Abstract
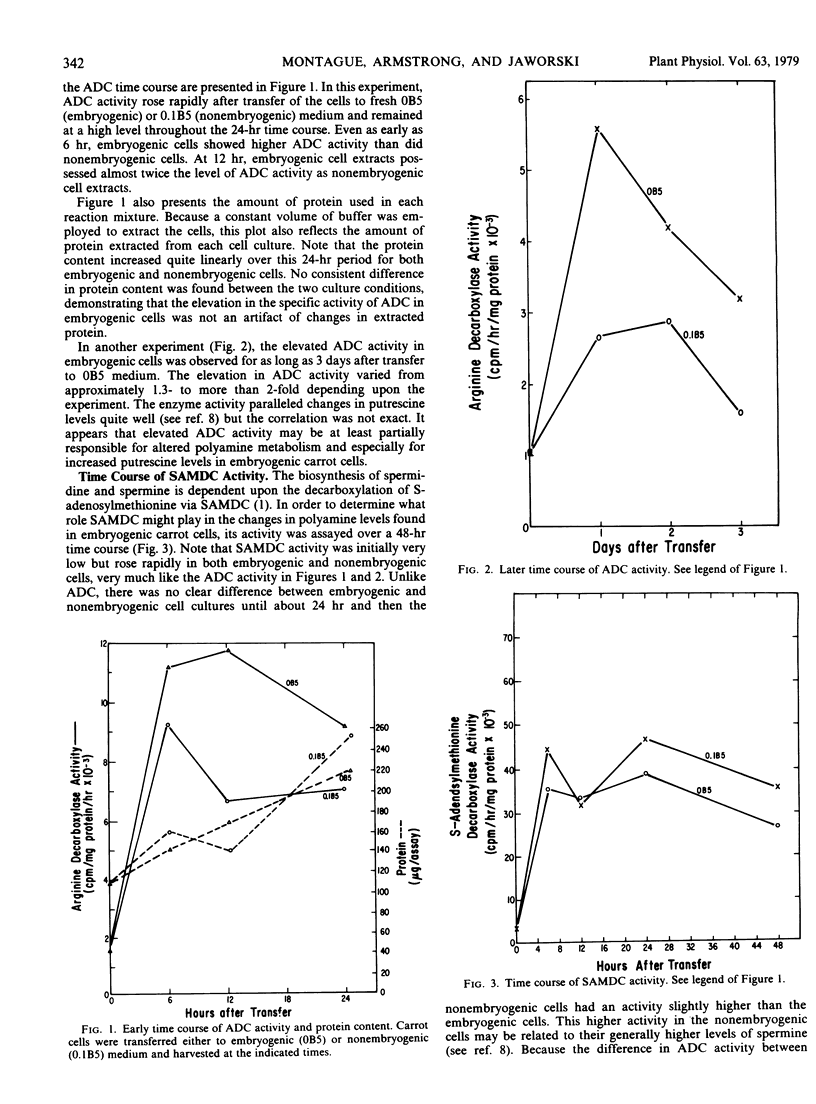
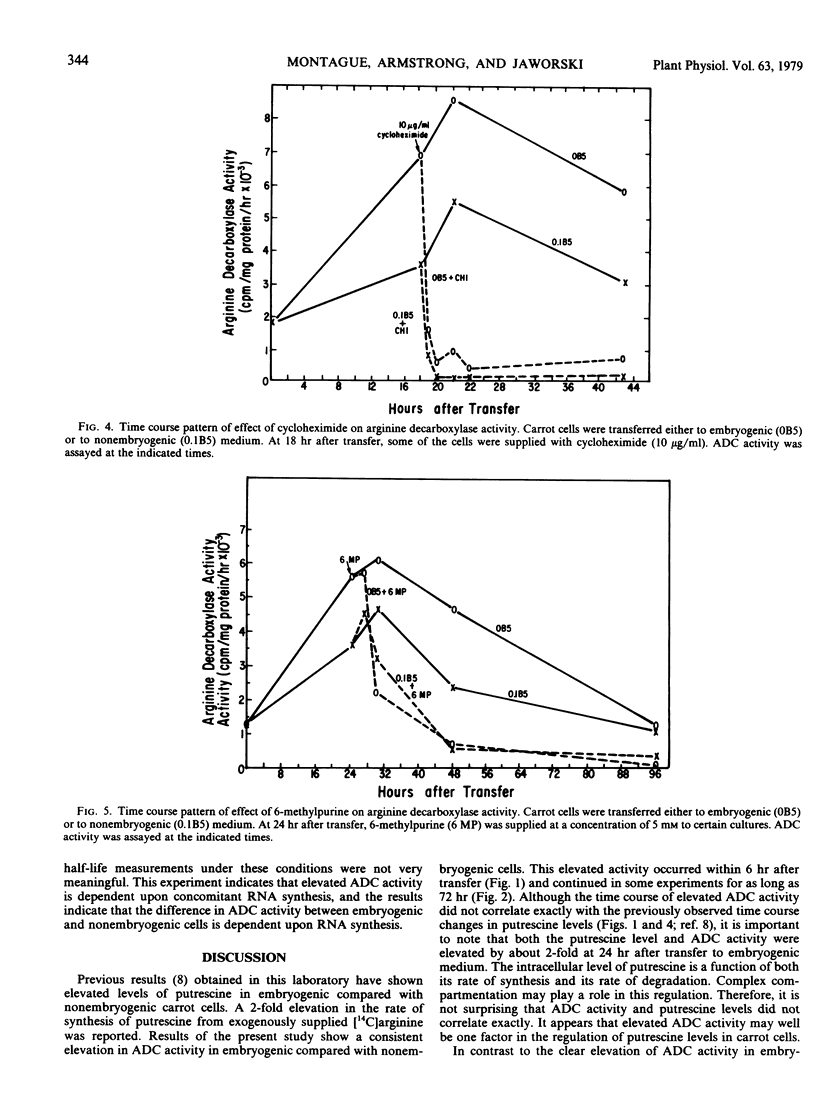
Embryogenic cultured cells of Daucus carota have been shown to synthesize putrescine from exogenously supplied [14C]arginine at twice the rate of control nonembryogenic cells. In the present paper, the activity of arginine decarboxylase (arginine carboxy-lyase, EC 4.1.1.19), an important enzyme in the synthesis of putrescine, was assayed and also found to be elevated by as much as 2-fold in embryogenic cells. This difference between embryogenic and nonembryogenic cells was observed as early as 6 hours after the induction of embryogenesis and appeared not to result from the presence of a diffusible inhibitor or activator. It seemed to be dependent upon concomitant RNA and protein synthesis, as judged using 6-methyl-purine and cycloheximide. After cycloheximide addition to the culture medium, arginine decarboxylase activity declined with a half-time of about 30 minutes in both embryogenic and nonembryogenic cells. It is suggested that elevated arginine decarboxylase activity is involved in the mechanism leading to elevated putrescine levels in these cells and hence may play a role in the embryogenic process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McAnulty P. A., Williams J. P. Polyamines and their biosynthetic decarboxylases in various tissues of the young rat during recovery from undernutrition. Biochem J. 1977 Jan 15;162(1):109–121. doi: 10.1042/bj1620109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: I. Changes in Intracellular Content and Rates of Synthesis. Plant Physiol. 1978 Sep;62(3):430–433. doi: 10.1104/pp.62.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis and their specificity for tumor promotion. Cancer Res. 1975 Sep;35(9):2426–2433. [PubMed] [Google Scholar]
- Prouty W. F. Ornithine decarboxylase inactivation in HeLa cells. J Cell Physiol. 1976 Sep;89(1):65–76. doi: 10.1002/jcp.1040890107. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]
- Yamasaki Y., Ichihara A. Induction of ornithine decarboxylase in cultured mouse L cells. I. Effects of cellular growth, hormones, and actinomycin D. J Biochem. 1976 Sep;80(3):557–562. doi: 10.1093/oxfordjournals.jbchem.a131311. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Ichihara A. Induction of ornithine decarboxylase in cultured mouse L cells. II. Effects of additions of amino acids and serum. J Biochem. 1977 Feb;81(2):461–465. doi: 10.1093/oxfordjournals.jbchem.a131479. [DOI] [PubMed] [Google Scholar]


