Abstract
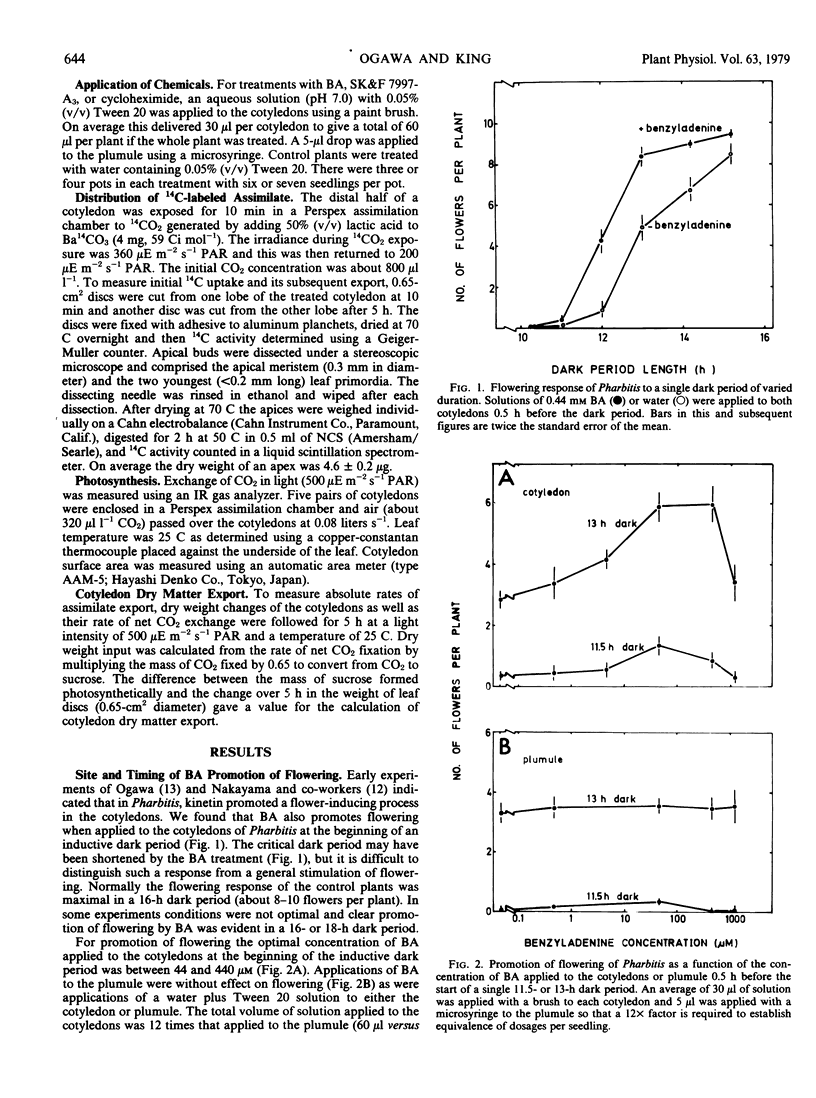
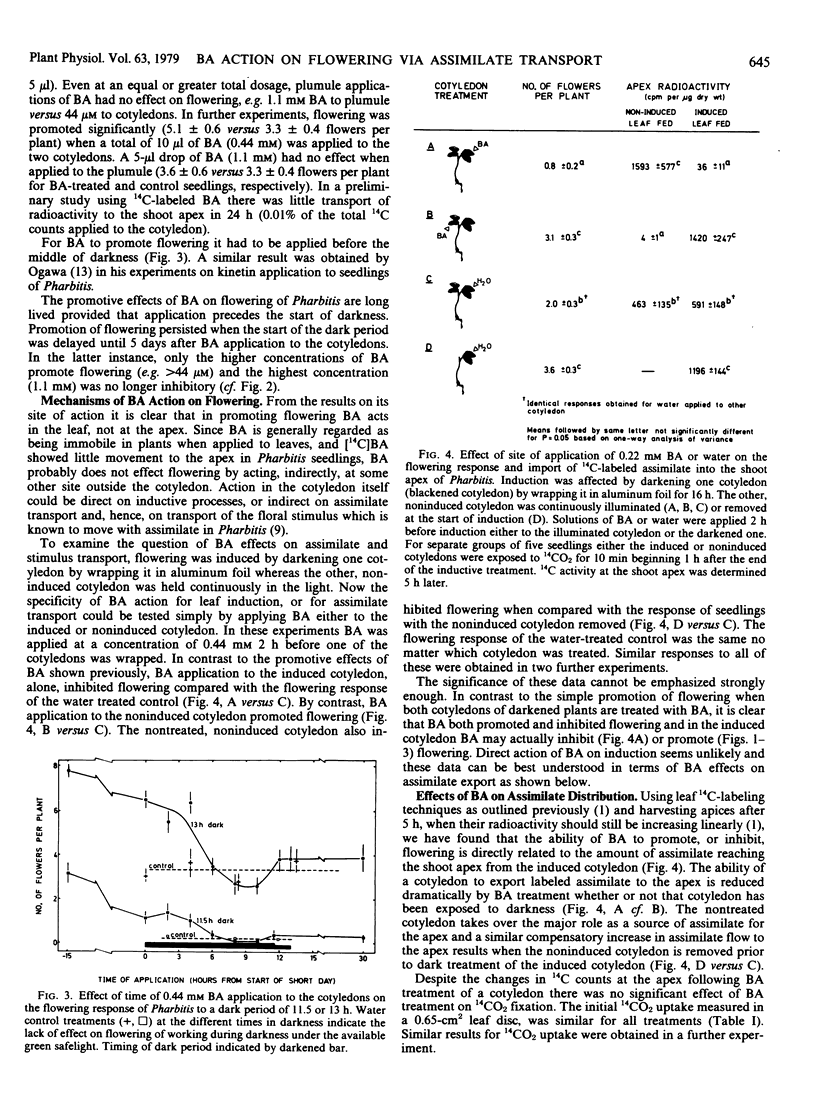
Benzyladenine (BA) brushed on the cotyledons of 4-day-old seedlings of Pharbitis nil Chois. markedly stimulates flowering. Greates response is obtained for concentrations between 44 and 440 micromolar. The action of BA is on processes in the cotyledon as shown by the response to its site of application, to the dosage applied and to the requirement for its application prior to the dark period. There was little or no effect of BA treatment on either the time measurement processes of photoperiodic induction or on the generation of floral stimulus. Transport of photosynthetic assimilate from the cotyledons to the shoot apex was altered.
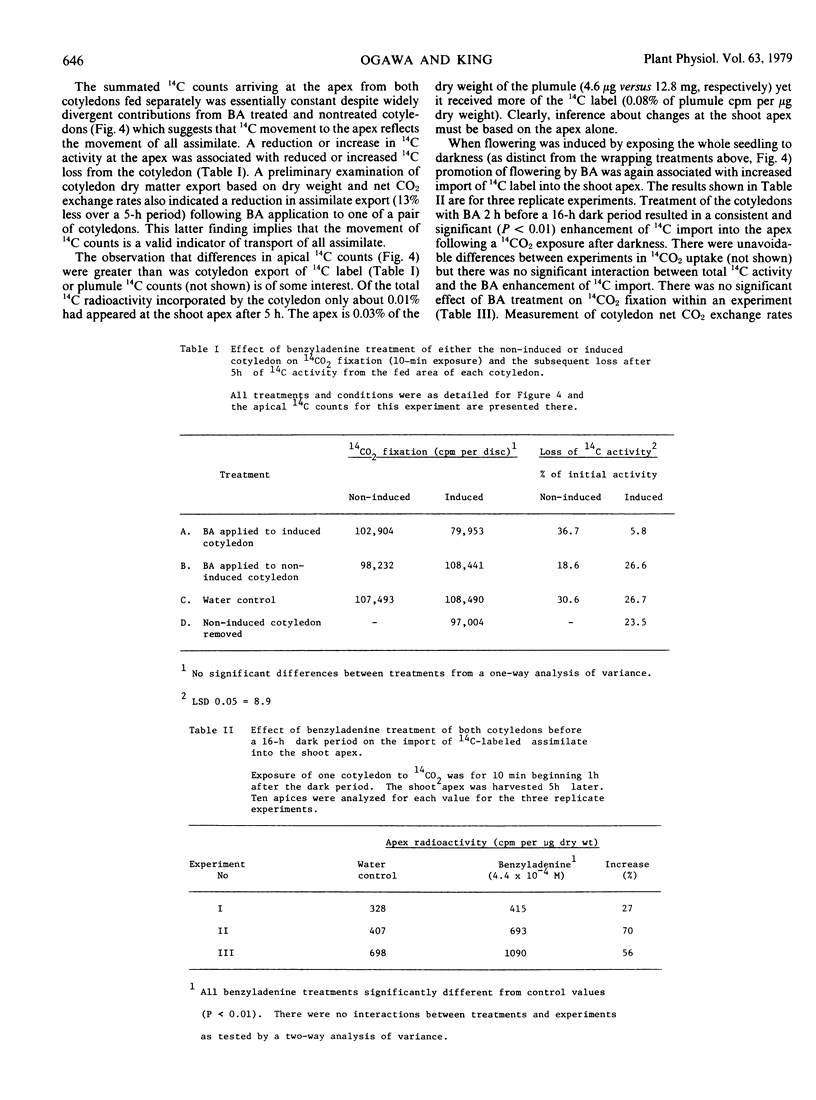
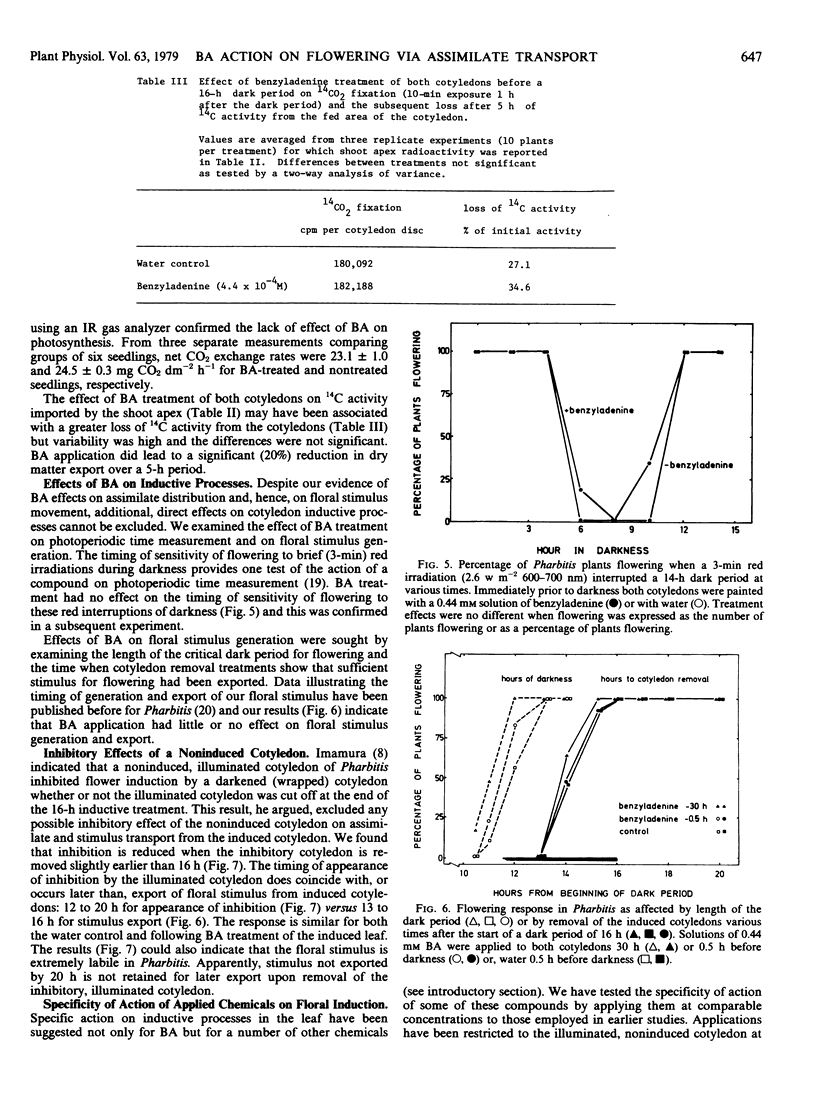
When only one of the pair of cotyledons was treated with BA it exported less 14C-labeled assimilate to the shoot apex and there was a compensatory increase in assimilate outflow from the other cotyledon. When BA was applied to a cotyledon exposed to an inductive dark period, flowering was inhibited in association with the reduced export of assimilate. Conversely, when BA was applied to the noninduced cotyledon, flowering was promoted in association with an enhanced export of assimilate from the induced leaf. Clearly, cytokinins can have an indirect effect on photoperiodic induction by altering assimilate and, hence, floral stimulus translocation to the shoot apex.
Two other chemicals which were previously considered as specific inhibitors of processes of floral induction in the cotyledon [Tris(2-diethylaminoethyl)phosphate trihydrochloride (SK&F 7997-A3) and cycloheximide] acted in the same manner as BA. Inhibitory effects of an illuminated cotyledon on flowering of Pharbitis were also shown to be mediated by interference of assimilate flow with transport of the stimulus for flowering.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner J., Zeevaart J. A. Ribonucleic Acid Synthesis in the Bud Essential Component of Floral Induction in Xanthium. Plant Physiol. 1962 Jan;37(1):43–49. doi: 10.1104/pp.37.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Floral stimulus movement in perilla and flower inhibition caused by noninduced leaves. Plant Physiol. 1973 Apr;51(4):727–738. doi: 10.1104/pp.51.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindy W. W., Weaver R. J. Export of photosynthate affected when leaves are pretreated with growth substances. Nature. 1970 Jul 18;227(5255):301–302. doi: 10.1038/227301a0. [DOI] [PubMed] [Google Scholar]
- Zeevaart J. A., Brede J. M., Cetas C. B. Translocation patterns in xanthium in relation to long day inhibition of flowering. Plant Physiol. 1977 Nov;60(5):747–753. doi: 10.1104/pp.60.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]


