Abstract
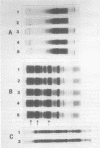
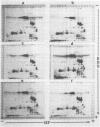
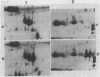
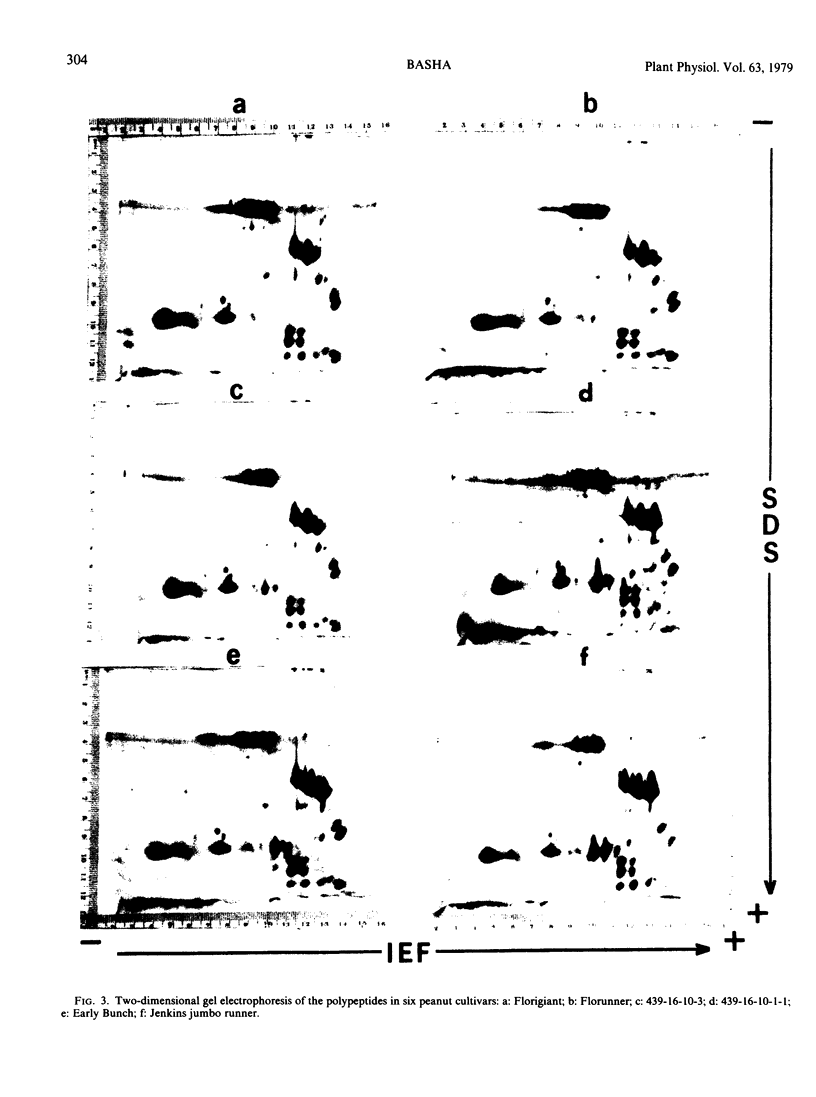
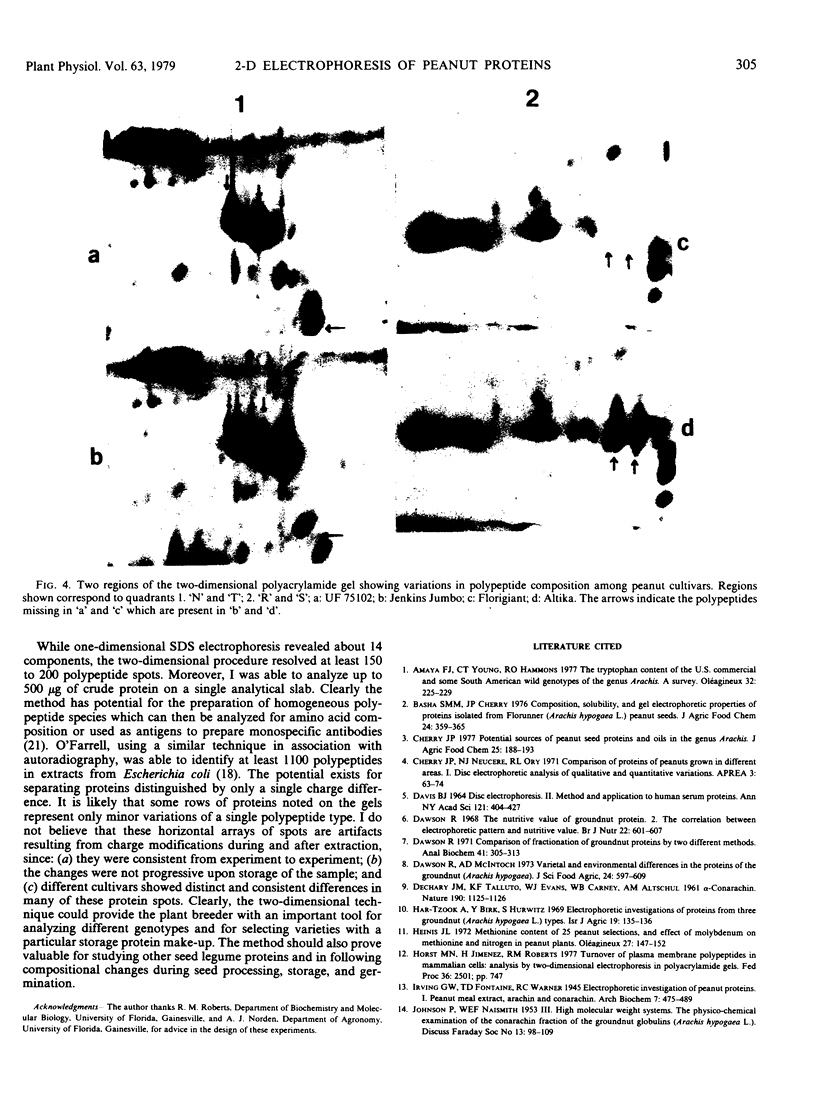
Seed polypeptides from several cultivars of peanut (Arachis hypogaea L.) have been compared by means of a two-dimensional polyacrylamide gel electrophoresis. Protein was extracted from the defatted peanut meal by homogenizing in 5 millimolar K2CO3-9.5 molar urea. After addition of Nonidet P-40 (2%, v/v) and dithiothreitol (0.5%, w/v) the solution was centrifuged at 25,000 g. This procedure led to solubilization of more than 95% of the total protein. The clear supernatant fraction was then subjected to two-dimensional polyacrylamide gel electrophoresis, employing isoelectric focusing in the first dimension and electrophoresis in presence of sodium dodecyl sulfate in the second. After examining several cultivars, it was possible to construct a composite map to include all of the polypeptide species found among all of the cultivars examined. At least 74 major and between 100 and 125 minor components were detectable by Coomassie blue staining. The majority of these had isoelectric points between pH 4.4 and 8.0, and molecular weights between 16,000 and 75,000. Several different cultivars have been compared using this method and it has been shown that considerable variation exists among the major polypeptides present. The method should prove valuable for analyzing different genotypes and selecting varieties with a particular storage protein make-up, as well as for following compositional changes that occur during seed development and germination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basha S. M., Al-Wandawi H. Composition, solubility, and gel electrophoretic properties of proteins isolated from Florunner (Arach is hypogaea L.) peanut seeds. J Agric Food Chem. 1976 Mar-Apr;24(2):359–365. doi: 10.1021/jf60204a058. [DOI] [PubMed] [Google Scholar]
- Cherry J. P. Potential sources of peanut seed proteins and oil in the genus Arachis. J Agric Food Chem. 1976 Jan-Feb;25(1):186–193. doi: 10.1021/jf60209a033. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dawson R. Comparison of fractionation of groundnut proteins by two different methods. Anal Biochem. 1971 Jun;41(2):305–313. doi: 10.1016/0003-2697(71)90147-3. [DOI] [PubMed] [Google Scholar]
- Dawson R. The nutritive value of groundnut protein. 2. The correlation between electrophoretic pattern and nutritive value. Br J Nutr. 1968 Dec;22(4):601–607. doi: 10.1079/bjn19680070. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neucere N. J. Isolation of alpha-arachin, the major peanut globulin. Anal Biochem. 1969 Jan;27(1):15–24. doi: 10.1016/0003-2697(69)90215-2. [DOI] [PubMed] [Google Scholar]
- Tombs M. P., Newsom B. G., Wilding P. Protein solubility: phase separation in arachin-salt-water systems. Int J Pept Protein Res. 1974;6(4):253–277. doi: 10.1111/j.1399-3011.1974.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Hengartner H. Sodium dodecyl sulfate electrophoresis of high molecular weight proteins in N, N'-diallyltartardiamide-cross-linked polyacrylamide slab gels. Eur J Immunol. 1977 Oct;7(10):690–690. doi: 10.1002/eji.1830071007. [DOI] [PubMed] [Google Scholar]