Abstract
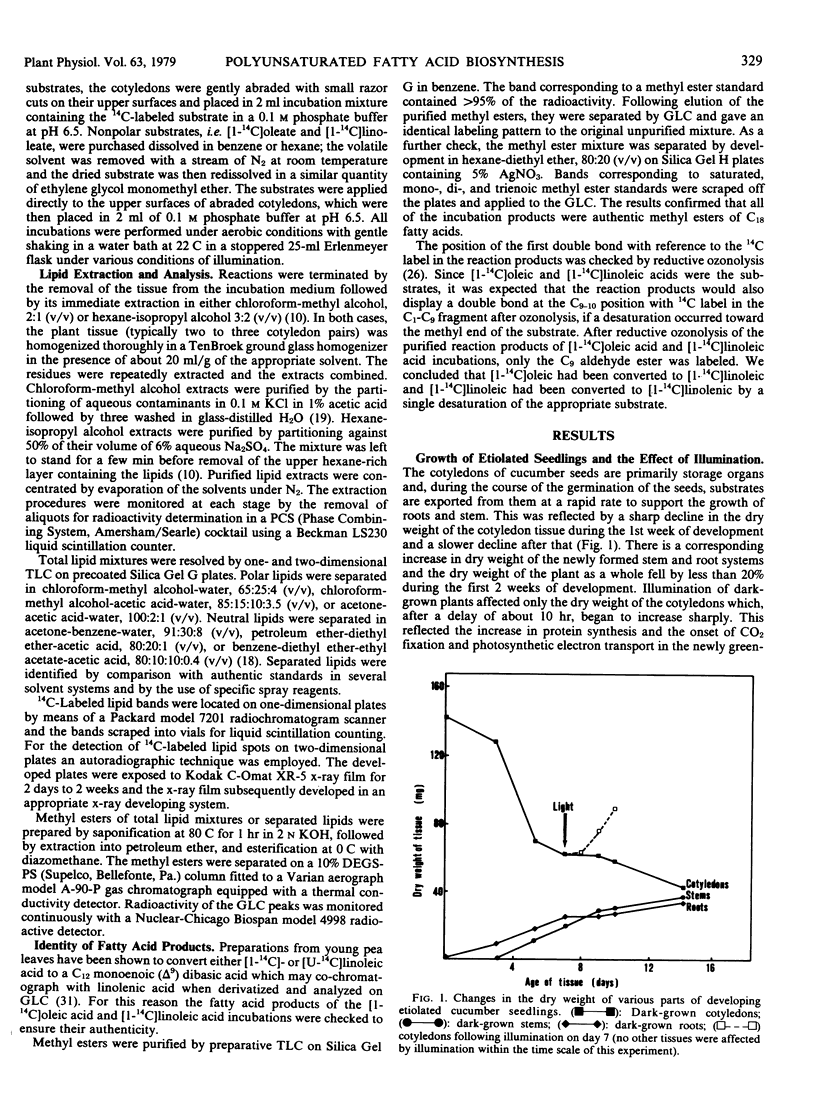
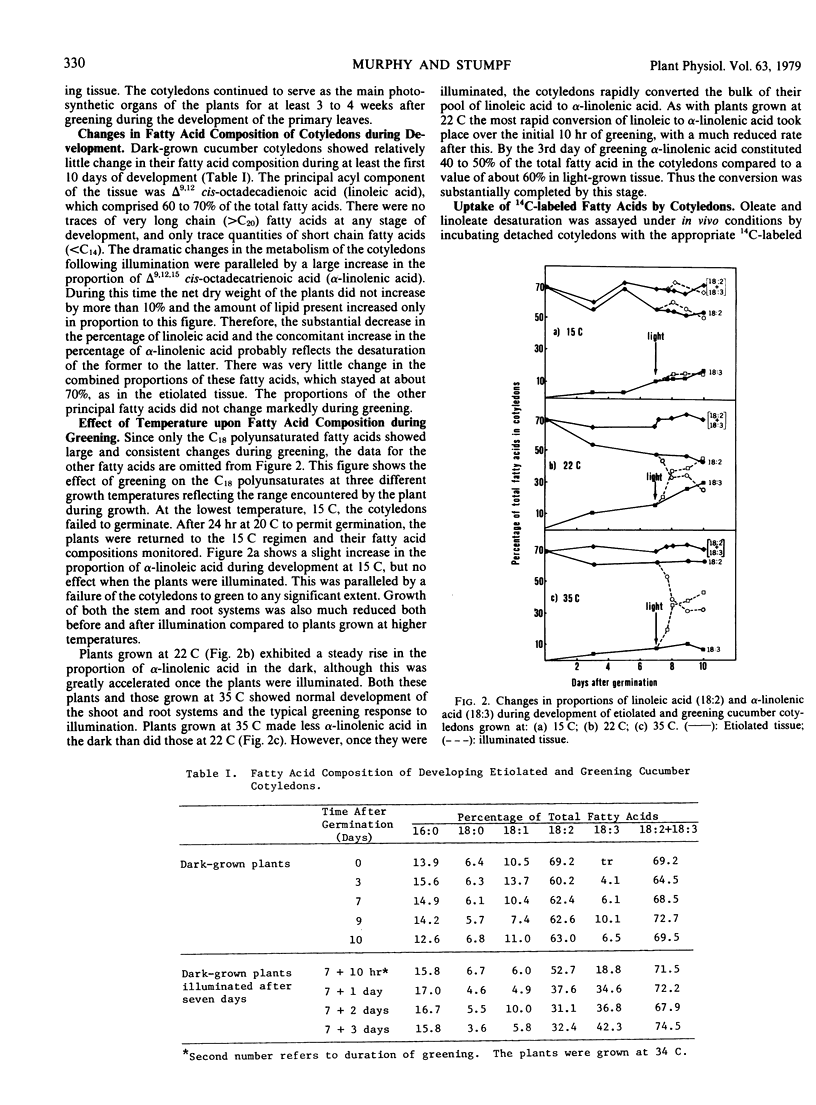
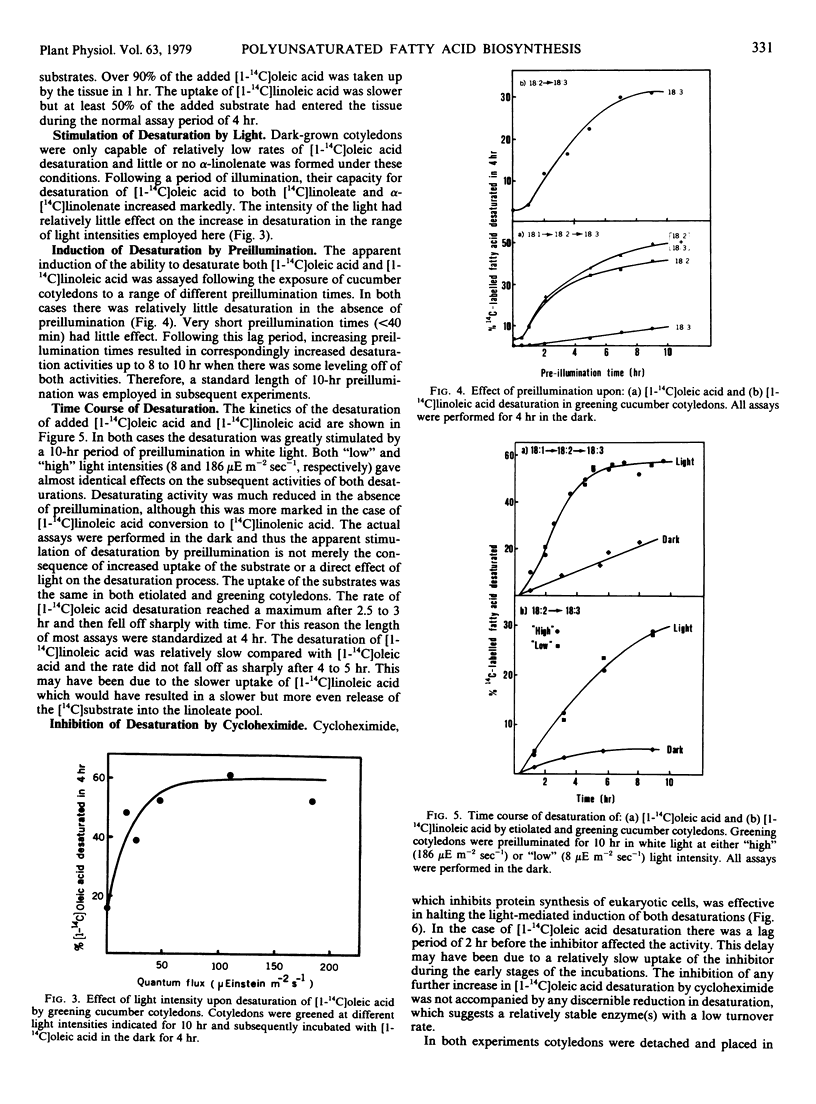
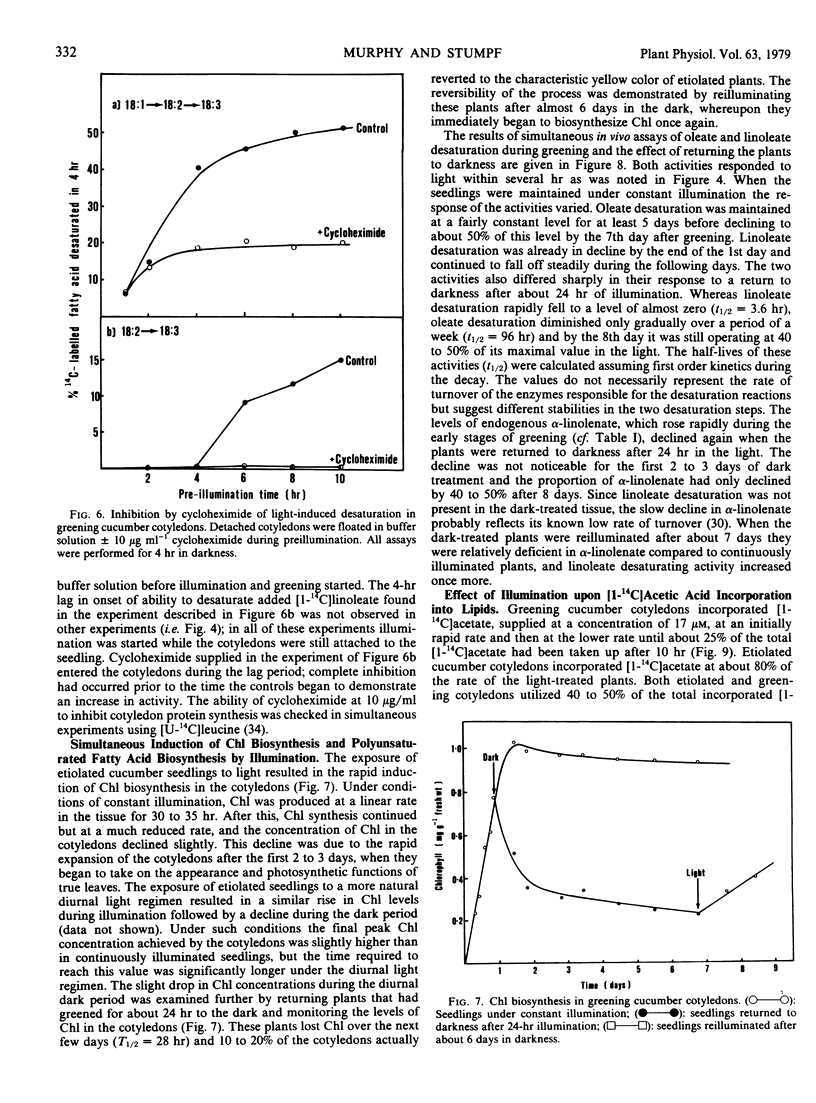
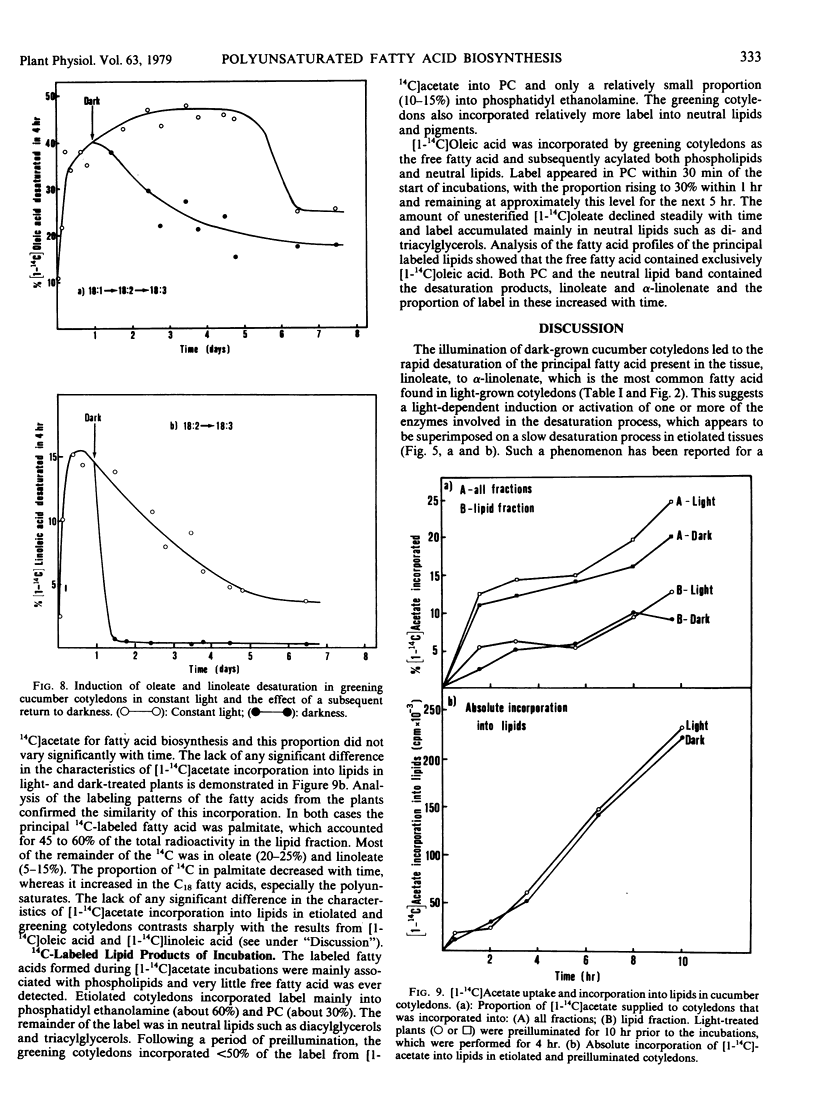
Greening cucumber (Cucumis sativus L.) cotyledons exhibited dramatic increases in the ability to desaturate exogenously added [1-14C]oleic acid and [1-14C]linoleic acid within 2 to 3 hours of illumination. These increases were effectively inhibited by 10 micrograms per milliliter cycloheximide. Oleate desaturation remained at a high level in constant light for 5 to 6 days after induction and then declined by about 50%; when returned to the dark, the tissue showed a sharp decrease in conversion of [14C]oleate to [14C]linoleate. Linoleate desaturation reached a maximum about 15 hours after induction and declined immediately thereafter while the tissue still was in the light; after induction had peaked return of the tissue to the dark showed a dramatic fall of linoleate desaturation. The changes in desaturation were correlated with the conversion of the principal fatty acid in the etiolated cotyledons, linoleate, to α-linolenate, and with the assembly of the chlorophyll-containing photosynthetic membranes. The incorporation of [1-14C]acetate into lipids showed no significant light stimulation. The role of light in the regulation of certain aspects of plant metabolism during development is discussed.
Full text
PDF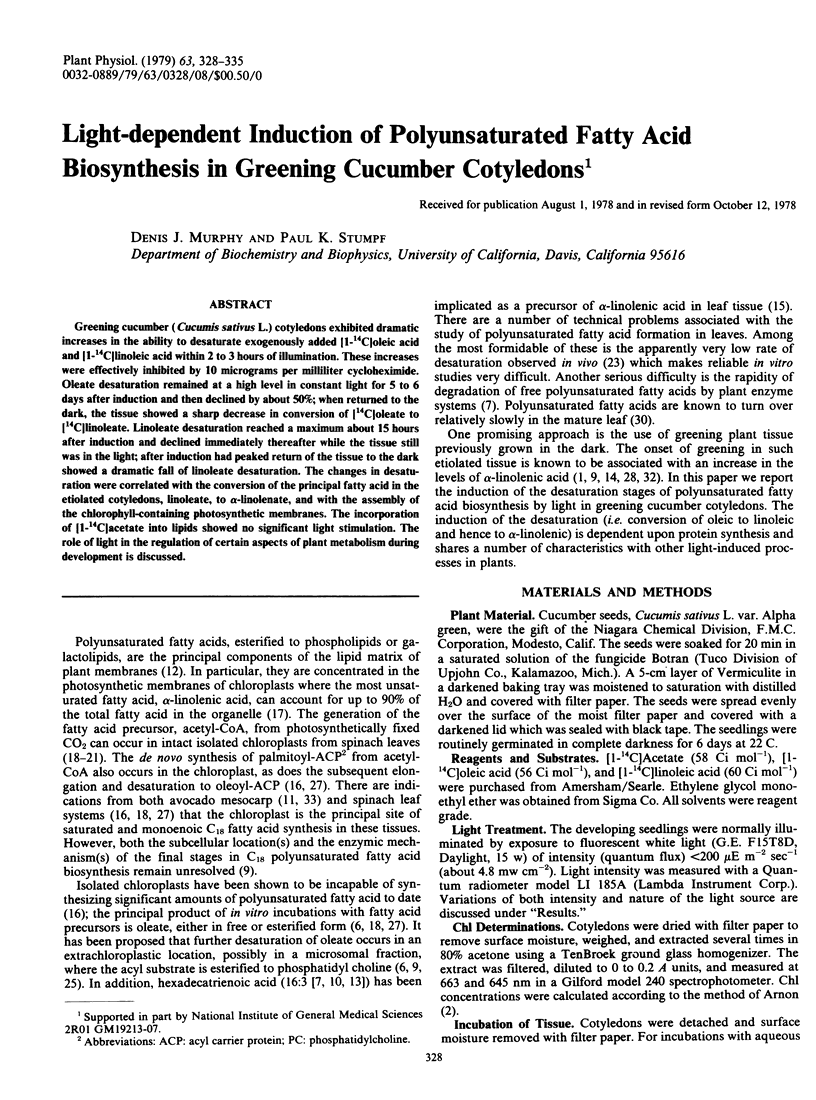


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelqvist L. A., Boynton J. E., Stumpf P. K., von Wettstein D. Lipid biosynthesis in relation to chloroplast development in barley. J Lipid Res. 1968 Jul;9(4):425–436. [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Henningsen K. W., Stumpf P. K., Appelqvist L. A., von Wettstein D. Lipid biosynthesis by isolated barley chloroplasts in relation to plastid development. Plant Physiol. 1971 Nov;48(5):526–531. doi: 10.1104/pp.48.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Jacobson B. S., Stumpf P. K. In vivo biosynthesis of -linolenic acid in plants. Biochem Biophys Res Commun. 1973 May 15;52(2):648–655. doi: 10.1016/0006-291x(73)90762-6. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. I. The biosynthesis of polyunsaturated fatty acids by isolated spinach chloroplasts. Arch Biochem Biophys. 1972 Feb;148(2):414–424. doi: 10.1016/0003-9861(72)90159-2. [DOI] [PubMed] [Google Scholar]
- Murphy D. J., Leech R. M. Lipid biosynthesis from [14C]bicarbonate, [2(-14)C]pyruvate and [1(-14)C]acetate during photosynthesis by isolated spinach chloroplasts. FEBS Lett. 1977 May 15;77(2):164–168. doi: 10.1016/0014-5793(77)80226-3. [DOI] [PubMed] [Google Scholar]
- Nicholas J. C., Harper J. E., Hageman R. H. Nitrate Reductase Activity in Soybeans (Glycine max [L.] Merr.): I. Effects of Light and Temperature. Plant Physiol. 1976 Dec;58(6):731–735. doi: 10.1104/pp.58.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidyl choline: Donor of 18-carbon unsaturated fatty acids for glycerolipid biosynthesis. Lipids. 1975 Oct;10(10):609–614. doi: 10.1007/BF02532725. [DOI] [PubMed] [Google Scholar]
- Roughan P. G. Turnover of the glycerolipids of pumpkin leaves. The importence of phosphatidylcholine. Biochem J. 1970 Mar;117(1):1–8. doi: 10.1042/bj1170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler C. R., Purvis A. C., Jordan W. R. Factors Involved in in Vitro Stabilization of Nitrate Reductase from Cotton (Gossypium hirsutum L.) Cotyledons. Plant Physiol. 1978 May;61(5):714–717. doi: 10.1104/pp.61.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trémolières A., Lepage M. Changes in Lipid Composition during Greening of Etiolated Pea Seedlings. Plant Physiol. 1971 Feb;47(2):329–334. doi: 10.1104/pp.47.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemot C., Stumpf P. K. Fat metabolism in higher plants. XXXIV. Development of fatty acid synthetase as a function of protein synthesis in aging potato tuber slices. Plant Physiol. 1967 Mar;42(3):391–397. doi: 10.1104/pp.42.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine ammonia-lyase in Xanthium leaf disks. Photosynthetic requirement and effect of daylength. Plant Physiol. 1969 Jun;44(6):912–922. doi: 10.1104/pp.44.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]


