Abstract
Auxin signaling events in plants play important role in developmental regulation as well as gravitropic responses and plays crucial role in the development of root, lateral root and root hairs. The gene that is known to be most important in the development of root, lateral root and root hairs is commonly known as auxin efflux carrier (PIN). Being commonly known as orphan plant, the genome sequence of Eleusine coracana is not known yet, and hence it was very difficult to conduct advanced research in root development in this plant. As PIN gene plays crucial role in root development, to have some advanced study we proposed to clone the PIN genes from E. coracana. We cloned two PIN genes in E. coracana and named them as EcPIN1a and EcPIN1b. The coding sequence (CDS) of EcPIN1a was 1779 bp and EcPIN1b was 1788 bp long that encodes for 593 and 596 amino acids, respectively. In-silico analysis shows the presence of transmembrane domain in EcPIN1a and EcPIN1b protein. Multiple sequence alignment of EcPIN1a and EcPIN1b protein shows the presence of several conserved motifs. Phylogenetic analysis of EcPIN1a and EcPIN1b grouped with the PIN gene of monocot plant Oryza sativa. This shows that EcPIN genes were monocot specific, and closely match with the PIN genes of O. sativa. The transcript analysis of EcPIN1a gene in leaf tissue shows gradual up-regulation from 7th to 28th days of developmental time period while the transcript level was found to be lower in root tissue. The transcript abundance of EcPIN1b was not detected. Gradual up-regulation of EcPIN1a gene in developmental stages signifies its important role in root development in E. coracana.
Keywords: Auxin, Auxin influx carrier, Auxin efflux carrier, PIN, Transmembrane domain
Introduction
The sessile organism plant uses its roots to acquire water and nutrient molecules from soil as well as monitor the underground soil for a wide range of environmental conditions (Chapman et al. 2012; Craine and Dybzinski 2013; Barberon and Geldner 2014; Kong and Ma 2014). Moreover, the degree of root branching has lots of impact in the efficiency of the nutrient acquisition, water uptake and anchorage to the plant (López-Bucio et al. 2003; Hermans et al. 2006). Therefore, it is very important to understand the agronomic importance of root development. The cellular and molecular basis of root formation and its development has been studied extensively in model plants Oryza sativa and Arabidopsis thaliana (Coudert et al. 2010; Petricka et al. 2012; Mohanta et al. 2015; Singh et al. 2015). Lots of progress has been made to identify the important genes involved in root, lateral root and root hair formation in these plants (Bañoc et al. 2000; Casimiro et al. 2003; Péret et al. 2009). The model organism Arabidopsis possesses primary root that continuously branches to generate several lateral roots (Péret et al. 2009), whereas the monocot plant O. sativa, Zea mays and others predominantly contain adventitious roots (Lorbiecke 1999; Rebouillat et al. 2009; Liu et al. 2009). Availability of genomic data led to the identification of several genes that are common to lateral, adventitious and crown root development (Casimiro et al. 2003; Petricka et al. 2012). Availability of genomic data of crop plant O. sativa led to the development of several novel agronomic traits with superior root development and nutrient acquisition potential (Majumder et al. 1990; Wissuwa and Ae 2001; de Dorlodot et al. 2007; Shrawat et al. 2008). These agronomic traits are very useful for plants growing in hilly areas as well as in drought and dry soil to withstand adverse conditions. In hilly area, the roots provide proper anchorage to the plants while in drought and water starved area, roots help to absorb the underground soil moisture and help the plant to withstand the drought condition. Availability of genome sequence data in model plant like O. sativa led to decipher the potential role of different agronomic important traits. However, the genome sequence data of the orphan crop plant Eleusine coracana is not available yet. Eleusine coracana is an important monocot crop plant belonging to family Poaceae and cultivated throughout the world and used as a staple food crop in Africa and India as well. The grains of this plant contain important nutritional value as they are rich in vitamins (Shobana et al. 2013; Chandra et al. 2016; Gull and Ahmad 2016). Besides this, it is also very attractive source of dietary calcium content (Chandra et al. 2016). Therefore, the grains of E. coracana used to make dietary food and food product as well (Ramulu and Udayasekhara Rao 1997; Mangala et al. 1999; Mahadevamma and Tharanathan 2004). However, the crop yield of E. coracana is largely affected by soil texture, nutrient supplement and proper irrigation. In majority of cases, this plant is cultivated in dry and loamy conditions. Therefore, improvement of the rooting system in E. coracana will be very valuable to enhance the potential of crop yield.
The root development in plants is largely regulated by the phytohormone auxin (Vanneste and Friml 2009; Zazímalová et al. 2010; Mohanta and Mohanta 2013; Mohanta et al. 2014; Gururani et al. 2015). Several studies have demonstrated in model plants regarding root, and root hair formation. The phytohormone auxin is regulated by different auxin signaling genes including Aux/IAA (Indole-3-acetic acid) (Lewis et al. 2011; Carraro et al. 2012), LAX (auxin influx carrier) (Bainbridge et al. 2008; Swarup et al. 2008; Vandenbussche et al. 2010) and PIN (auxin efflux carrier) (Friml et al. 2003; Forestan and Varotto 2012). Previous studies demonstrated that auxin efflux carrier (PIN) protein conducts directional auxin flow and regulate root development and plant morphogenesis (Péret et al. 2013; Drdová et al. 2013; Band et al. 2014; Singh et al. 2015). Therefore, it was highly important to study the role of PIN genes in E. coracana to understand their potential role in root development. Hence, we cloned and characterized the auxin efflux carrier (EcPIN) genes from E. coracana and reported here.
Materials and methods
Plant material and growth conditions
To grow E. coracana, first of all the compost soil were autoclaved and sterilized to remove any potential bacterial and fungal growth. Later, the seeds were surface sterilized by 1% (v/v) sodium hypochlorite for 5 min followed by washing with 70% (v/v) ethanol for one minute. To remove the residual ethanol, seeds were washed with sterilized double distilled water. Later the seeds were germinated in pot and allowed to grow in the greenhouse at 50–60% of relative humidity. The light intensity for growth of E. coracana was kept around 700 µmol m−2 S−1. Later the plants were harvested at different developmental stages of 7th, 14th, 21st, and 28th days, respectively (at the interval of 1 week). The harvested plant samples (leaves and roots) were immediately transferred to the liquid nitrogen for further analysis. Three biological replicates of samples were harvested each time for the study.
Extraction of total RNA and cDNA synthesis
Total RNA was extracted from the root and leaf samples of E. coracana using Trizol method following manufacturer instructions. The isolated RNA was treated with DNase to remove the presence of any residual and contaminated DNA. The isolated RNA was used to synthesize cDNA for further use. RevertAid first strand synthesis kit was used to synthesize the first strand of cDNA. The reaction mixture was prepared by taking 2 µg of RNA sample followed by gentle heating and subsequent addition of oligo dT primers (500 ng/µl) and MLV reverse transcriptase (Promega, Madison, WI, USA). The resulted cDNA was diluted ten times with nuclease free water and kept at −20 °C for further use. A PCR reaction was run with actin gene to ensure that synthesis of cDNA was accomplished. The PCR reaction was checked in agarose gel electrophoresis. To confirm whether the DNase treatment was successful, another reaction was run with primers of actin gene in non-reverse transcribed total RNA. It was failed to amplify any product thus suggesting that the RNA and cDNA did not had any contaminated genomic DNA.
Primer design and cloning
Eleusine coracana is a monocot plant and the genome sequence of this orphan plant is not available yet. However, the genome sequence of monocot plant O. sativa is available and highly matches with the genome of E. coracana. Therefore, we used the OsPIN1a and OsPIN1b genes of O. sativa as orthologous gene to design the primers to clone the EcPIN genes in E. coracana. The forward and reverse primers of EcPIN1a were 5′-ATGATAACGGGGGC-3′ and 5′-CCCCAGCAGGATGTAGTACACC-3′, respectively, and forward and reverse primers of EcPIN1b were 5′-ATGATCACGGTGGT-3′ and 5′-GAGCCCCAGCAGTATGTAGTAG-3′, respectively. For qRT-PCR analysis, full length sequences of EcPIN1a and EcPIN1b were used to design the primers using Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/primer3/). The forward and reverse primers of EcPIN1a for qRT-PCR analysis were 5′-CATCGTCCTCGCGCTCCTCA-3′ and 5′-CCCATGACGAGCGTGTTGGG-3′, respectively, while the forward and reverse primers for EcPIN1b were 5′-GATGGTGCTGGCCATGCTCA-3′ and 5′-TGTTGGCGGCGGTGTCCGGG-3′, respectively. About 20 µl of PCR reaction mixture was prepared that contained 4 µl of high fidelity phusion buffer (5×), 0.5 µl of 10 mM dNTPs, 1 µl of 10 µM forward and reverse primer, 1 µl of cDNA template, 0.1 µl of phusion polymerase, 2 µl of DMSO solution, and 10.4 µl of nuclease free water. The thermal profile of the PCR reaction was as follows; initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 30 s, primer annealing at 58 °C for 30 s, polymerization at 72 °C for 2 min and final extension at 72 °C for 5 min. The resulted PCR products were separated by agarose gel electrophoresis and cloned into pGEMT vector. The cloned genes were subsequently sequenced to get their full length sequence.
In silico analysis of EcPIN genes
The resulted sequences of E. coracana were subjected to BLASTN search in O. sativa genome database and NCBI database. The BLAST result has shown similarity with PIN genes. The nucleotide sequences were translated to protein sequence using online server of Expasy bioinformatics portal (http://web.expasy.org/translate/) and Emboss Transeq of EMBL-EBI (https://www.ebi.ac.uk/Tools/st/emboss_transeq/). The resulted protein sequences were subjected to study the molecular masses and isoelectric point using protein calculator v3.4 (http://protcalc.sourceforge.net/). The presence of transmembrane domains in EcPIN proteins were studied using TMHMM server (http://www.cbs.dtu.dk/services/TMHMM/). The presence of different motifs in EcPIN protein was analyzed by MEME suit (http://meme-suite.org/) and motif scan software (http://myhits.isb-sib.ch/cgi-bin/motif_scan).
Multiple sequence alignment
To find out the sequence similarity with other PIN proteins, we conducted multiple sequence alignment of EcPIN proteins using Multalin server (http://multalin.toulouse.inra.fr/multalin/). Statistical parameters used to run the analysis were sequence input method, auto; alignment matrix, Blosum62-12-2; gap penalty, default; high consensus, 90% and low consensus was 50%.
Phylogenetic analysis
To construct the phylogenetic tree, PIN protein sequences of O. sativa and A. thaliana were downloaded from the rice genome annotation database and The Arabidopsis Information Resource portal, respectively. The EcPIN proteins along with the AtPINs and OsPINs were subjected to generate a clustal file using MUSCLE in EBI-EMBL (http://www.ebi.ac.uk/Tools/msa/muscle/) database. The resulted clustal file of PIN proteins was downloaded and converted to MEGA file format using MEGA6 software (Tamura et al. 2013). The MEGA file of the PIN protein was used to construct the phylogenetic tree. Following parameters were used to construct the phylogenetic tree; analysis, phylogeny reconstruction; statistical method, maximum likelihood; test of phylogeny, bootstrap method; number of bootstrap replicate, 1000, and gaps/missing data treatment, partial deletion.
Expression analysis by qRT-PCR
Transcript level of cloned EcPIN genes was studied using quantitative real-time PCR (qRT-PCR) using Mx3000P real-time PCR system (Stratagene, Santa Clara, CA, USA). The qRT-PCR was run using 25 µl reaction mixture that contained 12.5 µl of SYBR green/ROX master mix (Fermentas, USA), 1 µl of cDNA template, 1 µl of each forward and reverse primer, and 8.5 µl of nuclease free water. The actin gene of E. coracana was used as an internal control to normalize the gene of interest. Each sample was amplified in three biological replicates where each biological replicate contained three technical replicates as well. The thermal profile for qRT-PCR analysis was as follows: an initial step of denaturation at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and polymerization at 72 °C for 30 s. The expression levels of EcPIN1a and EcPIN1b were calculated using the 2−ΔΔCT method (Schmittgen and Livak 2008).
Results and discussion
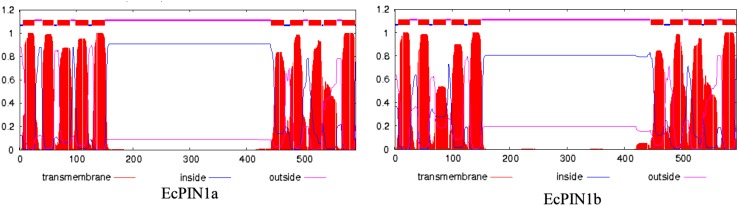
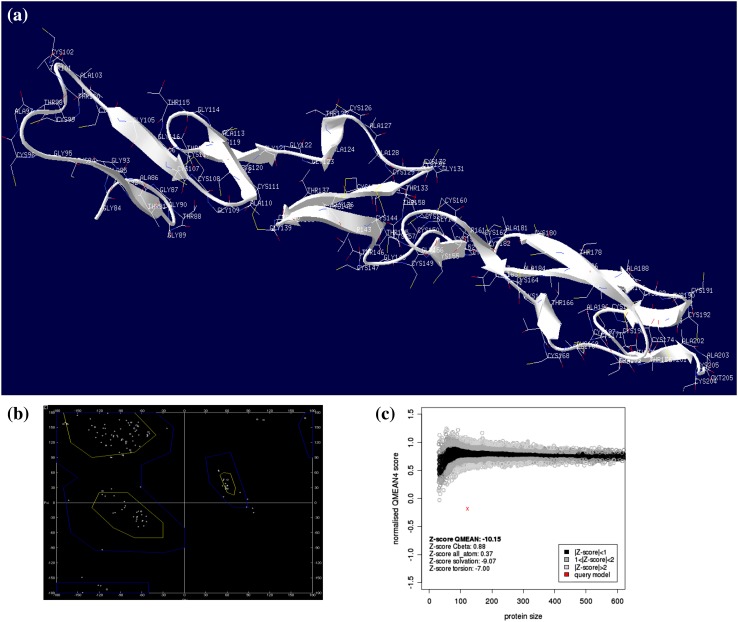
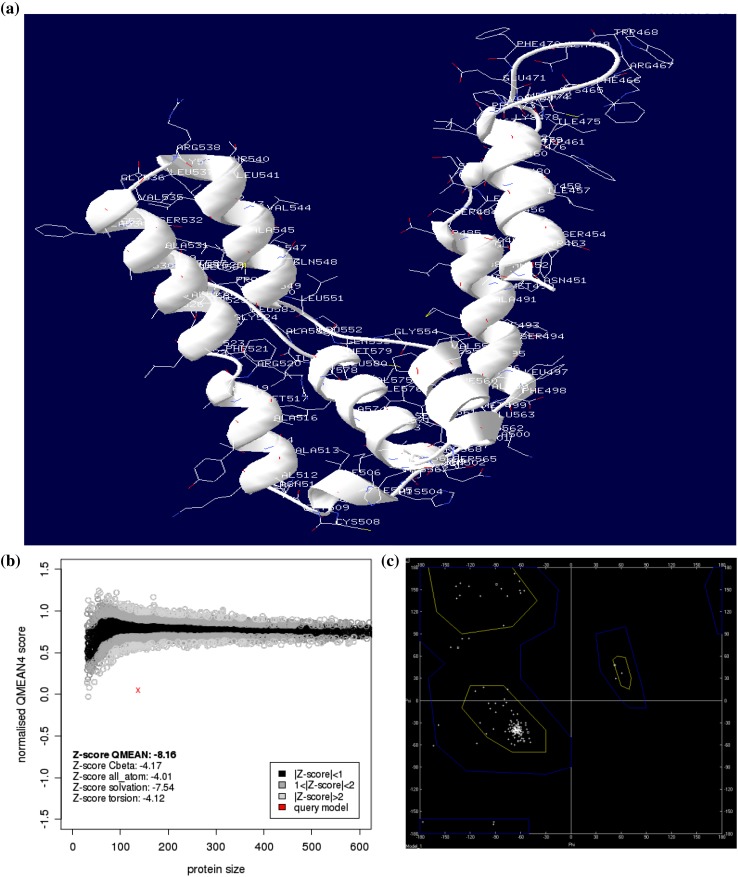
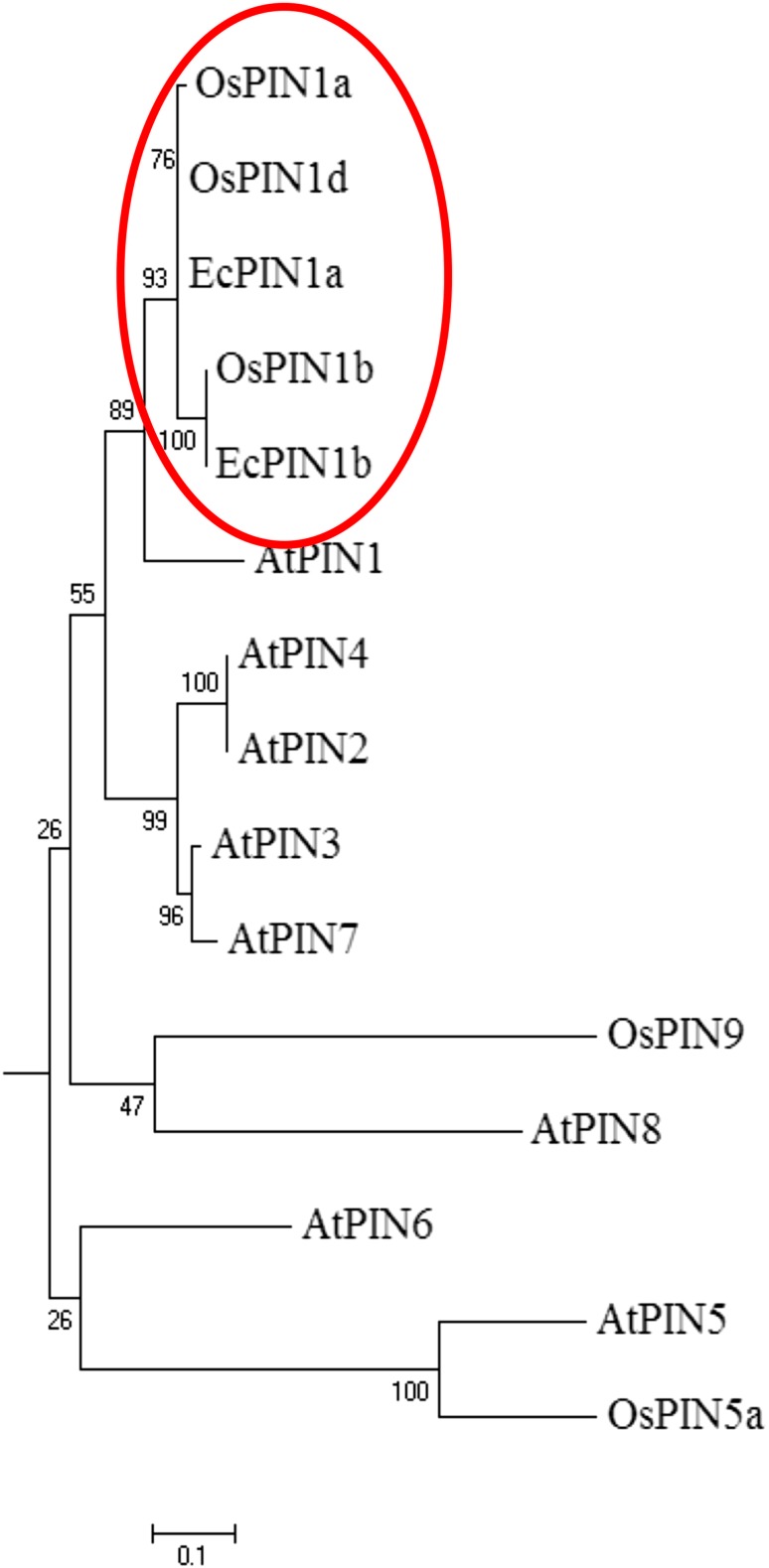
The O. sativa PIN genes available in public genome database were used to design the EcPIN family gene specific primers in E. coracana. The PCR reaction carried out with specific primers to clone two EcPIN genes resulted in amplification of two bands from E. coracana. The sequencing results followed by BLAST analysis of these PCR products showed similarity with PIN genes of other organism in NCBI, TAIR and rice genome annotation database. This confirms that the cloned genes belonged to EcPIN genes. The sequencing report revealed that EcPIN1a was 1779 bp and PIN1b was 1788 bp long, and was predicted to contain 592 and 595 amino acids, respectively (GenBank accession number for PIN1a and PIN1b are KY305428 and KY305429, respectively) (Fig. 1). Pairwise nucleotide sequence alignment between EcPIN1a and EcPIN1b genes show 84.5% similarity while pairwise sequence alignment of amino acid shows 87.9% similarity. The molecular mass of EcPIN1a and EcPIN1b was found to be 64.368 and 64.809 kDa, respectively, while the iso-electric point (pI) of EcPIN1a and EcPIN1b was 8.96 and 8.93, respectively. The charge at pH 7 of EcPIN1a and EcPIN1b was found to be 9.0 and 8.5, respectively. Motif analysis using MEME suit v4.11.2 shows the presence of at least 14 motifs in EcPIN1a and 12 motifs in EcPIN1b. Multiple sequence alignment with PIN proteins of O. sativa and A. thaliana shows several conserved motifs. Few of the conserved motifs of EcPIN1a and EcPIN1b along with PIN proteins of O. sativa and A. thaliana were F-x2-D/E-Q-C, I-I-W, F-x2-E, W-R-K-L-x2-P-N-x-Y, G-x2-W-x10-L-P-x5-S and V-A-I-x-Q-L-L-A-P-x2-I-x2-F-V-F-A-x-E-Y-x2-H-x4-T-x-V-I-x-G (Fig. 2). The transmembrane domain analysis revealed the presence of membrane spanning transmembrane domains in EcPIN1a and EcPIN1b (Fig. 3) protein. The EcPIN1a and EcPIN1b proteins were found to contain nine transmembrane helices. The crystal structure of PIN protein is not available yet. Hence, to know the probable structure of PIN protein, we undertake modeling approach to model EcPIN1a and EcPIN1b protein in SWISS model automatic modeling mode (Figs. 4, 5). From several models obtained during the modeling process, we selected the best one based on its lowest QMEAN Z-score (Z-score: −10.15). Further motif analysis shows the presence of one membrane transport and amidation site, three Asn glycosylation sites, one CAMP phospho site, five CK2 phospho sites, nine PKC phospho sites, and 12 myristoylation sites in EcPIN1a protein. Unlike EcPIN1a, EcPIN1b was also found to contain one membrane transport and amidation site, one Asn glycosylation site, one CAMP phospho site, four CK2 phospho sites, ten PKC phospho sites, 12 myristoylation and alanine rich sites (Table 1). The EcPIN1a protein contained three Asn glycosylation sites, whereas EcPIN1b protein contained only one Asn glycosylation site. Further, EcPIN1a protein contained five CK2 phospho sites while EcPIN1b protein contained only four CK2 phospho sites. The EcPIN1a protein contained only nine PKC phospho sites while EcPIN1b contained ten. Alanine rich region was not found in EcPIN1a while it was present in EcPIN1b protein. The phylogenetic analysis of EcPIN proteins with PIN proteins of A. thaliana and O. sativa shows closer relationship of EcPIN1a with OsPIN1d and OsPIN1a, while EcPIN1b shows close relationship with OsPIN1b (Fig. 6). The PIN proteins of E. coracana grouped with the PIN protein of monocot plant O. sativa while the PIN proteins of dicot plant A. thaliana grouped separately. This shows that EcPIN proteins are monocot specific and belonged to E. coracana.
Fig. 1.
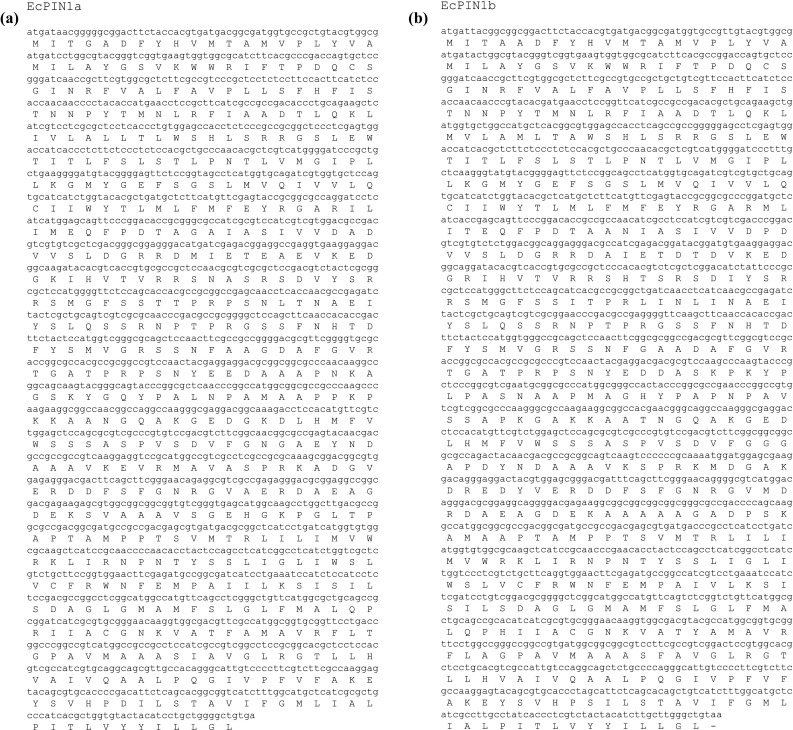
a Cloned nucleotide sequence of EcPIN1a. It contains 1779 nucleotides that encode for 592 amino acids. b Cloned nucleotide sequence of EcPIN1b. It contains 1788 nucleotides that encodes for 595 amino acids
Fig. 2.
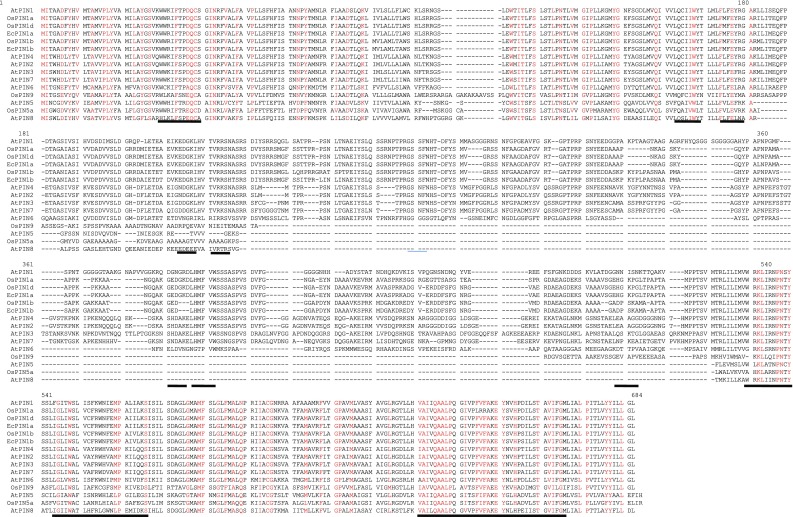
Multiple sequence alignment of EcPIN1a and EcPIN1b proteins with PIN proteins of model plant Arabidopsis thaliana and Oryza sativa. Alignment shows the presence of several conserved motifs in PIN proteins. Conserved motifs are underlined in the figure
Fig. 3.
Analysis for the presence of membrane bound transmembrane domain in EcPIN1a and EcPIN1b protein. Prediction shows the presence of membrane bound transmembrane helices in EcPIN1a and EcPIN1b. Prediction was conducted using online TMHMM server
Fig. 4.
In silico modeling of EcPIN1a protein. a Molecular model of EcPIN1a protein. b Ramachandran plot of EcPIN1a model. c It shows the model quality and QMEAN Z-score. The model quality along with distribution of amino acids in Ramachandran plot shows that the resulted model was the best model of EcPIN1a protein. Molecular modeling was conducted in SWISS-MODEL automatic mode
Fig. 5.
In silico modeling of EcPIN1b protein. a Molecular model of EcPIN1b protein. b Ramachandran plot of EcPIN1b model. c It shows the model quality and QMEAN Z-score. The model quality along with distribution of amino acids in Ramachandran plot shows that the resulted model was the best model of EcPIN1b protein. Molecular modeling was conducted in SWISS-MODEL automatic mode
Table 1.
In-silico prediction of possible functional domains of EcPIN1a and EcPIN1b protein
| EcPIN1a | EcPIN1b | ||
|---|---|---|---|
| Position | Domain | Position | Domain |
| 9–587 | Membrane transporter | 9–590 | Membrane transporter |
| 185–188 | Amidation | 185–188 | Amidation |
| 211–214 | Asn glycosylation | 257–260 | Asn glycosylation |
| 234–237 | Asn glycosylation | ||
| 257–260 | Asn glycosylation | ||
| 94–97 | CAMP phospho site | 94–97 | CAMP phospho site |
| 3–6 | CK2 phospho site | 3–6 | CK2 phospho site |
| 193–196 | CK2 phospho site | 193–196 | CK2 phospho site |
| 213–216 | CK2 phospho site | 213–216 | CK2 phospho site |
| 236–239 | CK2 phospho site | 288–291 | CK2 phospho site |
| 288–291 | CK2 phospho site | ||
| 27–29 | PKC phospho site | 27–29 | PKC phospho site |
| 93–95 | PKC phospho site | 93–95 | PKC phospho site |
| 206–208 | PKC phospho site | 206–208 | PKC phospho site |
| 219–221 | PKC phospho site | 212–214 | PKC phospho site |
| 229–231 | PKC phospho site | 219–221 | PKC phospho site |
| 245–247 | PKC phospho site | 229–231 | PKC phospho site |
| 250–252 | PKC phospho site | 245–247 | PKC phospho site |
| 284–286 | PKC phospho site | 250–252 | PKC phospho site |
| 373–375 | PKC phospho site | 284–286 | PKC phospho site |
| 170–175 | Myristoylation | 272–274 | PKC phospho site |
| 253–258 | Myristoylation | 253–258 | Myristoylation |
| 278–283 | Myristoylation | 272–277 | Myristoylation |
| 301–306 | Myristoylation | 278–283 | Myristoylation |
| 326–331 | Myristoylation | 326–331 | Myristoylation |
| 355–360 | Myristoylation | 334–339 | Myristoylation |
| 455–460 | Myristoylation | 415–420 | Myristoylation |
| 484–489 | Myristoylation | 458–463 | Myristoylation |
| 493–498 | Myristoylation | 487–492 | Myristoylation |
| 506–511 | Myristoylation | 496–501 | Myristoylation |
| 533–538 | Myristoylation | 509–514 | Myristoylation |
| 575–580 | Myristoylation | 536–541 | Myristoylation |
| 578–583 | Myristoylation | ||
| 403–427 | Alanine rich | ||
Fig. 6.
Phylogenetic relationship of EcPIN1a and EcPIN1b with PIN proteins of model plants A. thaliana and O. sativa. Phylogenetic result shows close relationship of EcPIN1a with OsPIN1a and OsPIN1d and EcPIN1b with OsPIN1b. This show the EcPIN proteins were closer to monocot plant O. sativa. Phylogenetic tree was constructed using MEGA6 software with 1000 bootstrap replicates
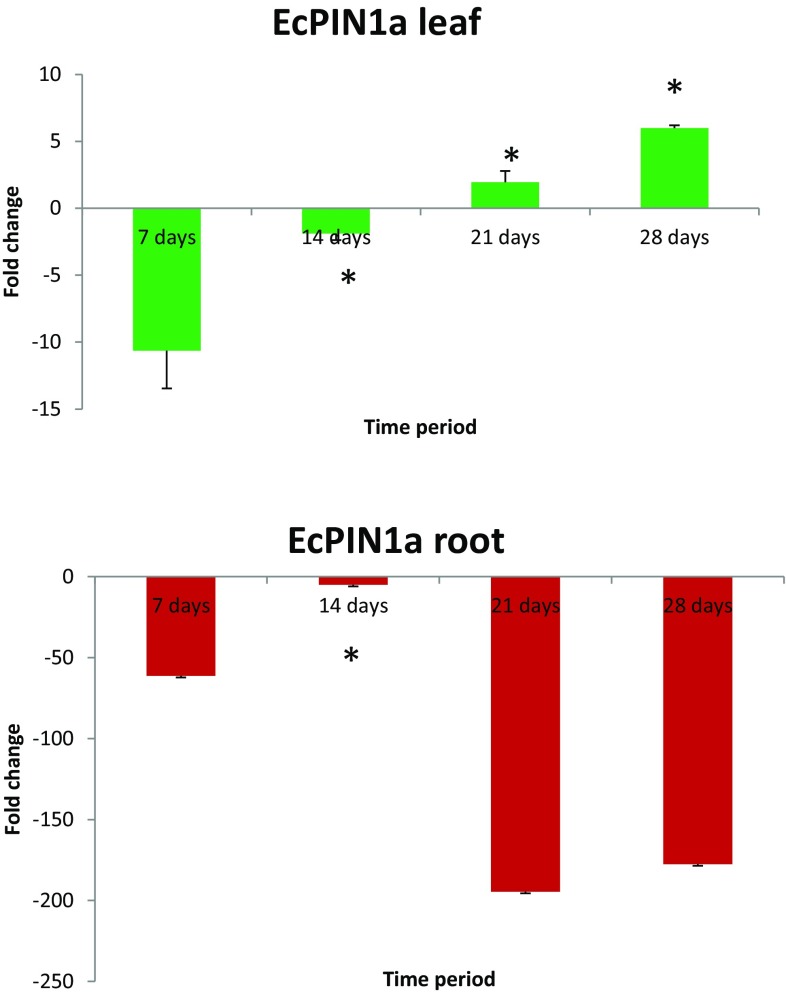
Transcriptome analysis was performed to understand their transcript abundance at different developmental stages. The transcript level of EcPIN1a in leaf was found to be down-regulated at 7- and 14-day time periods while they were up-regulated at 21- and 28-day time periods (Fig. 7). The EcPIN1a was down-regulated 10.63-fold at 7 days followed by 1.88-fold down-regulation while it was up-regulated 1.95-fold at 21 days and 5.98-fold at 28-day time period. The transcript abundance gradually increased with increasing time period in leaf tissue. However, the transcript abundance of ECPIN1a in root was found to be down-regulated at all the four time points. At 7-day time period it was found to be down-regulated 61.23-fold while it was down-regulated 5.08-fold in the 14 days. Although it was down-regulated in both the time periods, transcript level was comparatively up-regulated at 14 days compared to the 7-day time period. The EcPIN1b was down-regulated at 21- and 28-day time periods as well. The transcript abundance of EcPIN1b was detected very minutely in both root and leaf tissue.
Fig. 7.
Transcript analysis of EcPIN1a gene. a The transcript profile of EcPIN1a gene was found to be down-regulated at 7- and 14-day (1st and 2nd week) time periods while it was up-regulated at 21- and 28-day (3rd and 4th week) time periods. The transcript level was gradually up-regulated by increase in time period. b The transcript profile of EcPIN1a in root tissue shows down-regulated in all four time periods. At the 14-day time period, it was comparatively up-regulated compared to 7-day time period. Transcript profile of EcPIN1b gene was not detected during this study. Metric bars indicated the standard error (SE). Asterisk in the graph indicates statistically significant differences: * p < 0.05. Statistical analysis was done using unpaired t test
Development of plant organs and its morphogenesis largely depends upon the dynamic relationship between the regulation of gene expression and fine tuning of physico-chemical processes. The phytohormone auxin plays a major role in vegetative (Ljung et al. 2001; Reinhardt et al. 2003), reproductive (Sundberg and Østergaard 2009; Jain and Khurana 2009) and root development (Mohanta and Mohanta 2013; Singh et al. 2015) in plants. Auxin was found to play important role in root hair formation (Lee and Cho 2006; Band et al. 2014), root gravitropism (Luschnig et al. 1998; Friml et al. 2002b), phyllotactic patterning (Vernoux et al. 2000; Reinhardt et al. 2000; Furutani et al. 2004) and leaf vein formation (Rolland-Lagan and Prusinkiewicz 2005; Wenzel et al. 2007; Scarpella et al. 2010). It is synthesized in the localized aerial parts of the plant and mediates uneven distribution throughout the plant and maintains homeostasis with the help of auxin efflux carrier (PIN) proteins that leads to proper growth and development of plants (Mohanta et al. 2015; Singh et al. 2015). The regulated and directional polar auxin transport within different plant tissues is unique to auxin and it has not been found in any other signaling molecules. Therefore, cloning of EcPIN1a and EcPIN1b gene was uncalled for and reported in the manuscript. Previous studies reported that PIN protein contains membrane spanning transmembrane domain which help to flux the auxin molecules in directional manner (Friml and Palme 2002; Petrásek and Friml 2009). Our analysis using TMHMM software shows that EcPIN1a and EcPIN1b proteins contain transmembrane domains which closely match with the previous studies (Chen et al. 1998; Friml et al. 2002a). Both EcPIN1a and EcPIN1b contain ten transmembrane helices. Chen et al. (1998) also reported the presence of same number of transmembrane helices in A. thaliana (Chen et al. 1998). Arabidopsis thaliana AGRAVITROPIC 1 (AGR1) encodes for auxin efflux carrier and mutation in AGR1 conferred increased root-growth sensitivity auxin and decreased sensitivity to ethylene, thus causing retention of exogenously added auxin in root tip cells (Chen et al. 1998). Chen et al. (1998) used positional cloning method to know whether AGR1 encodes putative transmembrane domain and its homologies shares with bacterial transporter protein. Upon expression in Saccharomyces cerevisiae, AGR1 promoted an increase in efflux of radiolabeled IAA from the cells and confers increased resistant to toxic fluoro-IAA (Chen et al. 1998). Li et al. (2012) cloned and characterized auxin efflux carrier MiPIN1 in Mangifera indica associated with adventitious root formation in cotyledon segment (Li et al. 2012). Chawla and DeMason (2004) cloned PsPIN1 gene, a putative auxin efflux carrier from Pisum sativum (Chawla and DeMason 2004). The PsPIN1 gene was found to be ubiquitously expressed throughout the plant, more specifically in growing tissues. The phylogenetic analysis of EcPIN1a and EcPIN1b with PIN proteins of monocot and dicot plant grouped them in monocot lineage, suggesting their monocot specific origin in plant kingdom.
Conclusion
Cloning of auxin efflux carrier gene EcPIN1a and EcPIN1b were done in E. coracana and their expression analysis revealed gradual up-regulation of PIN1a genes from 7th to 28 days of developmental stages. Although EcPIN1a was down regulated at 7th and 14th days, they were up-regulated at 21 and 28-day time periods. Auxin efflux carrier genes play significant roles in plant growth and development in model plant including A. thaliana and O. sativa. Our study will help to understand the role of EcPIN1a and EcPIN1b gene in root and vegetative development in E. coracana plant.
Acknowledgement
This work was carried out with the support of the Next-Generation Biogreen 21 Program (PJ011113), Rural Development Administration, Korea.
Compliance with ethical standards
Conflict of interest
Author declares that there is no competing interest towards the publication of this manuscript.
Contributor Information
Tapan Kumar Mohanta, Email: nostoc.tapan@gmail.com.
Hanhong Bae, Email: hanhongbae@ynu.ac.kr.
References
- Bainbridge K, Guyomarc’h S, Bayer E, et al. Auxin influx carriers stabilize phyllotactic patterning. Genes Dev. 2008;22:810–823. doi: 10.1101/gad.462608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band LR, Wells DM, Fozard JA, et al. Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell. 2014;26:862–875. doi: 10.1105/tpc.113.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañoc DM, Yamauchi A, Kamoshita A, et al. Genotypic variations in response of lateral root development to fluctuating soil moisture in rice. Plant Prod Sci. 2000;3:335–343. doi: 10.1626/pps.3.335. [DOI] [Google Scholar]
- Barberon M, Geldner N. Radial transport of nutrients: the plant root as a polarized epithelium. Plant Physiol. 2014;166:528–537. doi: 10.1104/pp.114.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro N, Tisdale-Orr TE, Clouse RM, et al. Diversification and expression of the PIN, AUX/LAX, and ABCB families of putative auxin transporters in Populus. Front Plant Sci. 2012;3:17. doi: 10.3389/fpls.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, et al. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003;8:165–171. doi: 10.1016/S1360-1385(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Chandra D, Chandra S, Sharma AK. Review of Finger millet (Eleusine coracana (L.) Gaertn): a power house of health benefiting nutrients. Food Sci Hum Wellness. 2016;5:149–155. doi: 10.1016/j.fshw.2016.05.004. [DOI] [Google Scholar]
- Chapman N, Miller AJ, Lindsey K, Whalley WR. Roots, water, and nutrient acquisition: let’s get physical. Trends Plant Sci. 2012;17:701–710. doi: 10.1016/j.tplants.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Chawla R, DeMason DA. Molecular expression of PsPIN1, a putative auxin efflux carrier gene from pea (Pisum sativum L.) Plant Growth Regul. 2004;44:1–14. doi: 10.1007/s10725-004-2139-9. [DOI] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, et al. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y, Périn C, Courtois B, et al. Genetic control of root development in rice, the model cereal. Trends Plant Sci. 2010;15:219–226. doi: 10.1016/j.tplants.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Craine JM, Dybzinski R. Mechanisms of plant competition for nutrients, water and light. Funct Ecol. 2013;27:833–840. doi: 10.1111/1365-2435.12081. [DOI] [Google Scholar]
- de Dorlodot S, Forster B, Pagès L, et al. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007;12:474–481. doi: 10.1016/j.tplants.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Drdová EJ, Synek L, Pečenková T, et al. The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J. 2013;73:709–719. doi: 10.1111/tpj.12074. [DOI] [PubMed] [Google Scholar]
- Forestan C, Varotto S. The role of PIN auxin efflux carriers in polar auxin transport and accumulation and their effect on shaping maize development. Mol Plant. 2012;5:787–798. doi: 10.1093/mp/ssr103. [DOI] [PubMed] [Google Scholar]
- Friml J, Palme K. Polar auxin transport-old questions and new concepts? Plant Mol Biol. 2002;49:273–284. doi: 10.1023/A:1015248926412. [DOI] [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/S0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, et al. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Furutani M, Vernoux T, Traas J, et al. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development. 2004;131:5021–5030. doi: 10.1242/dev.01388. [DOI] [PubMed] [Google Scholar]
- Gull A, Ahmad NG. Technological, processing and nutritional approach of finger millet (Eleusine coracana)—a mini review. J Food Process Technol. 2016;7:8–11. doi: 10.4172/2157-7110.1000593. [DOI] [Google Scholar]
- Gururani M, Mohanta T, Bae H. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int J Mol Sci. 2015;16:19055–19085. doi: 10.3390/ijms160819055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006;11:610–617. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Jain M, Khurana JP. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J. 2009;276:3148–3162. doi: 10.1111/j.1742-4658.2009.07033.x. [DOI] [PubMed] [Google Scholar]
- Kong D, Ma C. Acquisition of ephemeral module in roots: a new view and test. Sci Rep. 2014;4:4–7. doi: 10.1038/srep05078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Cho H. PINOID positively regulates auxin efflux in arabidopsis root hair cells and tobacco cells. Plant Cell. 2006;18:1604–1616. doi: 10.1105/tpc.105.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development. 2011;138:3485–3495. doi: 10.1242/dev.065102. [DOI] [PubMed] [Google Scholar]
- Li YH, Zou MH, Feng BH, et al. Molecular cloning and characterization of the genes encoding an auxin efflux carrier and the auxin influx carriers associated with the adventitious root formation in mango (Mangifera indica L.) cotyledon segments. Plant Physiol Biochem. 2012;55:33–42. doi: 10.1016/j.plaphy.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang J, Wang L, et al. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res. 2009;19:1110–1119. doi: 10.1038/cr.2009.70. [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001;28:465–474. doi: 10.1046/j.1365-313X.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6:280–287. doi: 10.1016/S1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- Lorbiecke R. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999;119:21–30. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevamma S, Tharanathan RN. Processing of legumes: resistant starch and dietary fiber contents. J Food Qual. 2004;27:289–303. doi: 10.1111/j.1745-4557.2004.00620.x. [DOI] [Google Scholar]
- Majumder ND, Rakshit SC, Borthakur DN. Genetic effect on uptake of selected nutrients in some rice (O. sativa L.) varieties in phosphorus deficient soils. Plant Soil. 1990;123:117–120. doi: 10.1007/BF00009935. [DOI] [Google Scholar]
- Mangala SL, Malleshi NG, Tharanathan RN. Resistant starch from differently processed rice and ragi (finger millet) Eur Food Res Technol. 1999;209:32–37. doi: 10.1007/s002170050452. [DOI] [Google Scholar]
- Mohanta TK, Mohanta N. Genome wide identification of auxin efflux carrier gene family in Physcomitrella patens. J Biotechnol Sci. 2013;1:54–64. [Google Scholar]
- Mohanta T, Mickael M, Nibedita M, Chidananda NK. In-silico identification and phylogenetic analysis of auxin efflux carrier gene family in Setaria italica L. Afr J Biotechnol. 2014;13:211–225. doi: 10.5897/AJB2014.13617. [DOI] [Google Scholar]
- Mohanta T, Mohanta N, Bae H. Identification and expression analysis of PIN-like (PILS) gene family of rice treated with auxin and cytokinin. Genes. 2015;6:622–640. doi: 10.3390/genes6030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, et al. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Péret B, Middleton AM, French AP, et al. Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Mol Syst Biol. 2013;9:699. doi: 10.1038/msb.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- Petricka JJ, Winter CM, Benfey PN. Control of Arabidopsis root development. Annu Rev Plant Biol. 2012;63:563–590. doi: 10.1146/annurev-arplant-042811-105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramulu P, Udayasekhara Rao P. Effect of processing on dietary fiber content of cereals and pulses. Plant Foods Hum Nutr. 1997;50:249–257. doi: 10.1007/BF02436061. [DOI] [PubMed] [Google Scholar]
- Rebouillat J, Dievart A, Verdeil JL, et al. Molecular genetics of rice root development. Rice. 2009;2:15–34. doi: 10.1007/s12284-008-9016-5. [DOI] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce E-R, Stieger P, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Rolland-Lagan A-G, Prusinkiewicz P. Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis. Plant J. 2005;44:854–865. doi: 10.1111/j.1365-313X.2005.02581.x. [DOI] [PubMed] [Google Scholar]
- Scarpella E, Barkoulas M, Tsiantis M. Control of leaf and vein development by auxin. Cold Spring Harb Perspect Biol. 2010;2:a001511. doi: 10.1101/cshperspect.a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shobana S, Krishnaswamy K, Sudha V, et al. Finger millet (Ragi, Eleusine coracana L.). A review of its nutritional properties, processing, and plausible health benefits. Adv Food Nutr Res. 2013;69:1–39. doi: 10.1016/B978-0-12-410540-9.00001-6. [DOI] [PubMed] [Google Scholar]
- Shrawat AK, Carroll RT, DePauw M, et al. Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol J. 2008;6:722–732. doi: 10.1111/j.1467-7652.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- Singh P, Mohanta TK, Sinha AK. Unraveling the intricate nexus of molecular mechanisms governing rice root development: OsMPK3/6 and Auxin-Cytokinin Interplay. PLoS ONE. 2015;10:e0123620. doi: 10.1371/journal.pone.0123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg E, Østergaard L. Distinct and dynamic auxin activities during reproductive development. Cold Spring Harb Perspect Biol. 2009;1:1–15. doi: 10.1101/cshperspect.a001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benkova E, Swarup R, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrásek J, Zádníková P, et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development. 2010;137:597–606. doi: 10.1242/dev.040790. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, et al. PIN-FORMED 1 regulates cell fate at the perifery of the shoot apical meristem. Development. 2000;127:5157–5165. doi: 10.1242/dev.127.23.5157. [DOI] [PubMed] [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J. 2007;49:387–398. doi: 10.1111/j.1365-313X.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- Wissuwa M, Ae N. Further characterization of two QTLs that increase phosphorus uptake of rice (Oryza sativa L.) under phosphorus deficiency. Plant Soil. 2001;237:275–286. doi: 10.1023/A:1013385620875. [DOI] [Google Scholar]
- Zazímalová E, Murphy AS, Yang H, et al. Auxin transporters—why so many? Cold Spring Harb Perspect Biol. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]