Abstract
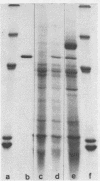
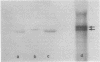
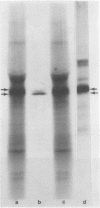
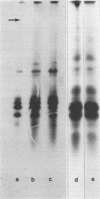
α-Amylase from wheat aleurone (Triticum aestivum) was synthesized in a S-150 wheat germ readout system using polysomes, and a messenger RNA-dependent reticulocyte lysate system using polyadenylic acid [poly(A)]-enriched RNA. The product was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, precipitation by specific λ-globulin for α-amylase, and proteolysis. Two immunoprecipitated products were synthesized from the readout system, the predominant species migrating coincidentally with authentic α-amylase on sodium dodecyl sulfate-polyacrylamide gels. A putative precursor, 1,500 daltons larger, was evident but was less abundant. The relationship between the two polypeptides was established by proteolytic analysis using Staphylococcus aureus V8 protease. At least nine fragments were generated and were identical in both species. The poly(A)-enriched RNA synthesized only the putative precursor in the reticulocyte lysate system. Attempts to process the precursor to the mature size of α-amylase failed. These findings are discussed in connection with the signal hypothesis (proposed for the transport of proteins across membranes) and the mode of secretion of α-amylase in aleurone cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartel D. M., Howell S. H. Changes in polypeptide initiation and elongation rates during the cell cycle of Chlamydomonas reinhardi. Biochemistry. 1977 Jul 12;16(14):3182–3189. doi: 10.1021/bi00633a022. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Czichi U., Lennarz W. J. Localization of the enzyme system for glycosylation of proteins via the lipid-linked pathway in rough endoplasmic reticulum. J Biol Chem. 1977 Nov 25;252(22):7901–7904. [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M. Cell surface saccharides of Trypanosoma lewis i. II. Lectin-mediated agglutination and fine-structure cytochemical detection of lectin-binding sites. J Cell Sci. 1976 Oct;22(1):1–19. doi: 10.1242/jcs.22.1.1. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Lingrel J. B. Hemoglobin messenger ribonucleic acid. Distribution of the 9S ribonucleic acid in polysomes of different sizes. Biochemistry. 1969 Mar;8(3):829–831. doi: 10.1021/bi00831a010. [DOI] [PubMed] [Google Scholar]
- Evins W. H. Enhancement of polyribosome formation and induction of tryptophan-rich proteins by gibberellic acid. Biochemistry. 1971 Nov;10(23):4295–4303. doi: 10.1021/bi00799a022. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Heizmann P., Gelvin S., Walker L. L. Identification and Properties of the Messenger RNA Activity in Chlamydomonas reinhardi Coding for the Large Subunit of d-ribulose-1,5-bisphosphate Carboxylase. Plant Physiol. 1977 Mar;59(3):464–470. doi: 10.1104/pp.59.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Chen R. F. Immunohistochemical localization of alpha-amylase in barley aleurone cells. J Cell Sci. 1976 Jan;20(1):183–198. doi: 10.1242/jcs.20.1.183. [DOI] [PubMed] [Google Scholar]
- Kruppa J., Sabatini D. D. Release of poly A(+) messenger RNA from rat liver rough microsomes upon disassembly of bound polysomes. J Cell Biol. 1977 Aug;74(2):414–427. doi: 10.1083/jcb.74.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Jones R. A., Tsai C. Y. Isolation and in vitro translation of zein messenger ribonucleic acid. Biochemistry. 1976 Dec 14;15(25):5506–5511. doi: 10.1021/bi00670a014. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanovich M. P., Hill R. D. Affinity chromatography of cereal alpha-amylase. Anal Biochem. 1976 Jun;73(2):430–433. doi: 10.1016/0003-2697(76)90191-3. [DOI] [PubMed] [Google Scholar]
- Sun S. M., Buchbinder B. U., Hall T. C. Cell-free Synthesis of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):780–785. doi: 10.1104/pp.56.6.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L., Ruddat M. Effect of gibberellic Acid and actinomycin d on the formation and distribution of rough endoplasmic reticulum in barley aleurone cells. Plant Physiol. 1973 Mar;51(3):549–558. doi: 10.1104/pp.51.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]