Abstract
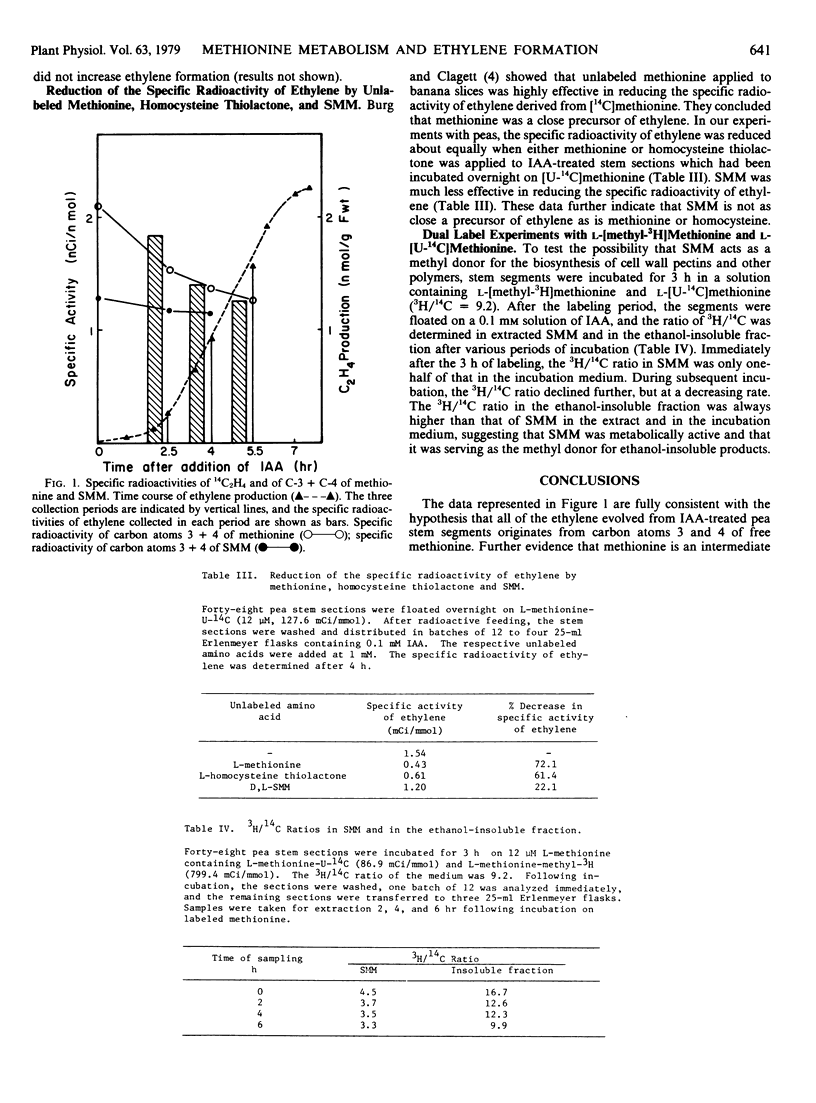
Stem sections of etiolated pea seedlings (Pisum sativum L. cv. Alaska) were incubated overnight on tracer amounts of l-[U-14C]methionine and, on the following morning, on 0.1 millimolar indoleacetic acid to induce ethylene formation. Following the overnight incubation, over 70% of the radioactivity in the soluble fraction was shown to be associated with S-methylmethionine (SMM). The specific radioactivity of the ethylene evolved closely paralleled that of carbon atoms 3 and 4 of methionine extracted from the tissue and was always higher than that determined for carbon atoms 3 and 4 of extracted SMM.
Overnight incubation of pea stem sections on 1 millimolar methionine enhanced indoleacetic acid-induced ethylene formation by 5 to 10%. Under the same conditions, 1 millimolar homocysteine thiolactone increased ethylene synthesis by 20 to 25%, while SMM within a concentration range of 0.1 to 10 millimolar did not influence ethylene production. When unlabeled methionine or homocysteine thiolactone was applied to stem sections which had been incubated overnight in l-[U-14C]methionine, the specific radioactivity of the ethylene evolved was considerably lowered. Application of unlabeled SMM reduced the specific radioactivity of ethylene only slightly.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Methionine metabolism in apple tissue: implication of s-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol. 1977 Dec;60(6):892–896. doi: 10.1104/pp.60.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Methionine metabolism and ethylene biosynthesis in senescent flower tissue of morning-glory. Plant Physiol. 1976 Apr;57(4):528–537. doi: 10.1104/pp.57.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze J. R., Schilling N., Kende H. Enhancement of ethylene formation by selenoamino acids. Plant Physiol. 1978 Sep;62(3):397–401. doi: 10.1104/pp.62.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. T. Ethylene-forming Systems in Etiolated Pea Seedling and Apple Tissue. Plant Physiol. 1975 Jun;55(6):1074–1078. doi: 10.1104/pp.55.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr D. P., Yang S. F. Inhibition of in Vivo Conversion of Methionine to Ethylene by l-Canaline and 2,4-Dinitrophenol. Plant Physiol. 1975 Jan;55(1):79–82. doi: 10.1104/pp.55.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F., Ku H. S., Pratt H. K. Photochemical production of ethylene from methionine and its analogues in the presence of flavin mononucleotide. J Biol Chem. 1967 Nov 25;242(22):5274–5280. [PubMed] [Google Scholar]


