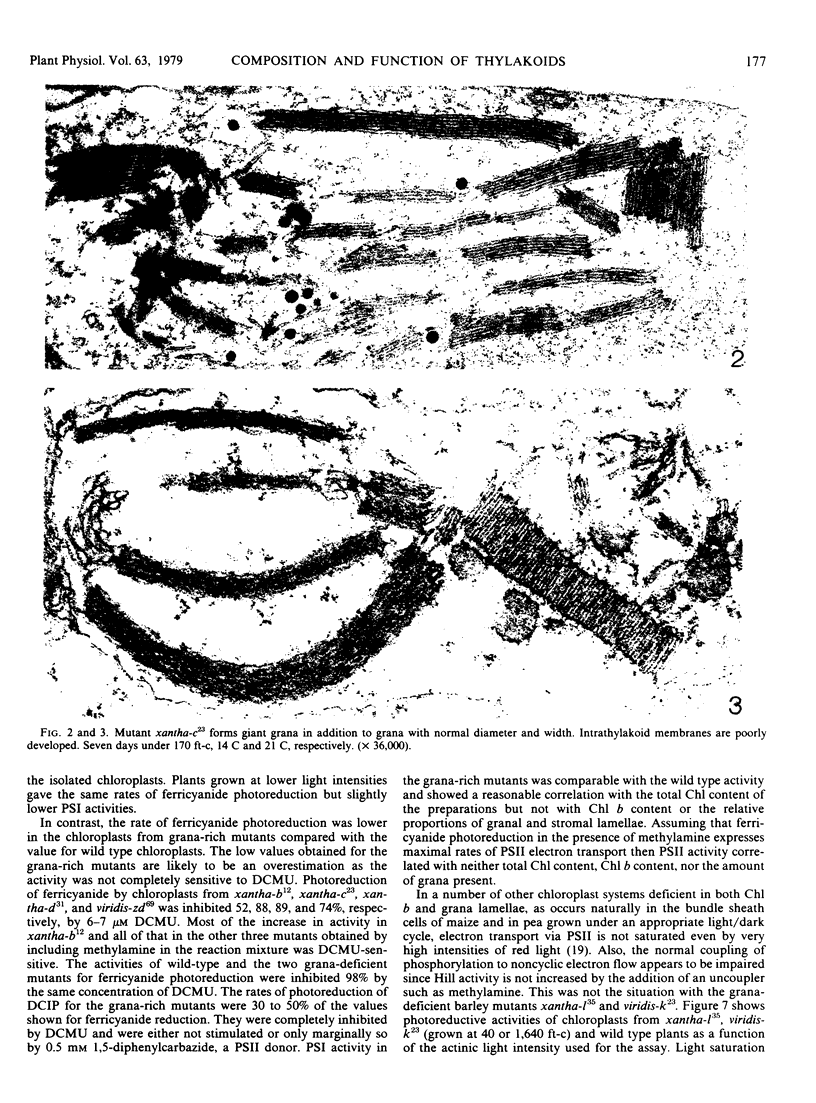
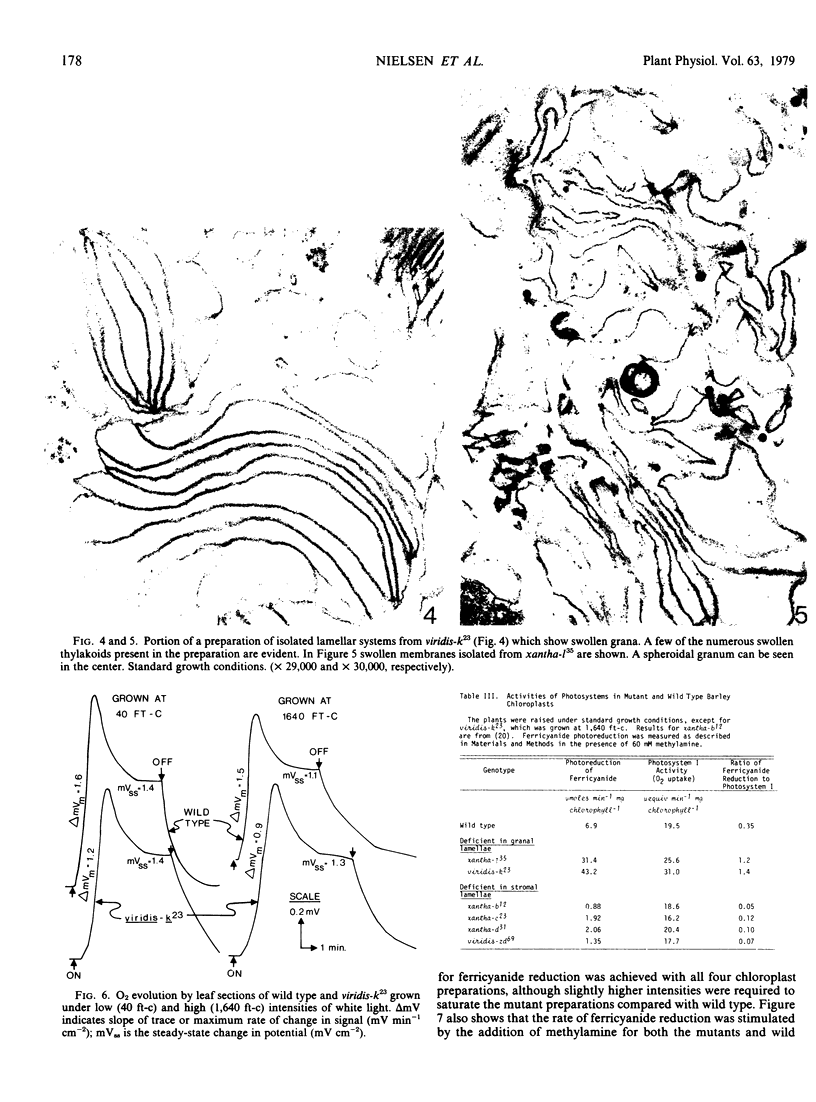
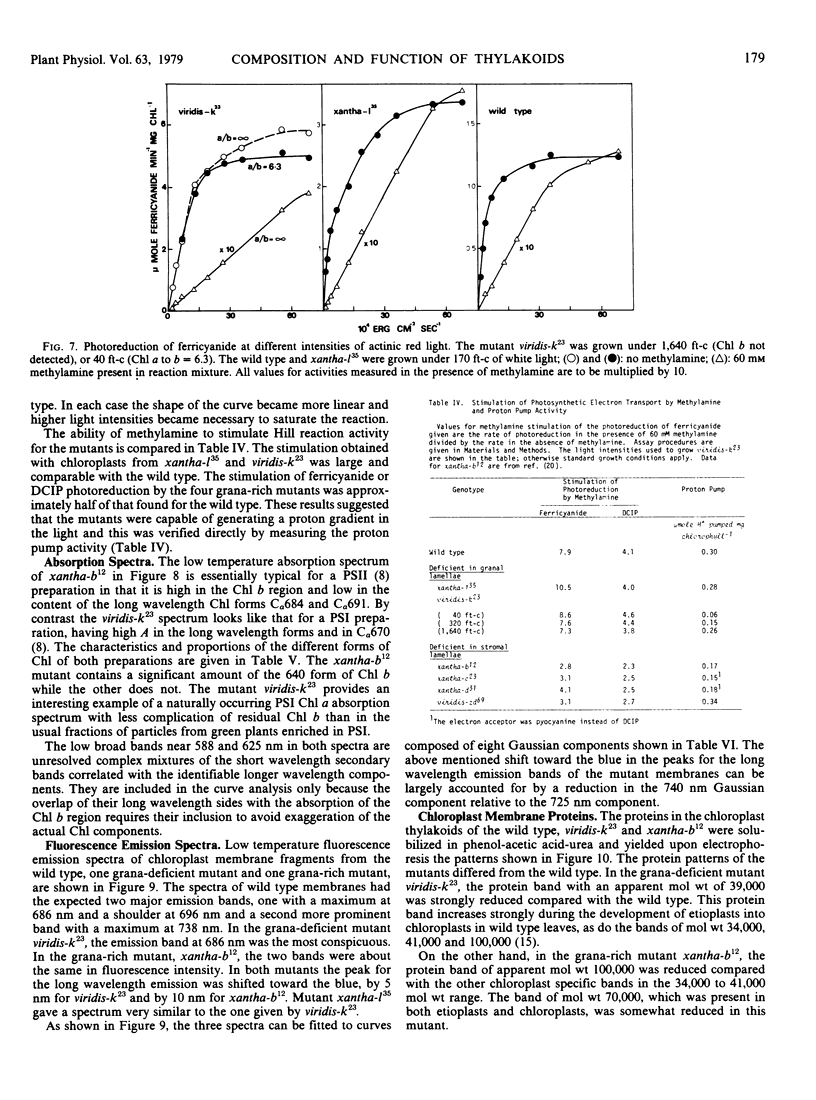

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelqvist L. A., Stumpf P. K., von Wettstein D. Lipid synthesis and ultrastructure of isolated barley chloroplasts. Plant Physiol. 1968 Feb;43(2):163–187. doi: 10.1104/pp.43.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W., Anderson J. M. Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Aug;56(2):586–593. doi: 10.1073/pnas.56.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C. S., Brown J. S., Lawrence M. C. Four universal forms of chlorophyll a. Plant Physiol. 1972 Mar;49(3):421–429. doi: 10.1104/pp.49.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W., Dilley R. A., Ke B. Photochemical characteristics in a soybean mutant. Plant Physiol. 1970 Nov;46(5):699–704. doi: 10.1104/pp.46.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N. C. Electrophoretic characterization of membrane proteins during chloroplast development in barley. Eur J Biochem. 1975 Jan 15;50(3):611–623. doi: 10.1111/j.1432-1033.1975.tb09902.x. [DOI] [PubMed] [Google Scholar]
- Nielsen N. C., Smillie R. M. The effect of p-phenylenediamine and dibromothymoquinone on the postosynthetic electron transport and proton pump activities of isolated barley chloroplats. Arch Biochem Biophys. 1978 Feb;186(1):52–59. doi: 10.1016/0003-9861(78)90462-9. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]