Abstract
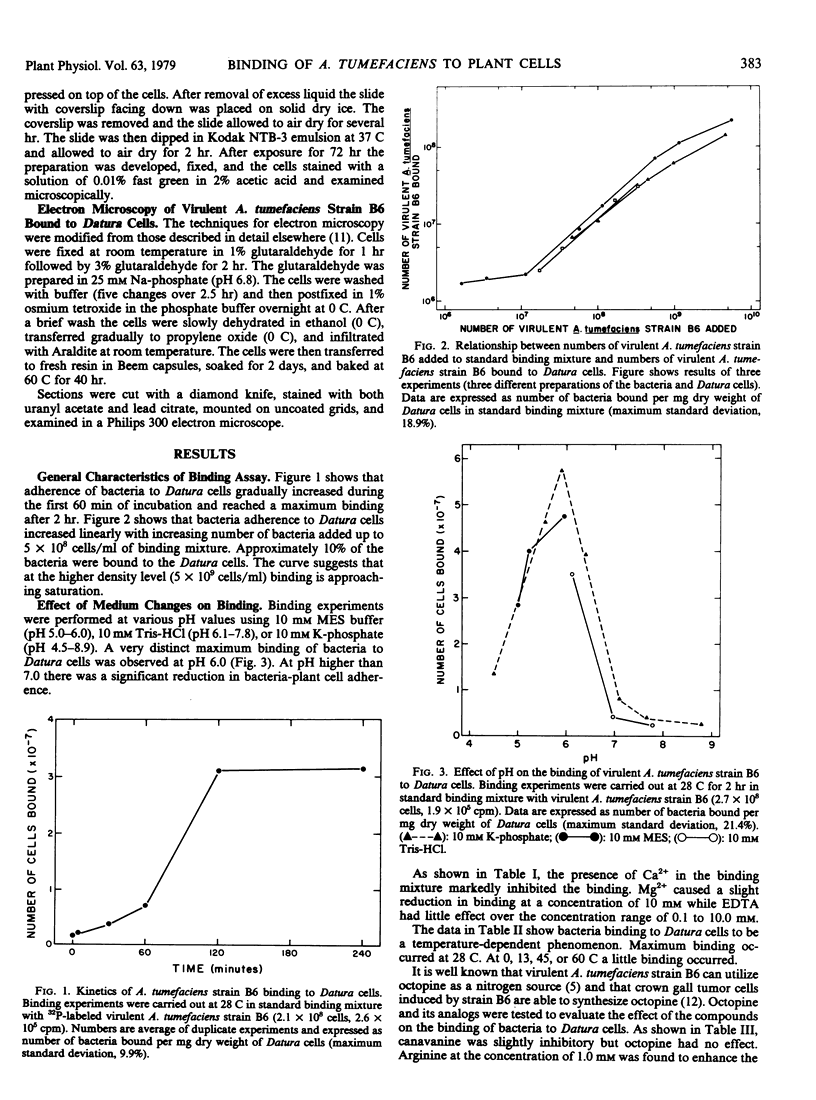
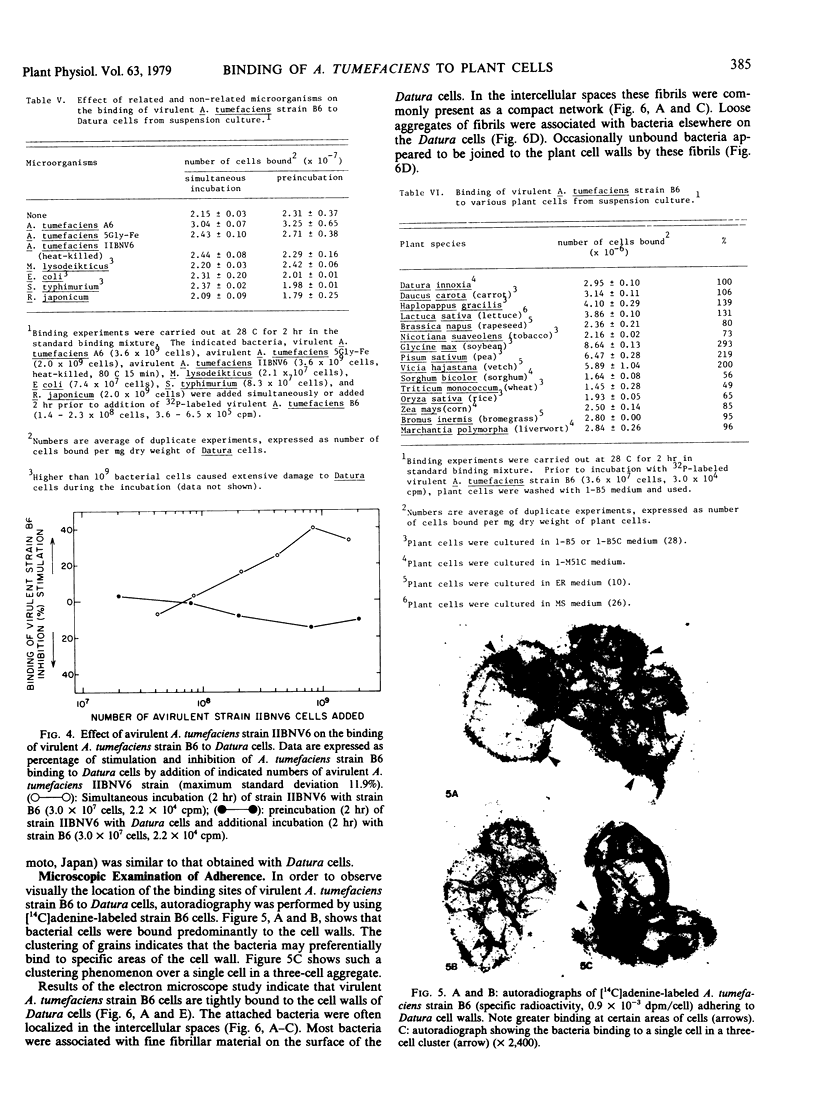
In vitro binding experiments were carried out using 32P-labeled cells of the virulent Agrobacterium tumefaciens strain B6 and Datura innoxia cells from suspension culture. Binding kinetics showed that adherence of bacteria to Datura cells increased gradually during the first 60 minutes and attained a maximum level within 120 minutes of incubation. Maximum binding occurred at pH 6.0. The presence of Ca2+ and Mg2+ reduced binding slightly and EDTA had little effect at concentrations of 0.1 to 10 millimolar. The binding of bacteria to Datura cells was temperature-dependent. Escherichia coli, Salmonella typhimurium, Rhizobium japonicum, and Micrococcus lysodeikticus did not compete with virulent A. tumefaciens strain B6 for binding to Datura cells. The admixture of avirulent A. tumefaciens strain IIBNV6 enhanced adherence of virulent A. tumefaciens strain B6 to Datura cells. Octopine had no effect on the binding of virulent A. tumefaciens strain B6 to Datura cells, but 10 millimolar canavanine was inhibitory. Arginine enhanced the adherence of the bacteria at concentrations higher than 0.1 millimolar. Incubation with DNase, RNase, and lipase did not affect the binding, but protease stimulated the adherence of bacteria to Datura cells. Concanavaline A and soybean lectin had little effect whereas lecithin and lysolecithin enhanced binding slightly. Poly-l-lysine markedly stimulated the bacteria-plant cell adherence. Cells from suspension cultures of pea, vetch, and soybean had a 2- to 3-fold higher binding capacity than Datura cells, whereas cells from wheat, corn, rice, and sorghum had a considerably lower affinity for binding with virulent A. tumefaciens strain B6. Bacterial adherence to plant cells was confirmed by autoradiography and electron microscopy. Autoradiographic analysis showed that bacteria were associated with the cell wall, and that often binding of bacteria was localized. Electron micrographs clearly illustrated a tight association of virulent A. tumefaciens strain B6 cells to the Datura cell wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki S., Takebe I. Infection of tobacco mesophyll protoplasts by tobacco mosaic virus ribonucleic acid. Virology. 1969 Nov;39(3):439–448. doi: 10.1016/0042-6822(69)90092-0. [DOI] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Braun A. C., Wood H. N. On the inhibition of tumor inception in the crown-gall disease with the use of ribonuclease A. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1417–1422. doi: 10.1073/pnas.56.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D. Fine structure and distribution of extracellular polymer surrounding selected aerobic bacteria. Can J Microbiol. 1975 Mar;21(3):395–408. doi: 10.1139/m75-055. [DOI] [PubMed] [Google Scholar]
- Leppard G. G., Colvin J. R. Electron-opaque fibrils and granules in and between the cell walls of higher plants. J Cell Biol. 1972 Jun;53(3):695–703. doi: 10.1083/jcb.53.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppard G. G., Colvin J. R., Rose D., Martin S. M. Lignofibrils on the external cell wall surface of cultured plant cells. J Cell Biol. 1971 Jul;50(1):63–80. doi: 10.1083/jcb.50.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott B. B., Lippincott J. A. Bacterial attachment to a specific wound site as an essential stage in tumor initiation by Agrobacterium tumefaciens. J Bacteriol. 1969 Feb;97(2):620–628. doi: 10.1128/jb.97.2.620-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott B. B., Margot J. B., Lippincott J. A. Plasmid content and tumor initiation complementation by Agrobacterium tumefaciens IIBNV6. J Bacteriol. 1977 Dec;132(3):824–831. doi: 10.1128/jb.132.3.824-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott B. B., Whatley M. H., Lippincott J. A. Tumor induction by agrobacterium involves attachment of the bacterium to a site on the host plant cell wall. Plant Physiol. 1977 Mar;59(3):388–390. doi: 10.1104/pp.59.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. A., Lippincott B. B. Cell walls of crown-gall tumors and embryonic plant tissues lack agrobacterium adherence sites. Science. 1978 Mar 10;199(4333):1075–1078. doi: 10.1126/science.199.4333.1075. [DOI] [PubMed] [Google Scholar]
- Lippincott J. A., Lippincott B. B., Chang C. C. Promotion of crown-gall tumor growth by lysopine, octopine, nopaline, and carnosine. Plant Physiol. 1972 Feb;49(2):131–137. doi: 10.1104/pp.49.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. A., Lippincott B. B. The genus Agrobacterium and plant tumorigenesis. Annu Rev Microbiol. 1975;29:377–405. doi: 10.1146/annurev.mi.29.100175.002113. [DOI] [PubMed] [Google Scholar]
- MENAGE A., MOREL G. SUR LA PR'ESENCE D'OCTOPINE DANS LES TISSUS DE CROWN-GALL. C R Hebd Seances Acad Sci. 1964 Dec 21;259:4795–4796. [PubMed] [Google Scholar]
- Manasse R. J., Corpe W. A. Chemical composition of cell envelopes from Agrobacterium tumefaciens. Can J Microbiol. 1967 Dec;13(12):1591–1603. doi: 10.1139/m67-209. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Genetello C., Schell J., Schilperoort R. A., Hermans A. K., Van Montagu M., Hernalsteens J. P. Acquisition of tumour-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature. 1975 Jun 26;255(5511):742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Hegeman G. D. Tumor induction by Agrobacterium tumefaciens: specific transfer of bacterial deoxyribonucleic acid to plant tissue. J Bacteriol. 1971 Dec;108(3):973–979. doi: 10.1128/jb.108.3.973-979.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]