Abstract
Aim
To describe the 1-year neurobehavioral outcome of survivors of cardiac arrest secondary to drowning, compared with other respiratory etiologies, in children enrolled in the Therapeutic Hypothermia after Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) trial.
Methods
Exploratory analysis of survivors (ages 1–18 years) who received chest compressions for ≥2 minutes, were comatose, and required mechanical ventilation after return of circulation (ROC). Participants recruited from 27 pediatric intensive care units in North America received targeted temperature management [therapeutic hypothermia (33°C) or therapeutic normothermia (36.8°C)] within 6 hours of ROC. Neurobehavioral outcomes included 1-year Vineland Adaptive Behavior Scales, Second Edition (VABS-II) total and domain scores and age-appropriate cognitive performance measures (Mullen Scales of Early Learning or Wechsler Abbreviated Scale of Intelligence).
Results
Sixty-six children with a respiratory etiology of cardiac arrest survived for 1-year; 60/66 had broadly normal premorbid functioning (VABS-II ≥ 70). Follow up was obtained on 59/60 (30 with drowning etiology). VABS-II composite and domain scores declined significantly from premorbid scores in drowning and non-drowning groups (p <0.001), although declines were less pronounced for the drowning group. Seventy-two percent of children had well below average cognitive functioning at 1-year. Younger age, fewer doses of epinephrine, and drowning etiology were associated with better VABS-II composite scores. Demographic variables and treatment with hypothermia did not influence neurobehavioral outcomes.
Conclusions
Risks for poor neurobehavioral outcomes were high for children who were comatose after out-of-hospital cardiac arrest due to respiratory etiologies; survivors of drowning had better outcomes than those with other respiratory etiologies.
Keywords: drowning, respiratory arrest, cardiac arrest, therapeutic hypothermia, functional outcome, pediatrics, cognition
INTRODUCTION
Multicenter or population-based studies of pediatric out-of-hospital cardiac arrest (OHCA) are limited. In a recent, prospective, multicenter, randomized controlled trial, Therapeutic Hypothermia after Pediatric Cardiac Arrest, Out-of-Hospital (THAPCA-OH), the efficacy of two targeted temperature management strategies [therapeutic hypothermia (33°C) to therapeutic normothermia (36.8°C)] was examined. Children recruited to THAPCA-OH were comatose and required mechanical ventilation after return of circulation (ROC), and were at high risk for neurologic disability. Trial results showed that neither treatment arm conferred a significant benefit on survival with favorable functional outcome. (1)
Planned secondary analyses of this trial found substantial neurobehavioral morbidity in survivors one year later. (2, 3) Neurobehavioral outcomes included caregiver-reported functional skills in a range of domains [Vineland Adaptive Behavior Scales, Second Edition (VABS-II)] and age-appropriate cognitive performance measures (Mullen Scales of Early Learning or Wechsler Abbreviated Scale of Intelligence). While many children displayed severe to profound impairments on these measures, there was a range of outcomes; half functioned broadly within normal limits (within 2 SD of VABS-II means) and one third functioned similarly well on cognitive testing. Variables associated with worse outcome included older age and respiratory (compared with cardiac) etiologies. Neither reported outcomes separately for children with OHCA secondary to drowning or other respiratory etiologies.
Drowning cases enrolled in THAPCA-OH were described in secondary studies. (4, 5) Hypothermia did not confer significant outcome benefit in comparison with normothermia and there was a high rate of culture-proven bacterial infection in both groups. (4) Among respiratory etiologies, drowning was associated with better outcomes. (5) Similarly, in an observational cohort, children with drowning etiologies were more likely to survive to hospital discharge compared to all other OHCA etiologies. (6) This cohort included children with lower risk for poor neurological outcome than those enrolled in the THAPCA-OH trial; all children with ≥ 1 minute of chest compressions were included (versus ≥ 2 minutes with coma after ROC in THAPCA-OH). Detailed neurobehavioral outcomes were not examined in these reports.
In a broader group of children with OHCA due to drowning, who received emergency medical services, most had unfavorable short-term neurologic outcome. (7) Long-term outcomes, however, varied in a similar group. Suominen and colleagues explored neurobehavioral outcome several years after OHCA due to drowning in children resuscitated and then admitted to intensive care. In that group, 57% had neurological dysfunction and 40% had below average full-scale IQs; (8) longer submersion time was associated with worse cognitive outcome. There is also evidence that length of resuscitation is predictive of global outcome after drowning in children. (9)
To date, no study has examined detailed neurobehavioral outcomes one year after pediatric OHCA due to drowning or other respiratory etiologies, in children who remained comatose following ROC. Better understanding of the range of outcomes and outcome predictors in these children would be helpful for neuroprognostication. The objective of this study, restricted to a distinct and well-characterized sub-group of THAPCA-OH enrolled subjects who survived for at least one year, is to compare neurobehavioral outcomes in OHCA cases due to drowning with those due to other respiratory etiologies. Based on the limited literature available, we hypothesized that children with OHCA due to drowning would have better neurobehavioral outcomes than those with OHCA due to other respiratory etiologies.
METHODS
Study Setting and Population
The THAPCA-OH trial was conducted in 36 pediatric intensive care units (PICUs) in the United States (U.S.) and Canada from September 1, 2009 through December 31, 2012. Details of the THAPCA-OH trial were previously published. (10, 11) The trial was approved by the Institutional Review Boards at all sites, the Data Coordinating Center, and Outcome Center.
Children >48 hours and <18 years of age who had an OHCA with chest compressions for ≥2 minutes, and required mechanical ventilation after ROC met the original trial inclusion criteria. Major exclusion criteria for the THAPCA-OH Trial included the inability to be randomized within 6 hours of ROC, a Glasgow Coma Scale motor score of 5 or 6, a decision by clinicians to withhold aggressive treatment, OHCA due to trauma, and drowning in ice water. The inclusion and exclusion criteria are detailed in the primary outcome paper supplemental appendix. (1)
Of the 66 survivors who had respiratory etiologies, 60 had pre-OHCA VABS-II scores ≥70 and were eligible for the THAPCA-OH primary outcome analysis. This report analyzes 1-year neurobehavioral outcomes in 59 of these 60 survivors who were recruited from 27 of the 36 THAPCA-OH PICU sites. Children were grouped based on reported OHCA etiology (drowning versus other respiratory etiology).
Measures
Neurobehavioral Functioning
Vineland Adaptive Behavior Scales, Second Edition (VABS-II)
(12) VABS-II measures caregiver report of functional skills and provides age-corrected standard scores [mean=100, standard deviation(SD)=15] in four domains (communication, daily living, socialization, motor skills) and an overall adaptive behavior composite. Higher scores denote better functioning. In THAPCA-OH, a favorable outcome was defined as a score within 2SD of the age-corrected standard score mean (≥70). Each domain includes several subdomains with developmentally sequenced items, starting with skills typically observed in infancy. VABS-II includes a parent/caregiver rating form and a survey interview (using caregiver as informant) that yield comparable scores. (12)
Cognitive Performance
Wechsler Abbreviated Scale of Intelligence (WASI)
(13) WASI measures intellectual or general cognitive functioning. Normative data are based on a standardization sample highly representative of the English-speaking United States population aged from 6–89. The Vocabulary subtest requires individuals to orally define words. The Matrix Reasoning subtest, a measure of non-verbal fluid reasoning, requires individuals to view incomplete gridded patterns and select correct responses. Age-corrected standardized t-scores are available for both. When combined, these subtests yield age-corrected standard scores (mean=100, SD=15) for general intellectual functioning (Full Scale IQ).
Mullen Scales of Early Learning (Mullen)
(14) The Mullen, a measure of cognitive functioning designed for infants and young children, has four scales (visual reception, fine motor, receptive language, and expressive language). Normative data are available through age 5-years-8-months. Age-corrected standardized scores are available for each scale as t-scores and for overall early learning composite as a standard score.
For this report, all t-scores (Mullen and WASI) and v-scores (VABS-II) were transformed to standard scores. Scores >115 are above average, 85–115 are average, 70–84 are below average, and 50–69 are well below average. The lowest possible Mullen composite score is 49. For Mullen scales, raw scores below the lowest score on the normative table for age were referred to as lowest possible scores.
Other Descriptive Measures
Family Assessment Device
(15) Pre-OHCA family functioning was measured using the General Functioning Scale of the Family Assessment Device (FAD), a 12-item self-reported measure, scored 0–4; scores ≥2 indicate abnormal functioning.
Pediatric Cerebral Performance Category (PCPC) and Pediatric Overall Performance Category (POPC)
(16, 17) PCPC measures neurological functioning whereas POPC measures overall health (including neurological functioning). Although these clinician-rated scales (scored 1–6 with lower scores associated with better function) provide no detailed measurements, they have often been used to report pediatric cardiac arrest outcomes, and facilitated comparison between THAPCA-OH and other studies. (18)
Procedures
Within 24 hours of enrollment, a primary caregiver completed the VABS-II rating form to determine pre-OHCA baseline functioning. Site research coordinators reviewed instructions for form completion and responses for accuracy. In some cases, coordinators read items to caregivers and recorded responses. Demographic variables (age, sex, race, ethnicity, caregiver education level, and family functioning) were collected. Premorbid neurological and overall functioning (PCPC/POPC) was rated by research staff using medical records or caregiver report. OHCA-related variables (etiology, epinephrine doses, randomization treatment) were collected.
One year following OHCA, a trained research assistant at one site (Kennedy-Krieger Institute, Baltimore, MD), unaware of treatment group assignment, conducted a semi-structured telephone interview to assess neurobehavioral function (including VABS-II). Subsequently, children participated in on-site cognitive testing. Children ≥6 years who were reported to have no consistent means of functional communication on the 1-year VABS-II did not undergo additional testing and were assigned lowest possible scores for outcome analyses.
Data Analysis
Change in VABS-II scores were calculated for each child (1-year – pre-OHCA score). Distributions of continuous variables were compared between groups using the Wilcoxon rank sum test. Signed rank tests were used to test differences between two continuous variables (e.g. between premorbid and 1-year scores). Categorical variables were examined using Fisher’s exact test or the Cochran-Armitage test for trend. Standard linear regression models were employed to predict 1-year VABS-II score. Predictor variables included premorbid continuous and categorical factors. A multivariable regression model was fit using premorbid predictors which showed an association (p<0.05) in univariate models. No adjustments for multiple comparisons were made in these analyses. All analyses were performed using SAS software, version 9.4 (SAS Institute).
RESULTS
Demographics and Pre-OHCA Baseline Functioning
Thirty children were included in the Drowning group and 29 in the Other Respiratory Etiology (Other) group. Of the 29 children in the Other group, etiologies were classified as non-drowning asphyxia for eleven, near-miss sudden infant death syndrome for five, apnea for three, and other respiratory causes (e.g., asthma, tracheostomy tube issue, pneumonia, smoke inhalation) for ten.
Demographic variables are described in Table 1. Age distribution did not differ significantly; 19/30 (63%) of the Drowning group and 16/29 (55%) of the Other group were <4 years at randomization. At 12 month follow up, ages ranged from 1.1 to 16.9 years. The majority were white and not Hispanic. Mean premorbid VABS-II scores were average for age. Almost all children had normal PCPC ratings at pre-OHCA baseline.
Table 1.
Demographics by Group
| Primary respiratory etiology
|
P-value | ||
|---|---|---|---|
| Drowning (N = 30) | Other (N = 29) | ||
| Age at Randomization (years): Mean (SD) | 4.6 (4.16) | 5.1 (5.41) | 0.4261 |
| Male | 21 (70.0%) | 21 (72.4%) | 1.0002 |
| Race | 0.2042 | ||
| Black or African American | 7 (23.3%) | 9 (31.0%) | |
| White | 20 (66.7%) | 13 (44.8%) | |
| Other/Unknown | 3 (10.0%) | 7 (24.1%) | |
| Ethnicity | 0.7942 | ||
| Hispanic or Latino | 6 (20.0%) | 4 (13.8%) | |
| Not Hispanic or Latino | 23 (76.7%) | 23 (79.3%) | |
| Stated as Unknown | 1 (3.3%) | 2 (6.9%) | |
| Caregivers highest education received | 0.6663 | ||
| Some high school or less | 6 (20.0%) | 7 (24.1%) | |
| High school graduate or General Educational Development (GED) | 7 (23.3%) | 10 (34.5%) | |
| Vocational school or some college | 10 (33.3%) | 4 (13.8%) | |
| College degree | 3 (10.0%) | 4 (13.8%) | |
| Graduate or doctoral degree | 4 (13.3%) | 4 (13.8%) | |
| Average FAD score: Mean (SD) | 1.3 (0.42) | 1.5 (0.37) | 0.0341 |
| Pre-existing Conditions | |||
| Prenatal condition | 1 (3.3%) | 9 (31.0%) | 0.0062 |
| Lung or airway disease | 3 (10.0%) | 14 (48.3%) | 0.0022 |
| Congenital heart disease | 1 (3.3%) | 2 (6.9%) | 0.6122 |
| Gastrointestinal disorder | 1 (3.3%) | 6 (20.7%) | 0.0522 |
| Neurologic condition | 3 (10.0%) | 3 (10.3%) | 1.0002 |
| Other | 2 (6.7%) | 7 (24.1%) | 0.0802 |
| Any pre-existing condition | 9 (30.0%) | 18 (62.1%) | 0.0192 |
| Cardiac arrest witnessed | 7 (23.3%) | 14 (48.3%) | 0.0952 |
| Chest compressions administered by bystander | 26 (86.7%) | 17 (58.6%) | 0.0252 |
| Total number of doses of epinephrine administered by EMS and at hospital4: Mean (SD) | 2.6 (2.60) | 2.1 (1.25) | 0.8311 |
| Estimated duration of chest compressions5 | 0.4253 | ||
| Unable to determine | 1 (3.3%) | 2 (6.9%) | |
| Up to 15 minutes | 9 (30.0%) | 10 (34.5%) | |
| 15 to 30 minutes | 13 (43.3%) | 13 (44.8%) | |
| More than 30 minutes | 7 (23.3%) | 4 (13.8%) | |
| Pre-cardiac arrest VABS Adaptive Behavior Composite Score: Mean (SD) | 100.8 (12.81) | 99.7 (15.87) | 0.7961 |
| Pre-cardiac arrest PCPC | 0.5333 | ||
| Normal = 1 | 29 (96.7%) | 27 (93.1%) | |
| Mild disability = 2 | 1 (3.3%) | 2 (6.9%) | |
| Pre-cardiac arrest POPC | 0.0113 | ||
| Good = 1 | 29 (96.7%) | 21 (72.4%) | |
| Mild disability = 2 | 1 (3.3%) | 6 (20.7%) | |
| Moderate disability = 3 | 0 (0.0%) | 2 (6.9%) | |
| Treatment Assigned | 0.1872 | ||
| Hypothermia | 21 (70.0%) | 15 (51.7%) | |
| Normothermia | 9 (30.0%) | 14 (48.3%) | |
| First Recorded Temperature6: Mean (SD) | 36.6 (1.58) | 36.3 (1.35) | 0.4661 |
P-value is based on the Wilcoxon rank-sum test.
Fisher’s exact test.
Two-sided Cochran-Armitage test for trend.
The number of doses of Epinephrine received was missing for one patient in the ‘Other’ group.
The category ‘Unable to determine’ excluded from p-value calculation.
Temperature recorded measured at the time of targeted temperature management or shortly after.
There were differences in demographic variables between the Drowning and Other groups. The Other group had more pre-existing conditions prior to cardiac arrest (primarily due to lung/airway disease) and more children with mild to moderate POPC ratings. Additionally, fewer children in the Other group had chest compressions administered by a bystander. Premorbid family functioning was also perceived as more problematic in this group, although the mean scores for both groups were within the normal range.
Neurobehavioral Functioning
Table 2 displays mean pre-OHCA scores, 1-year follow-up, and mean absolute change from baseline to follow up for VABS-II adaptive behavior composite and domain scores for the Drowning and Other groups. Composite and domain scores declined significantly for both groups; however, the Drowning group had significantly smaller declines in overall composite [Drowning/Other (median decline) −20.5/−44] as well as communication [Drowning/Other (median decline) −20/−51] and motor [Drowning/Other (median decline) −18/−54.5] domain scores.
Table 2.
Mean Vineland Adaptive Behavior Scales, Second Edition (VABS-II) Scores at Pre-OHCA Baseline and One Year Follow-Up and Mean Change by group
| Drowning (N=30)
|
Other (N=29)
|
P-value1 | |||||
|---|---|---|---|---|---|---|---|
| Baseline Mean | Follow-up Mean | Change | Baseline Mean | Follow-up Mean | Change | ||
| VABS Adaptive Behavior Composite Score | 100.8 | 71.4 | −29.4 | 99.7 | 56.3 | −43.4 | 0.049 |
| Communication Domain Score | 99.2 | 73.6 | −25.6 | 101.5 | 59.1 | −42.3 | 0.045 |
| Daily Living Domain Score | 105.9 | 73.0 | −32.9 | 100.3 | 56.2 | −44.2 | 0.150 |
| Socialization Domain Score | 100.8 | 77.7 | −23.1 | 99.6 | 66.1 | −33.4 | 0.170 |
| Motor Functioning Domain Score2 | 100.7 | 66.6 | −32.6 | 101.2 | 51.5 | −51.4 | 0.034 |
P-values compare the change in scores between etiology groups using the Wilcoxon rank-sum test.
One patient in each group had missing baseline scores.
Table S1a and S1b display the distributions of scores for the composite and domains. At 1-year follow-up, 53% (16/30) of the Drowning group had composite VABS-II scores ≥ 70, compared with 31% (9/29) for the Other group. Similar proportions were noted for most domains.
Similarly, 43% (13/30) of the Drowning group had composite scores within 1 SD (15 points) of their pre-OHCA baselines, in contrast with 14% (4/29) for the Other group. Trends for domain scores within 1 SD of baselines were similar [Drowning/Other - Communication (33%/17%); Daily Living, (40%/14%); Motor, (48%/14%)]. Within the Socialization Domain, the proportion of children within 1SD of baseline was similar between groups [Drowning/Other – (30%/28%)].
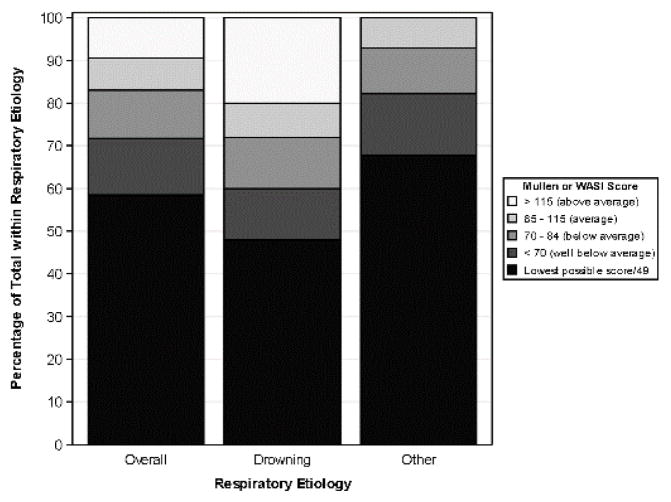
Figure 1 depicts percentage of children with cognitive functioning [Cognitive composite scores (early learning composite from Mullen or 2-subtest composite from the WASI)] in the above average, average, below average, and well below ranges, and percentage with lowest possible scores (for all cases and separately by etiology group). Forty-eight percent of the Drowning group was either not eligible for testing on the WASI or obtained the lowest possible Mullen score compared to 68% of the Other group. Similarly, 28% of Drowning group obtained scores that were average to above, whereas only 7% achieved average scores in the Other group and no child obtained an above average score.
Figure 1.
Overall Cognitive Performance 1 year after OHCA by etiology
Additional cognitive test performance details are presented in Tables S2 and S3. On Mullen Scales, 40% (6/15) in the Drowning group obtained overall composite scores in the average range, whereas no child in the Other group (0/17) obtained an average score (Table S2). For the children ≥ 6 years, very few children obtained average WASI scores (Drowning – 10% (1/10) and Other – 18% (2/11) (Table S3).
Predictors of Neurobehavioral Outcome
Table 3 displays results of univariate and multivariate regression analyses that examined predictors of 1-year VABS-II composite score. In the univariate analyses older age at OHCA, number of epinephrine doses, and non-drowning etiology of OHCA were associated with worse outcome. No other demographic variables predicted outcome. In a multivariate model which included these three predictors, all three variables remained significant and the overall model predicted 33% of the variance on the 1-year VABS-II score.
Table 3.
Predictors of Vineland Adaptive Behavior Scales, Second Edition (VABS-II) Overall Behavior Composite at 12-month Follow-Up for All Respiratory Etiologies
| Predictors | Univariate
|
Multivariable (R2 = 0.329)
|
|||
|---|---|---|---|---|---|
| Parameter Estimate (95% CI) | R2 | P-value | Parameter Estimate (95% CI) | P-value | |
| Age at Randomization (years) | −1.70 (−3.14, −0.26) | 0.090 | 0.021 | −1.74 (−2.99, −0.48) | 0.008 |
| Gender (male) | 1.07 (−14.68, 16.83) | 0.000 | 0.892 | ||
| Caregivers highest level of education | 0.138 | 0.086 | |||
| Some high school or less | Reference | ||||
| High school graduate or General Educational Development (GED) | −0.78 (−20.07, 18.51) | ||||
| Vocational school or some college | 23.41 (3.24, 43.57) | ||||
| College degree | 11.41 (−13.14, 35.95) | ||||
| Graduate or doctoral degree | 14.82 (−8.71, 38.35) | ||||
| Average FAD score | −8.43 (−26.25, 9.38) | 0.016 | 0.347 | ||
| Pre-cardiac arrest VABS Adaptive Behavior Composite Score | 0.25 (−0.25, 0.75) | 0.017 | 0.319 | ||
| Any pre-existing condition | −10.99 (−25.02, 3.03) | 0.041 | 0.122 | ||
| Cardiac arrest witnessed1 | −10.79 (−25.59, 4.00) | 0.037 | 0.149 | ||
| Chest compressions administered by bystander2 | 13.79 (−2.06, 29.64) | 0.051 | 0.087 | ||
| Estimated duration of chest compression (> 30 min)3 | −15.16 (−33.42, 3.10) | 0.049 | 0.102 | ||
| Total number of doses of epinephrine2 | −4.89 (−8.17, −1.62) | 0.138 | 0.004 | −5.71 (−8.69, −2.73) | <.001 |
| CA Etiology (drowning vs. other etiologies) | 15.12 (1.42, 28.82) | 0.079 | 0.031 | 16.06 (4.09, 28.04) | 0.010 |
| Diagnosed Seizures | −8.97 (−23.07, 5.12) | 0.028 | 0.208 | ||
| Hypothermia | 2.78 (−11.84, 17.39) | 0.003 | 0.705 | ||
Two patients with unable to determine status removed from analysis.
One patient with unable to determine status removed from analysis.
Three patients with unable to determine status removed from analysis.
DISCUSSION
This is the first detailed, prospective study of long-term neurobehavioral outcomes in pediatric OHCA survivors due to drowning and other respiratory etiologies who were admitted to a PICU and remained comatose within 6 hours of ROC. Caregiver-reported neurobehavioral functioning declined significantly for all domains, including communication, daily living, socialization, and motor skills. Overall, children with drowning etiology had better functional and cognitive outcome than those with other respiratory etiologies. Older age at CA, number of epinephrine doses, and non-drowning respiratory etiology predicted worse neurobehavioral outcome. When controlling for age and number of epinephrine doses, drowning etiology continued to be associated with better neurobehavioral outcome. Other demographic and CA-characteristics, including witnessed arrest and targeted temperature treatment group, were not predictive of outcomes.
Strengths of this study are the prospective design, high follow-up rate, and detailed neurobehavioral outcome measures that assess multiple domains of functioning, including caregiver-report and objective performance. Our sample was restricted to a well-characterized group of children who sustained OHCA of respiratory etiology and who were comatose within the first several hours after resuscitation (pain localization or responsiveness to commands were THAPCA-OH exclusion criteria).
Similar to the results of studies of all survivors of the THAPCA-OH Trial who were eligible for the primary outcome, (1–3) this subgroup of children with OHCA due to respiratory etiologies have considerable neurobehavioral morbidity, including significant declines in all domains of neurobehavioral functioning. Additionally, the majority of these children displayed well below average cognitive functioning or were assigned the lowest possible score. It is important to emphasize that the THAPCA-OH group was limited to children at very high risk for severe neurological injury after CA as inclusion criteria mandated at least two minutes of chest compressions and a GCS motor score of <5 after ROC. As a result, while our findings can help clinicians tasked with early prognostication to better understand the range of neurobehavioral outcomes in children at highest risk for neurobehavioral morbidity after OHCA due to drowning and other respiratory etiologies, they cannot be generalized to all OHCA survivors with respiratory etiologies. This point is highlighted by comparing drowning survivors from this report to a recent study of very long term neurocognitive outcome (median of 8 years) in 21 surviving children who required CPR after drowning. (8) In that study, only 4/20 (5%) had cognitive composite scores of <70, whereas 15/25 (60%) in our study fell into that category.
Despite the poor averaged group outcomes, it is important to emphasize that we found a broad range of outcomes, with 20% of cases displaying above average cognitive function in the Drowning group. Moreover, overall neurobehavioral outcomes were better in the Drowning group compared to those with other respiratory etiologies. While only children with broadly normal pre-OHCA baseline neurobehavioral functioning were included, more children in the Other group had pre-existing medical conditions that may have impacted recovery over the course of the 12 month follow-up period. Pre-OHCA family functioning was also significantly better in the Drowning group. Despite these differences, neither pre-existing conditions nor premorbid family functioning were significant predictors of 12-month outcome in the regression analyses.
Drowning etiology was associated with better outcome, even after controlling for other significant predictors of outcome (age and epinephrine doses). Better outcome following drowning relative to other OHCA etiologies has been previously reported. (4, 5) While previous reports cannot be directly compared to our population, as we only examined survivors, it is possible that those with certain respiratory etiologies (such as asthma) had a significant period of hypoxia due to respiratory distress prior to OHCA and therefore may have incurred a more severe hypoxic insult.
Other variables associated with worse outcome included older age and more epinephrine doses. The association between older age and worse outcome is consistent with findings from the larger group of THAPCA-OH survivors. (2, 3) In part, this association could reflect age-related differences in the sensitivity of the test measures, i.e., VABS-II scores may reflect less impairment at the younger age since expectations for functional skills are minimal in very young children and deficits relative to peers become more apparent over time. Greater number of epinephrine doses was associated with worse outcome at 12 months, congruent with prior studies. Higher number of epinephrine doses has been consistently associated with mortality following pediatric CA. (6, 19, 20) In survivors of the THAPCA-OH with normal premorbid functioning, number of epinephrine doses was associated with 12 month VABS-II scores in univariate analysis, and remained a significant predictor of outcome in a multivariate model controlling for age, etiology (respiratory, cardiac, or other). (3)
Our results need to be considered in the context of several limitations. The sample sizes are small and the Other group includes a range of etiologies which makes interpreting differences from the Drowning group difficult. Data collection in the THAPCA-OH protocol did not include some sources of variation in patient characteristics and treatment that could influence outcome (e.g. submersion duration for the Drowning group, EMS response time, temperature upon arrival, neuroimaging abnormalities, seizure burden, duration of coma, medications, rehabilitation services received). Moreover, the limited number of children aged >6 years who were eligible for testing precluded meaningful analysis of functioning in specific neuropsychological domains (e.g., executive functions, memory).
Conclusion
In this population of children who incurred OHCA due to respiratory etiologies and were comatose after resuscitation, and were enrolled in the THAPCA-OH trial, there was substantial neurobehavioral morbidity one year later. Younger age, fewer doses of epinephrine, and drowning etiology (versus all other respiratory etiologies) were associated with better 12-month neurobehavioral outcomes.
Supplementary Material
Acknowledgments
Source of Funding: Supported by the National Heart, Lung, and Blood Institute (NHLBI) grants HL094345 (to Dr. Moler) and HL094339 (to Dr. Dean). Support in part from the following federal planning grants contributed to the planning of the THAPCA Trials: HD044955 (to Dr. Moler) and HD050531 (to Dr. Moler). Additional in part support from the following research networks: Pediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Pediatric Critical Care Research Network (CPCCRN) from cooperative agreements U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934.
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Footnotes
Reprints will not be ordered.
CONFLICT OF INTEREST STATEMENT
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) grants HL094345 (to Dr. Moler) and HL094339 (to Dr. Dean). Support in part was also obtained from the following federal planning grants contributed to the planning of the THAPCA Trials: HD044955 (to Dr. Moler) and HD050531 (to Dr. Moler). Additional in part support was obtained from the following research networks: Pediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Pediatric Critical Care Research Network (CPCCRN) from cooperative agreements U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015 May 14;372(20):1898–908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slomine BS, Silverstein FS, Christensen JR, Holubkov R, Page K, Dean JM, et al. Neurobehavioral Outcomes in Children After Out-of-Hospital Cardiac Arrest. Pediatrics. 2016 Apr;137(4) doi: 10.1542/peds.2015,3412. Epub 2016 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverstein FS, Slomine BS, Christensen J, Holubkov R, Page K, Dean JM, et al. Functional Outcome Trajectories After Out-of Hospital Pediatric Cardiac Arrest. Crit Care Med. 2016 Aug 9; doi: 10.1097/CCM.0000000000002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moler FW, Hutchison JS, Nadkarni VM, Silverstein FS, Meert KL, Holubkov R, et al. Targeted Temperature Management After Pediatric Cardiac Arrest Due To Drowning: Outcomes and Complications. Pediatr Crit Care Med. 2016 Aug;17(8):712–20. doi: 10.1097/PCC.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meert KL, Telford R, Holubkov R, Slomine BS, Christensen JR, Dean JM, et al. Pediatric Out-of-Hospital Cardiac Arrest Characteristics and Their Association With Survival and Neurobehavioral Outcome. Pediatr Crit Care Med. 2016 Sep 27; doi: 10.1097/PCC.0000000000000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moler FW, Donaldson AE, Meert K, Brilli RJ, Nadkarni V, Shaffner DH, et al. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011 Jan;39(1):141–9. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitta M, Kitamura T, Iwami T, Nadkarni VM, Berg RA, Topjian AA, et al. Out-of-hospital cardiac arrest due to drowning among children and adults from the Utstein Osaka Project. Resuscitation. 2013 Nov;84(11):1568–73. doi: 10.1016/j.resuscitation.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suominen PK, Sutinen N, Valle S, Olkkola KT, Lonnqvist T. Neurocognitive long term follow-up study on drowned children. Resuscitation. 2014 Aug;85(8):1059–64. doi: 10.1016/j.resuscitation.2014.03.307. [DOI] [PubMed] [Google Scholar]
- 9.Kieboom JK, Verkade HJ, Burgerhof JG, Bierens JJ, Rheenen PF, Kneyber MC, et al. Outcome after resuscitation beyond 30 minutes in drowned children with cardiac arrest and hypothermia: Dutch nationwide retrospective cohort study. BMJ. 2015 Feb 10;350:h418. doi: 10.1136/bmj.h418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moler FW, Silverstein FS, Meert KL, Clark AE, Holubkov R, Browning B, et al. Rationale, timeline, study design, and protocol overview of the therapeutic hypothermia after pediatric cardiac arrest trials. Pediatr Crit Care Med. 2013 Sep;14(7):e304–15. doi: 10.1097/PCC.0b013e31828a863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holubkov R, Clark AE, Moler FW, Slomine BS, Christensen JR, Silverstein FS, et al. Efficacy outcome selection in the therapeutic hypothermia after pediatric cardiac arrest trials. Pediatr Crit Care Med. 2015 Jan;16(1):1–10. doi: 10.1097/PCC.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales: Survey Forms Manual. 2. Minneapolis, MN: NCS Pearson; 2005. [Google Scholar]
- 13.Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- 14.Mullen EM. Mullen scales of early learning. Circle Pine, MN: American Guidance Service; 1995. [Google Scholar]
- 15.Epstein NB, Baldwin LW, Bishop DS. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9(2):171. [Google Scholar]
- 16.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992 Jul;121(1):68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 17.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000 Jul;28(7):2616–20. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 18.Zaritsky A, Nadkarni V, Hazinski MF, Foltin G, Quan L, Wright J, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the Pediatric Utstein Style. A statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Resuscitation. 1995 Oct;30(2):95–115. doi: 10.1016/0300-9572(95)00884-v. [DOI] [PubMed] [Google Scholar]
- 19.Young KD, Gausche-Hill M, McClung CD, Lewis RJ. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004 Jul;114(1):157–64. doi: 10.1542/peds.114.1.157. [DOI] [PubMed] [Google Scholar]
- 20.Schindler MB, Bohn D, Cox PN, McCrindle BW, Jarvis A, Edmonds J, et al. Outcome of out-of-hospital cardiac or respiratory arrest in children. N Engl J Med. 1996 Nov 14;335(20):1473–9. doi: 10.1056/NEJM199611143352001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.