Abstract
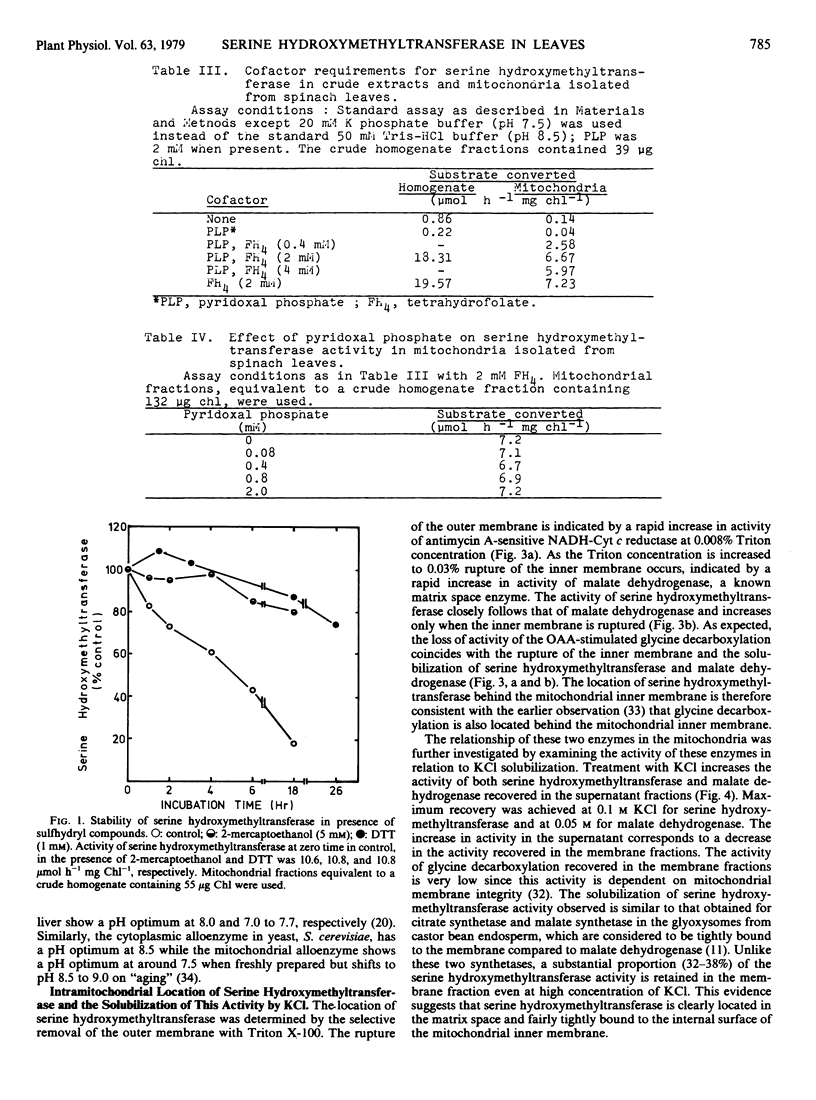
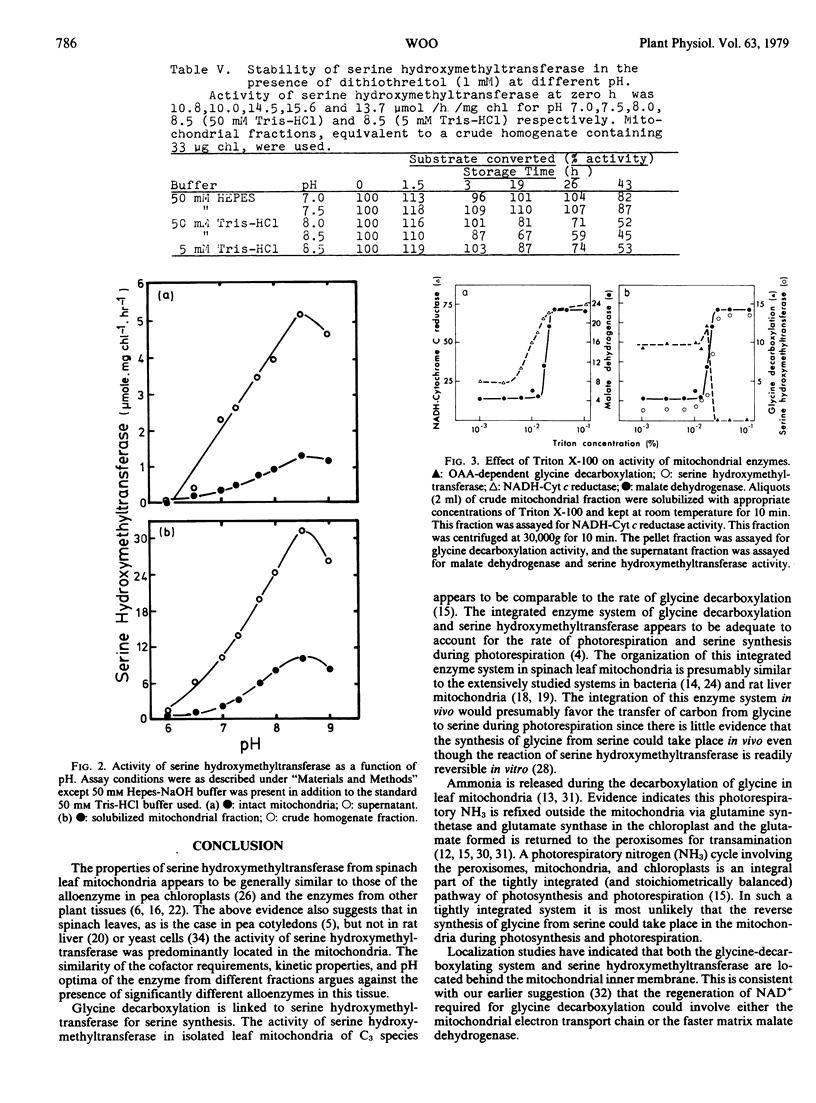
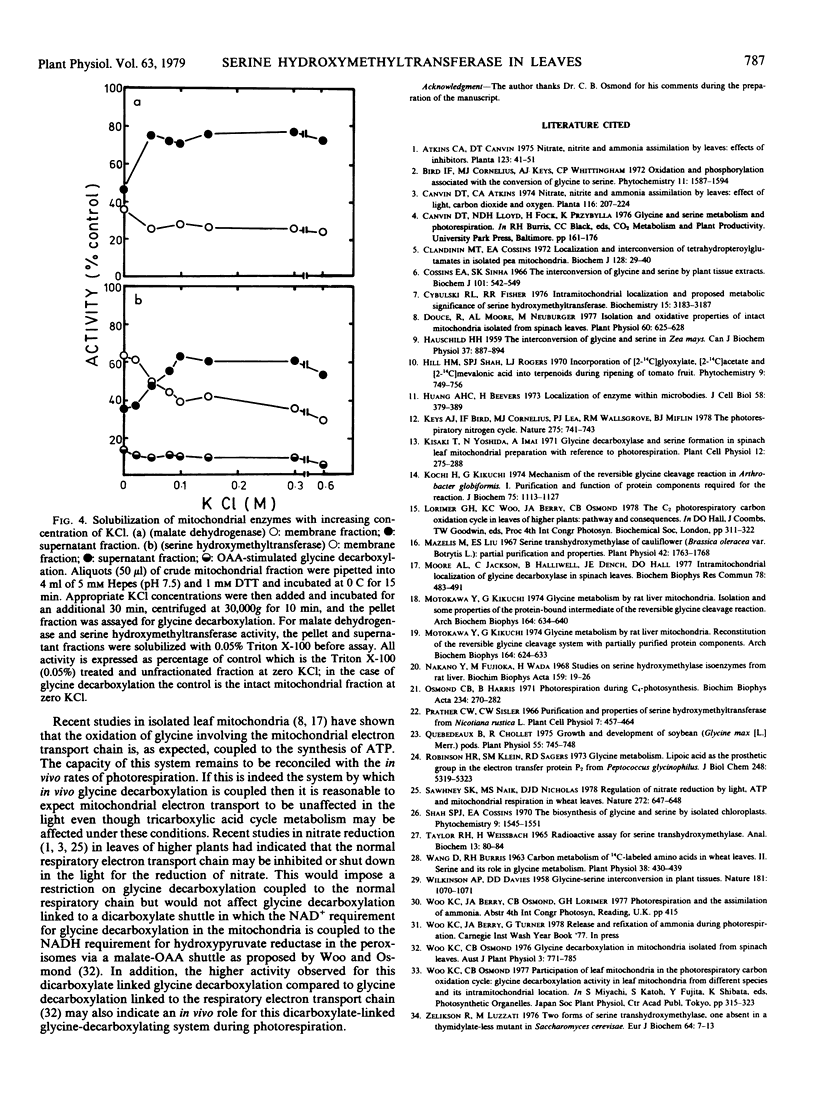
The activity of serine hydroxymethyltransferase in mitochondria isolated from spinach leaves was absolutely dependent on tetrahydrofolate; pyridoxal phosphate has no effect on the activity. The stability of this activity in the isolated mitochondria was dependent on the presence of sulfhydryl compounds. It was apparently more stable at pH 7.0 to 7.5 than at higher pH even though the pH optimum of serine hydroxymethyltransferase was 8.5 for both the mitochondrial and cytoplasmic fractions. Distribution studies have indicated that serine hydroxymethyltransferase was predominantly located in the mitochondria. The activity of serine hydroxymethyltransferase was observed to be co-compartmented with glycine decarboxylation and malate dehydrogenase behind the mitochondrial inner membrane. This activity could be solubilized by KCl from osmotically ruptured mitochondrial membrane fractions but substantial activity (35 to 40%) was still retained with the membrane fractions at 0.3 m KCl. This suggests that the glycine decarboxylation-serine hydroxymethyltransferase complex may be closely bound to the internal surface of the mitochondrial inner membrane.
The relationship of this integrated enzyme complex to CO2 evolution and serine synthesis during photorespiration and the physiological role of the dicarboxylate shuttle were discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clandinin M. T., Cossins E. A. Localization and interconversion of tetrahydropteroylglutamates in isolated pea mitochondria. Biochem J. 1972 Jun;128(1):29–40. doi: 10.1042/bj1280029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins E. A., Sinha S. K. The interconversion of glycine and serine by plant tissue extracts. Biochem J. 1966 Nov;101(2):542–549. doi: 10.1042/bj1010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski R. L., Fisher R. R. Intramitochondrial localization and proposed metabolic significance of serine transhydroxymethylase. Biochemistry. 1976 Jul 27;15(15):3183–3187. doi: 10.1021/bi00660a004. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSCHILD A. H. The interconversion of glycine and serine in Zea mays. Can J Biochem Physiol. 1959 Jul;37(7):887–894. [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Mechanism of the reversible glycine cleavage reaction in Arthrobacter globiformis. I. Purification and function of protein components required for the reaction. J Biochem. 1974 May;75(5):1113–1127. doi: 10.1093/oxfordjournals.jbchem.a130483. [DOI] [PubMed] [Google Scholar]
- Mazelis M., Liu E. S. Serine Transhydroxymethylase of Cauliflower (Brassica oleracea var. botrytis L.): Partial Purification and Properties. Plant Physiol. 1967 Dec;42(12):1763–1768. doi: 10.1104/pp.42.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism by rat liver mitochondria. Reconstruction of the reversible glycine cleavage system with partially purified protein components. Arch Biochem Biophys. 1974 Oct;164(2):624–633. doi: 10.1016/0003-9861(74)90074-5. [DOI] [PubMed] [Google Scholar]
- Nakano Y., Fujioka M., Wada H. Studies on serine hydroxymethylase isoenzymes from rat liver. Biochim Biophys Acta. 1968 Apr 24;159(1):19–26. doi: 10.1016/0005-2744(68)90240-4. [DOI] [PubMed] [Google Scholar]
- Osmond C. B., Harris B. Photorespiration during C 4 photosynthesis. Biochim Biophys Acta. 1971 May 11;234(2):270–282. doi: 10.1016/0005-2728(71)90082-x. [DOI] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Growth and Development of Soybean (Glycine max [L.] Merr.) Pods: CO(2) Exchange and Enzyme Studies. Plant Physiol. 1975 Apr;55(4):745–748. doi: 10.1104/pp.55.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. R., Klein S. M., Sagers R. D. Glycine metabolism. Lipoic acid as the prosthetic group in the electron transfer protein P2 from Peptococcus glycinophilus. J Biol Chem. 1973 Aug 10;248(15):5319–5323. [PubMed] [Google Scholar]
- WILKINSON A. P., DAVIES D. D. Serine-glycine interconversion in plant tissues. Nature. 1958 Apr 12;181(4615):1070–1071. doi: 10.1038/1811070a0. [DOI] [PubMed] [Google Scholar]
- Wang D., Burris R. H. Carbon Metabolism of C-Labeled Amino Acids in Wheat Leaves. II. Serine & its Role in Glycine Metabolism. Plant Physiol. 1963 Jul;38(4):430–439. doi: 10.1104/pp.38.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikson R., Luzzati M. Two forms of serine transhydroxymethylase, one absent in a thymidylate-less mutant in Saccharomyces cerevisiae. Eur J Biochem. 1976 Apr 15;64(1):7–13. doi: 10.1111/j.1432-1033.1976.tb10269.x. [DOI] [PubMed] [Google Scholar]


