Supplemental Digital Content is available in the text
Keywords: glucocorticoids, meta-analysis, pain control, total hip arthroplasty
Abstract
Background:
Glucocorticoids are increasingly used perioperatively, principally to prevent postoperative nausea and vomiting (PONV), and acute postoperative pain following total hip arthroplasty (THA). The authors hypothesized that preoperative intravenous glucocorticoids is associated with less pain scores and PONV without increasing the complications after THA.
Methods:
Four databases (PubMed, Embase, the Cochrane Central Register of Controlled Trials, and Web of Science) were searched with the limitations of randomized controlled trials (RCTs). The search cutoff date was set at November 6, 2016. Participants were patients who were prepared for primary THA. Intervention was preoperative intravenous glucocorticoids for postoperative pain control. Outcomes including the visual analog scale (VAS) scores at the postanesthesia care unit (PACU) and at 24 and 48 hours post operation, the occurrence of PONV and total morphine consumption were recorded. We calculated risk ratio (RR) with a 95% confidence interval (CI) for dichotomous outcomes, and the weighted mean difference (WMD) with a 95% CI for continuous outcomes.
Results:
A total of 6 studies were evaluated, which included 297 patients who underwent hip surgery with intravenous glucocorticoid treatment and control patients who underwent hip surgery without glucocorticoid treatment. Pooled results indicated that intravenous glucocorticoid treatment was associated with a reduction of VAS scores at the PACU (WMD = −9.06, 95% CI −12.67 to −5.45, P = .000) and total morphine consumption by 15.68 mg (WMD = −15.68, 95% CI −24.60 to −6.75, P = .001). No significant difference was observed in the VAS scores at 24 and 48 hours between the intravenous glucocorticoid and placebo treatments. Intravenous steroids can decrease the occurrence of PONV (RR = 0.46, 95% CI 0.26–0.82, P = .029).
Conclusion:
Intravenous glucocorticoid treatment can decrease early pain intensity and PONV after THA. However, the evidence for the use of glucocorticoids is limited by the low number of studies and variation in dosing regimens. Thus, additional high-quality RCTs are needed to identify the optimal drug protocol and determine the safety of intravenous glucocorticoids.
1. Introduction
In recent years, total hip arthroplasty (THA) has been used as an effective measure for the treatment of elderly patients with end-stage hip osteoarthritis. The incidence of postoperative nausea and vomiting (PONV) is significantly higher than that of postoperative pain and anemia.[1] Several studies have shown that the incidence of PONV in major orthopedic surgeries was between the range of 20% to 83%, and appropriately 85.9% of PONV occurred within 6 hours after surgery.[2–4] PONV seriously affects the subjective feelings of patients after surgery, reduces postoperative satisfaction, prolongs the length of hospital stay, and increases the psychological and economic burden of patients.[5–7] The use of opioids is classically used as the first alternative to control acute pain after THA; however, opioids will increase the occurrence of PONV and other intolerable complications.[8,9]
Glucocorticoids have potent anti-inflammatory, analgesic, and antiemetic effects. Glucocorticoids inhibit inflammatory gene expression and enhance oxidation activity to exert an analgesic effect.[10] These results indicate that intravenous glucocorticoids are potentially effective agents for reducing acute pain and PONV after THA. However, inconsistencies have been identified regarding pain relief and the morphine-sparing effects after intravenous glucocorticoids for THA.[11–13] Considering all of these issues, it is impossible to give clear advice regarding whether to adopt preoperative intravenous glucocorticoids as adjunct treatment to multimodal anesthetic management. In this study, we aimed to summarize the existing evidence from randomized controlled trials (RCTs) to determine whether preoperative intravenous glucocorticoid treatment was superior than control treatment with respect to pain scores, total morphine consumption, and PONV and additional postoperative complications. We hypothesized that preoperative intravenous glucocorticoid treatment results in lower pain scores, total morphine consumption, and PONV than controls.
2. Materials and methods
2.1. Search strategy and study selection
Four databases (PubMed, Embase, the Cochrane Central Register of Controlled Trials [CENTRAL], and Web of Science) were searched from inception to November 6, 2016 with the limitations of human subjects and RCTs. The details of the search strategy are shown in Supplement S1. There were no restrictions on language and publication status. Relevant review studies and reference lists were also manually searched for additional relevant missing studies. Gray academic studies are also identified from the reference of included studies. A meta-analysis was performed to collect relevant data from published articles, and thus no ethics committee was needed for approval.
2.2. Eligibility criteria
According to the PICOS rule, the eligible criteria were as follows:
-
(i)
Participants: Patients were prepared for primary THA.
-
(ii)
Interventions: The experimental group received preoperative intravenous administration of glucocorticoids. Dosage and time of intravenous glucocorticoids were not limited in our search process.
-
(iii)
Comparisons: The comparison group received a placebo or no intravenous treatment.
-
(iv)
Outcomes: The visual analog scale (VAS) scores were recorded at the postanesthesia care unit (PACU) and at 24 and 48 hours after the THA, and total morphine consumption and the occurrence of PONV were recorded.
-
(v)
Study design: Only RCTs were included. Any non-RCTs, quasi-RCTs, retrospective studies, reviews, and protocols were excluded. Disagreements were resolved by consensus.
2.3. Data extraction and outcome measures
Two authors (XL and ZS) independently extracted the first author name, publication year, the number of patients in intervention groups and control group, the proportion of male patients, and the mean age of the patients in the 2 groups, the anesthesia methods, the dose of glucocorticoids and equivalence to dexamethasone and controls, study type and duration of follow-up. The outcomes were assessed for the VAS scores at the PACU and at 24 and 48 hours post operation, the occurrence of PONV and total morphine consumption. Where papers only provide median (range) data, we convert these to mean (standard deviation) data following an established protocol.[14]
2.4. Methodological quality appraisal
Study methodological assessment was conducted by the 2 authors (BW and LH) using the modified Jadad scale following an established protocol.[15] There were a total of 8 items described: randomization, method of randomization, blinded analysis, blinding analysis methodology, withdrawal or dropouts, inclusion/exclusion criteria, adverse effects, and statistical analysis. The score for each item ranged from 0 to 1. In addition, total score ranges from 0 to 3 were identified as poor or low quality. Scores of 4 to 8 denoted high quality.
2.5. Quality of evidence assessment
Two reviewers (CH and LH) independently evaluated the quality of evidence assessment in accordance with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology.[16] The assessment items included the risk of bias, inconsistency, indirectness, imprecision, and publication bias.[16,17] Each result was classified as high, moderate, low, or very low. GRADE Pro software (GRADEpro, version 3.6) was used to construct summary tables for the included studies.
2.6. Statistical analysis
Weighted mean difference (WMD) and 95% confidence interval (CI) were calculated for continuous variables. Risk ratio (RR) and the corresponding 95% CI were calculated for discontinuous variables. Heterogeneity was divided into 3 grading categories (0%–30% = low heterogeneity, 30%–60% = middle heterogeneity, and 60%–100% = high heterogeneity). A random-effects model was applied to all of the results due to the heterogeneity between the studies. Funnel plots and Egger linear regression test was performed to test the publication bias. All statistical analyses were conducted using Stata 12.0 (Stata Corp., College Station, TX). Different types of glucocorticoid treatments were converted to equal dexamethasone dosage (0.75 mg dexamethasone = 4 mg methylprednisolone = 5 mg prednisolone = 20 mg hydrocortisone).[11]P < .05 was used to denote statistical significance.
3. Results
3.1. Search results
The literature search and selection processes are shown in Fig. 1. In the initial search, a total of 585 relevant studies were identified, of which 256 studies were from PubMed, 129 from Embase, 99 from CENTRAL, and 101 from Web of Science. All of the included studies were imported into the Endnote Software (Version X7; Thompson Reuters, Sunnyvale, CA) to remove duplicates. The titles and abstracts of a total of 462 papers were read, and 401 papers were excluded as they did not fulfill the inclusion criteria. Full-text studies were then obtained, and 55 papers were excluded. One study only compared the incidence of deep venous thrombosis between a glucocorticoid-treated group and a nonglucocorticoid-treated group and was thus excluded.[18] Finally, we included 6[12,19–23] RCTs (total = 297 patients, glucocorticoid treatment group = 152, and controls = 145) for current meta-analysis.
Figure 1.
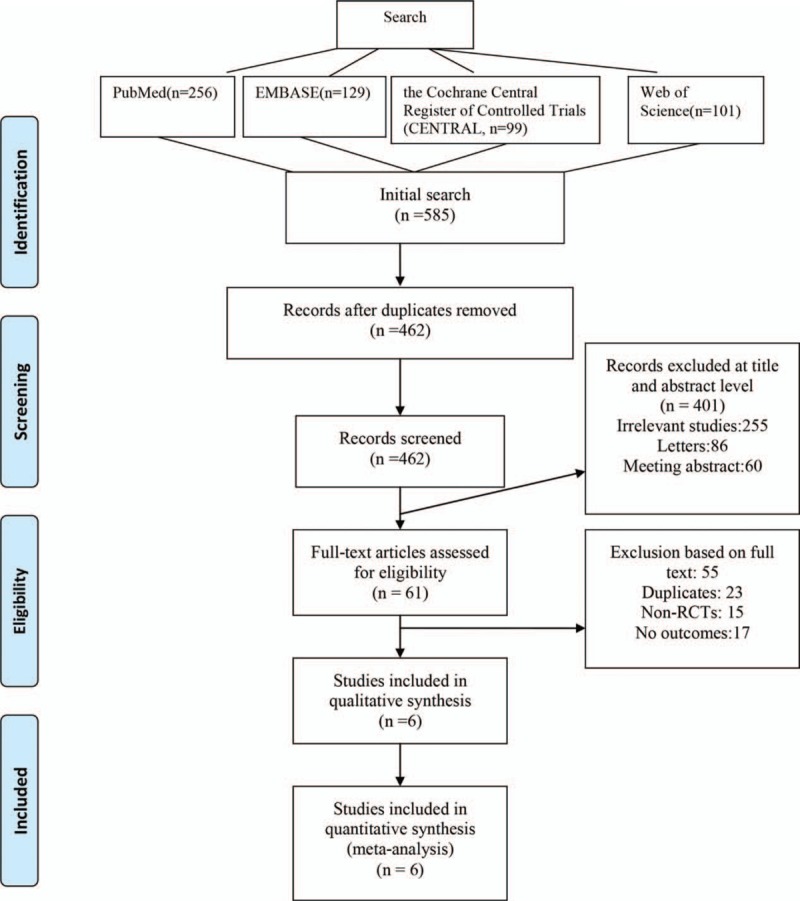
Flowchart of systematic database search and study selection.
3.2. General characteristic of the included studies
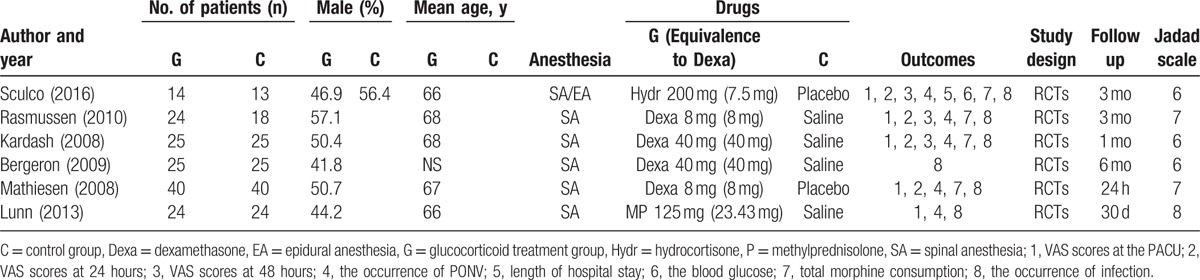
The detailed baseline characteristics of the included studies were presented in Table 1. Two studies were published in the year of 2008,[12,22] 1 study was published in the year of 2009,[21] 1 study was published in the year of 2010,[24] 1 study was published in the year of 2013,[23] and 1 was published in the year of 2016.[20] The sample size in the glucocorticoid-treated group ranged from 14 to 40, and sample size in the control group ranged from 13 to 40. The doses of glucocorticoids that are equivalent to dexamethasone ranged from 7.5 to 40 mg.
Table 1.
The general characteristic of the included studies.
3.3. Risk of bias
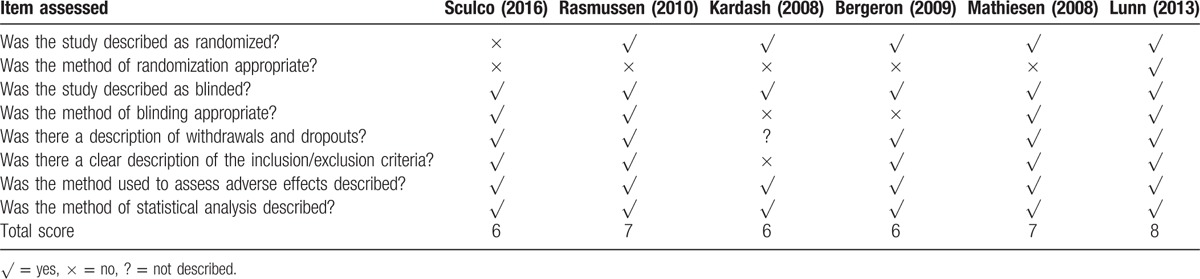
The details of the risk of bias assessment and the modified Jadad scores are shown in Table 2. The modified Jadad score for Sculco et al,[20] Rasmussen et al,[24] Kardash et al,[12] Bergeron et al,[21] Mathiesen et al,[22] and Lunn et al[23] was 6, 7, 6, 6, 7, and 8, respectively.
Table 2.
Methodological assessment of eligible studies using the modified Jadad scale.
3.4. Quality of evidence assessment
A summary of the quality of the evidence according to the GRADE approach is shown in Supplement S2. The GRADE level of evidence was low for the VAS scores at the PACU and at 24 hours and was very low for the VAS scores at 48 hours as well as for the occurrence of PONV and total morphine consumption.
3.5. Primary outcomes
3.5.1. VAS scores at the PACU
Five studies[12,20,22–24] (247 THAs) provided the data of preoperative intravenous glucocorticoids on the VAS scores at the PACU. Compared with the placebo group, intravenous glucocorticoids treatment was associated with a significant reduction in the VAS scores at the PACU (WMD = −9.06, 95% CI −12.67 to −5.45, P = .000), with low heterogeneity between the included studies (I2 = 9.0%, P = .355) (Fig. 2).
Figure 2.
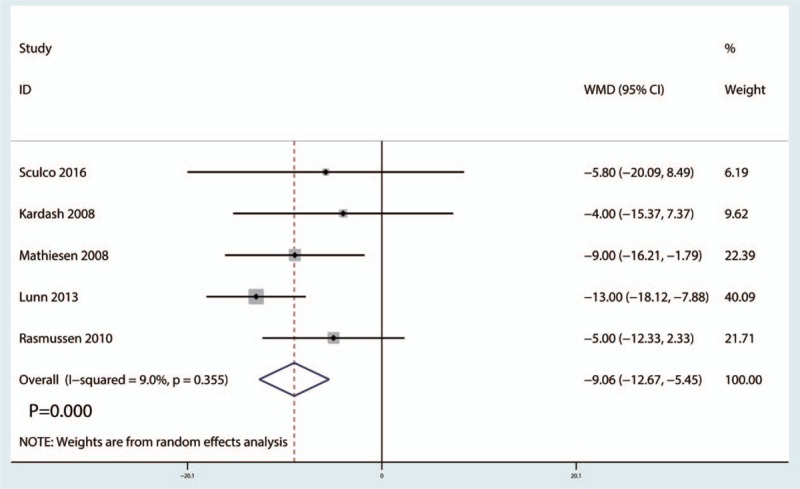
Forest plots of the included studies comparing the visual analog scale scores at the postanesthesia care unit.
3.5.2. VAS scores at 24 hours post operation
Five studies[12,20,22–24] (247 THAs) were included in this meta-analysis to estimate the effect of preoperative intravenous glucocorticoid treatment on the VAS at 24 hours. No statistically significant difference was observed in the VAS scores at 24 hours between the intravenous glucocorticoid-treated group and the placebo group (WMD = −3.59, 95% CI −7.34 to 0.55, P = .089), with no heterogeneity between the included studies (I2 = 0.0%, P = .880) (Fig. 3).
Figure 3.
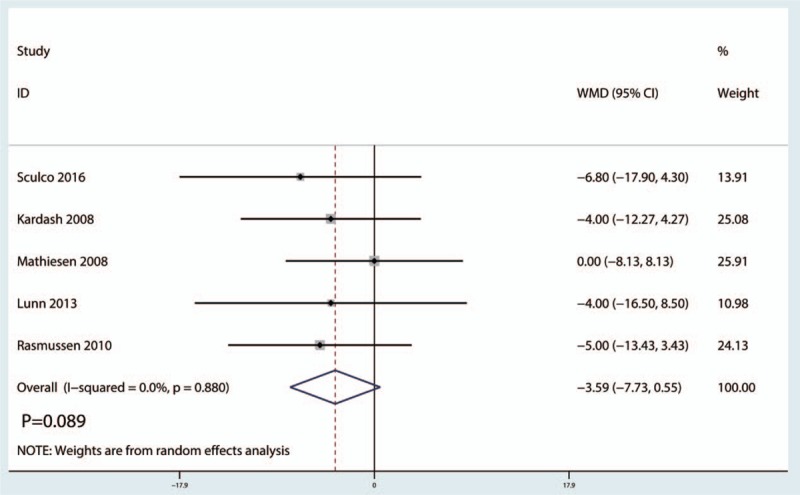
Forest plots of the included studies comparing the visual analog scale scores at 24-hour post operation.
3.5.3. VAS scores at 48 hours post operation
Three trials[12,20,24] (247 THAs) were available to provide the data of preoperative intravenous glucocorticoids on the VAS scores at 48 hours. The final results indicated that no statistically significant difference was found between the glucocorticoid treatment group and the control group in terms of the VAS scores at 48 hours (WMD = −0.74, 95% CI −2.82 to 1.35, P = .489), with low heterogeneity (I2 = 0.0%, P = .405) (Fig. 4).
Figure 4.
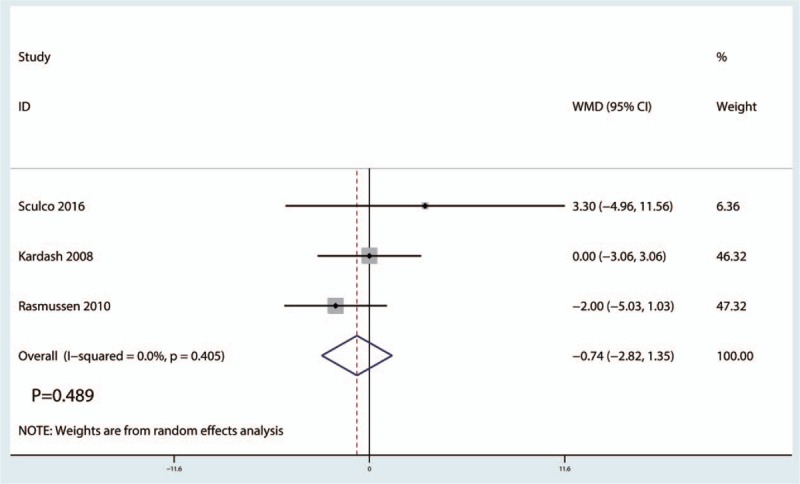
Forest plots of the included studies comparing the visual analog scale scores at 48-hour post operation.
3.5.4. The occurrence of PONV
Five studies[12,20,22–24] (247 participants) reported data on the occurrence of PONV. Compared with the placebo, intravenous glucocorticoid treatment significantly decreased the occurrence of PONV by 10.9% (RR = 0.46, 95% CI 0.26–0.82, P = .029), with low heterogeneity (I2 = 36.0%, P = .181) (Fig. 5).
Figure 5.
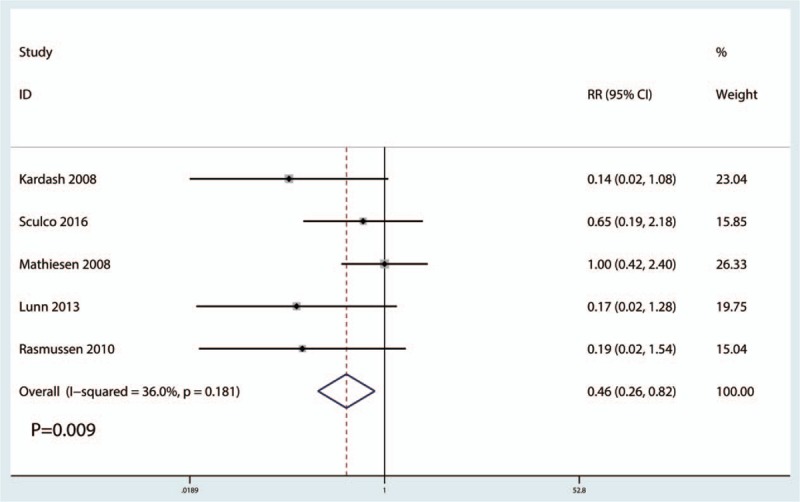
Forest plots of the included studies comparing the occurrence of postoperative nausea and vomiting.
3.5.5. Total morphine consumption
A total of 4 studies[12,20,22] (199 THAs) were available in the meta-analysis. Compared with the placebo group, intravenous glucocorticoid treatment was associated with a significant decrease in total morphine consumption by 15.68 mg (WMD = −15.68, 95% CI −24.60 to −6.75, P = .001), with moderate heterogeneity (I2 = 61.6%, P = .050) (Fig. 6).
Figure 6.
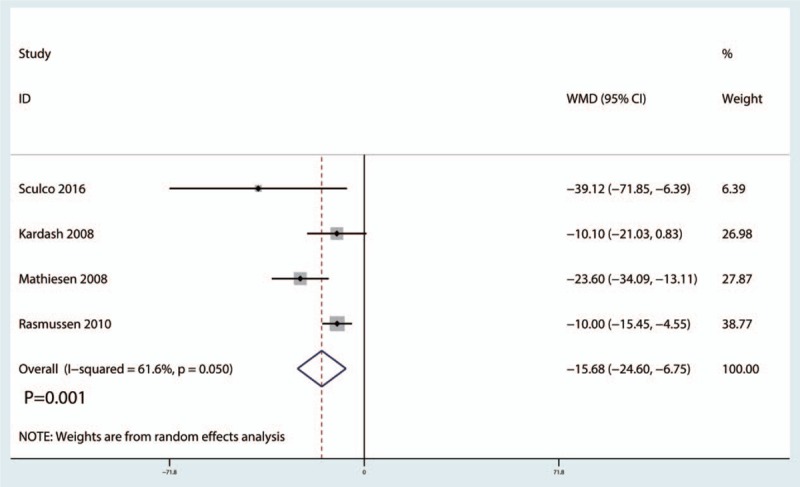
Forest plots of the included studies comparing the total morphine consumption.
3.6. Other outcomes
Sculco et al[20] reported the blood glucose and the length of hospital and found no significant difference between the glucocorticoid treatment group and the control group. Bergeron et al[21] reported the Harris scores at 6 weeks and 1 year follow-up and found that no significant difference between the glucocorticoid treatment group and the control group.
4. Discussion
To our knowledge, this is the first meta-analysis including only RCTs that compares the efficacy and safety of intravenous glucocorticoid treatment as an adjunct with multimodal anesthesia for patients prepared for primary THA. Pooled results indicated that intravenous glucocorticoid treatment, compared with the placebo group, was associated with a significant reduction in the VAS scores at the PACU, the occurrence of PONV, and total morphine consumption. There were no significant differences between the VAS scores at 24 and 48 hours between the glucocorticoid treatment group and the control group after THA; however, the efficacy of preoperative intravenous glucocorticoid treatment was limited in the first 24 hours. The level of evidence, which was undermined by heterogeneity, was low or very low, indicating that the advantage exists but the degree to which it does must be further studied.
Intravenous glucocorticoid treatment had a beneficial role on the VAS score at the PACU. There were no significant differences between the VAS score at 24 and 48 hours after THA. De Oliveira et al[25] compared the efficacy of intravenous glucocorticoid treatment for patients with all types of surgeries and found that preoperative intravenous glucocorticoid treatment was effective in reducing postoperative pain. The limitation of the above meta-analysis was that the participants had undergone all types of surgeries; thus, a large degree of heterogeneity in the group existed. The strength of the current meta-analysis was that we only included THA patients. Moreover, we used a random-effect model to analyze the relevant data to avoid heterogeneity between the included samples.
Intravenous glucocorticoid treatment was associated with a significant reduction of PONV. Our findings have important clinical implications, as intravenous glucocorticoids are commonly given intraoperatively at the time of anesthesia induction to reduce PONV.[26] Previously, updated meta-analysis including 60 RCTs with 6696 subjects indicated that the 4-mg to 5-mg dose regimen of systemic dexamethasone is beneficial in reducing the occurrence of PONV.[27] Fujii and Nakayama[28] found that the rates of emesis-free effects were increased in dexamethasone groups treated with 8 and 16 mg compared with those treated with 4 mg of dexamethasone. Liu et al[29] found 2.5 mg to be the minimum effective dose for antiemesis without discernible side effects.
Glucocorticoids are not, however, without harm.[30] The long-term side effects of glucocorticoids involve most major organ systems. However, the relative short-term use of glucocorticoids has mainly been focused on blood glucose, wound healing and wound superficial, and deep infection. Postoperative infections are important as they prolong hospital stay, increase costs, and impact postoperative mortality, which extends to at least to 30 days.[31] The outcomes did not include blood glucose levels, as there were no sufficient data to comprise for meta-analysis. Sculco et al[20] revealed that blood glucose levels were elevated in the glucocorticoid treatment group, with statistically significant differences at the PACU after THA. Many studies have shown that a single dose of dexamethasone (less than 20 mg) does not cause increased incidence of adverse reactions after surgery.[32,33] Among the included studies, there were no significant differences between blood glucose levels and the occurrence of infection. Waldron et al[34] found that intravenous glucocorticoids were not accompanied by an increased risk of infection or delayed wound healing. Toner et al[35] found that the use of perioperative glucocorticoid treatment was not associated with an increase of infection, hyperglycemia, or other adverse outcomes.
The limitations of this study were as follows: the relatively small sample size of each primary study, especially that of Sculco et al[20]; in some RCTs, the random sequence generation and allocation concealment methods were not described, which may influence the stability of our outcomes to some extent; differences in surgical time, technique, approaches, and postoperative pain protocols may have influenced the final results; the length of follow-up times differed and the complications were underestimated to some extent; and the dose and type of glucocorticoid used in each of the primary studies differed, though a subgroup analysis was performed. Additional RCTs are needed to identify the optimal dose and type of glucocorticoid treatment.
5. Conclusion
Current present meta-analysis favors intravenous glucocorticoid treatment for the alleviation of acute postoperative pain and the reduction of the occurrence of PONV in patients following THA. Another main finding was that the antiemesis effects were dose-dependent. However, the evidence for the use of glucocorticoid treatment is limited by the low quality of studies and variation dosing regimens. Thus, more RCTs are required to verify the optimal dose and type of glucocorticoid treatment for THA.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, GRADE = Grading of Recommendations Assessment, Development, and Evaluation, PACU = postanesthesia care unit, PONV = postoperative nausea and vomiting, RCT = randomized controlled trial, RR = risk ratio, THA = total hip arthroplasty, VAS = visual analog scale, WMD = weighted mean difference.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Jia XF, Ji Y, Huang GP, et al. Comparison of intrathecal and local infiltration analgesia by morphine for pain management in total knee and hip arthroplasty: a meta-analysis of randomized controlled trial. Int J Surg 2017;40:97–108. [DOI] [PubMed] [Google Scholar]
- [2].Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014;118:85–113. [DOI] [PubMed] [Google Scholar]
- [3].Fujii Y. Current review of ramosetron in the prevention of postoperative nausea and vomiting. Curr Drug Saf 2011;6:122–7. [DOI] [PubMed] [Google Scholar]
- [4].Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 2012;109:742–53. [DOI] [PubMed] [Google Scholar]
- [5].Macario A, Weinger M, Carney S, et al. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg 1999;89:652–8. [DOI] [PubMed] [Google Scholar]
- [6].Marques EM, Jones HE, Elvers KT, et al. Local anaesthetic infiltration for peri-operative pain control in total hip and knee replacement: systematic review and meta-analyses of short- and long-term effectiveness. BMC Musculoskelet Disord 2014;15:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Phillips C, Brookes CD, Rich J, et al. Postoperative nausea and vomiting following orthognathic surgery. Int J Oral Maxillofac Surg 2015;44:745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Camu F, Borgeat A, Heylen RJ, et al. Parecoxib, propacetamol, and their combination for analgesia after total hip arthroplasty: a randomized non-inferiority trial. Acta Anaesthesiol Scand 2017;61:99–110. [DOI] [PubMed] [Google Scholar]
- [9].Nguyen LC, Sing DC, Bozic KJ. Preoperative reduction of Opioid use before total joint arthroplasty. J Arthroplasty 2016;31(9 suppl):282–7. [DOI] [PubMed] [Google Scholar]
- [10].Smith C, Erasmus PJ, Myburgh KH. Endocrine and immune effects of dexamethasone in unilateral total knee replacement. J Int Med Res 2006;34:603–11. [DOI] [PubMed] [Google Scholar]
- [11].Lunn TH, Kristensen BB, Andersen LO, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth 2011;106:230–8. [DOI] [PubMed] [Google Scholar]
- [12].Kardash KJ, Sarrazin F, Tessler MJ, et al. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg 2008;106:1253–7. table of contents. [DOI] [PubMed] [Google Scholar]
- [13].Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am 2006;88:1361–72. [DOI] [PubMed] [Google Scholar]
- [14].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oremus M, Wolfson C, Perrault A, et al. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord 2001;12:232–6. [DOI] [PubMed] [Google Scholar]
- [16].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hogevold HE, Hoiseth A, Reikeras O. Effect of high-dose corticosteroids on the incidence of deep vein thrombosis after total hip replacement. Arch Orthop Trauma Surg 1991;111:29–31. [DOI] [PubMed] [Google Scholar]
- [19].Backes JR, Bentley JC, Politi JR, et al. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplasty 2013;28(8 suppl):11–7. [DOI] [PubMed] [Google Scholar]
- [20].Sculco PK, McLawhorn AS, Desai N, et al. The effect of perioperative corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplasty 2016;31:1208–12. [DOI] [PubMed] [Google Scholar]
- [21].Bergeron SG, Kardash KJ, Huk OL, et al. Perioperative dexamethasone does not affect functional outcome in total hip arthroplasty. Clin Orthop Relat Res 2009;467:1463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mathiesen O, Jacobsen LS, Holm HE, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth 2008;101:535–41. [DOI] [PubMed] [Google Scholar]
- [23].Lunn TH, Andersen LO, Kristensen BB, et al. Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth 2013;110:66–73. [DOI] [PubMed] [Google Scholar]
- [24].Rasmussen ML, Mathiesen O, Dierking G, et al. Multimodal analgesia with gabapentin, ketamine and dexamethasone in combination with paracetamol and ketorolac after hip arthroplasty: a preliminary study. Eur J Anaesthesiol 2010;27:324–30. [DOI] [PubMed] [Google Scholar]
- [25].De Oliveira GS, Jr, Almeida MD, Benzon HT, et al. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2011;115:575–88. [DOI] [PubMed] [Google Scholar]
- [26].Gan TJ, Meyer TA, Apfel CC, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2007;105:1615–28. table of contents. [DOI] [PubMed] [Google Scholar]
- [27].De Oliveira GS, Jr, Castro-Alves LJ, Ahmad S, et al. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg 2013;116:58–74. [DOI] [PubMed] [Google Scholar]
- [28].Fujii Y, Nakayama M. Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther 2005;27:740–5. [DOI] [PubMed] [Google Scholar]
- [29].Liu K, Hsu CC, Chia YY. The effective dose of dexamethasone for antiemesis after major gynecological surgery. Anesth Analg 1999;89:1316–8. [PubMed] [Google Scholar]
- [30].Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf 2016;15:457–65. [DOI] [PubMed] [Google Scholar]
- [31].Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326–41. discussion 341–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sauerland S, Nagelschmidt M, Mallmann P, et al. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf 2000;23:449–61. [DOI] [PubMed] [Google Scholar]
- [33].Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000;90:186–94. [DOI] [PubMed] [Google Scholar]
- [34].Waldron NH, Jones CA, Gan TJ, et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 2013;110:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Toner AJ, Ganeshanathan V, Chan MT, et al. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2017;126:234–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


