Abstract
Background
Prior studies suggest posttraumatic stress disorder (PTSD) is associated with elevated cardiovascular disease (CVD) risk, but effects of duration and remission of PTSD symptoms have rarely been evaluated.
Methods
We examined the association of time-updated PTSD symptom severity, remission, and duration with incident CVD risk (552 confirmed myocardial infarctions or strokes) over 20 years in 49,859 women in Nurses’ Health Study II. Among women who reported trauma on the Brief Trauma Questionnaire, PTSD symptoms, assessed by a screener, were classified by symptom severity and chronicity: a) no symptoms, b) 1–3 ongoing, c) 4–5 ongoing, d) 6–7 ongoing, e) 1–3 remitted, f) 4–7 remitted symptoms. Inverse probability weighting was used to estimate marginal structural logistic regression models, adjusting for time-varying and time-invariant confounders.
Results
Compared to women with no trauma exposure, women with trauma/no PTSD (OR=1.30; 95% CI: 1.03, 1.65) and women with trauma/6–7 symptoms (OR=1.69; 95% CI: 1.08, 2.63) had elevated risk of CVD; women with remitted symptoms did not have elevated CVD risk. Among women exposed to trauma, every 5 additional years of PTSD symptomology was associated with 9% higher CVD incidence compared to women with trauma/no PTSD.
Conclusion
Findings suggest alleviating PTSD symptoms shortly after onset may attenuate CVD risk.
Keywords: marginal structural models, posttraumatic stress disorder, severity, remission, duration
Posttraumatic stress disorder (PTSD) is a debilitating mental disorder that can occur after trauma exposure (American Psychiatric Association, 1994). Accumulating evidence indicates PTSD has cardiotoxic effects. For example, a recent meta-analysis found the estimated hazard ratio (HR) for cardiovascular disease (CVD) or cardiac deaths to be 1.55 (95% confidence interval (CI): 1.34, 1.80) with estimates from individual studies ranging from 1.39 to 3.28 (Edmondson and Cohen, 2013). Moreover, two studies demonstrated a dose response association between PTSD symptom severity and CVD risk (Kubzansky et al., 2007, Kubzansky et al., 2009). Despite consistent evidence for this association, how duration and remission of PTSD affect CVD risk remains unclear. Beyond having more detailed measures of PTSD, a major challenge to evaluating these questions is appropriately accounting for the role of health behaviors as possible time-varying confounders.
While often correlated, duration and severity are nonetheless distinct aspects of the PTSD experience. For example, trajectory models of PTSD symptoms have revealed that even among people who experience long duration, symptom severity differs (Lowe et al., 2014). No studies have directly considered whether duration of exposure (length of time experiencing PTSD symptoms) is relevant to CVD risk. Also unknown is whether CVD risk is mitigated for individuals whose PTSD symptoms remit. CVD risk conferred by other well-known risk factors increases with exposure duration and declines once the risk factor is eliminated. For example, longer smoking duration is associated with higher coronary heart disease risk (Burns, 2003), but excess risk due to smoking is halved one year after smoking cessation; 15 years after smoking cessation, risk is similar to that of a never smoker (United States, and Center for Chronic Disease Prevention and Health Promotion, 1990). A study in twins examining heart rate variability (HRV), a marker of CVD risk, found a dose response association between current PTSD severity and risk of low HRV but no association was evident with remitted PTSD symptoms (Shah et al., 2013). Understanding whether duration and remission of PTSD symptoms change CVD risk could provide additional insight into how PTSD alters cardiovascular function and when interventions might be most effective.
A more nuanced understanding of the role of health behaviors in the PTSD-CVD relationship may also add insight into how PTSD influences CVD risk. Health behaviors might exacerbate or lead to maintenance of PTSD symptoms, and may also act as mediators linking PTSD to CVD risk (Sumner et al., 2015). For example, PTSD is associated with increased risk of smoking (Fu et al., 2007), a major CVD risk factor (Burns, 2003), and post-trauma smoking increases risk of PTSD onset (van der Velden et al., 2008). Most longitudinal studies on PTSD and CVD adjust for health behaviors directly in regression models (Sumner et al., 2015, Kubzansky et al., 2007, Kubzansky et al., 2009, Scherrer et al., 2010, Beristianos et al., Turner et al., 2013). However, adjusting for time-varying confounders through direct inclusion in a regression model can bias estimates due to collider bias and over-control for mechanisms operating through health behaviors (Figure 1) (Robins, 1999, Robins et al., 2000). Inverse probability weighting provides control for time-varying confounders while avoiding conditioning on mediating pathways (Robins et al., 2000, Robins, 1999), but to our knowledge has not been used in PTSD and CVD research.
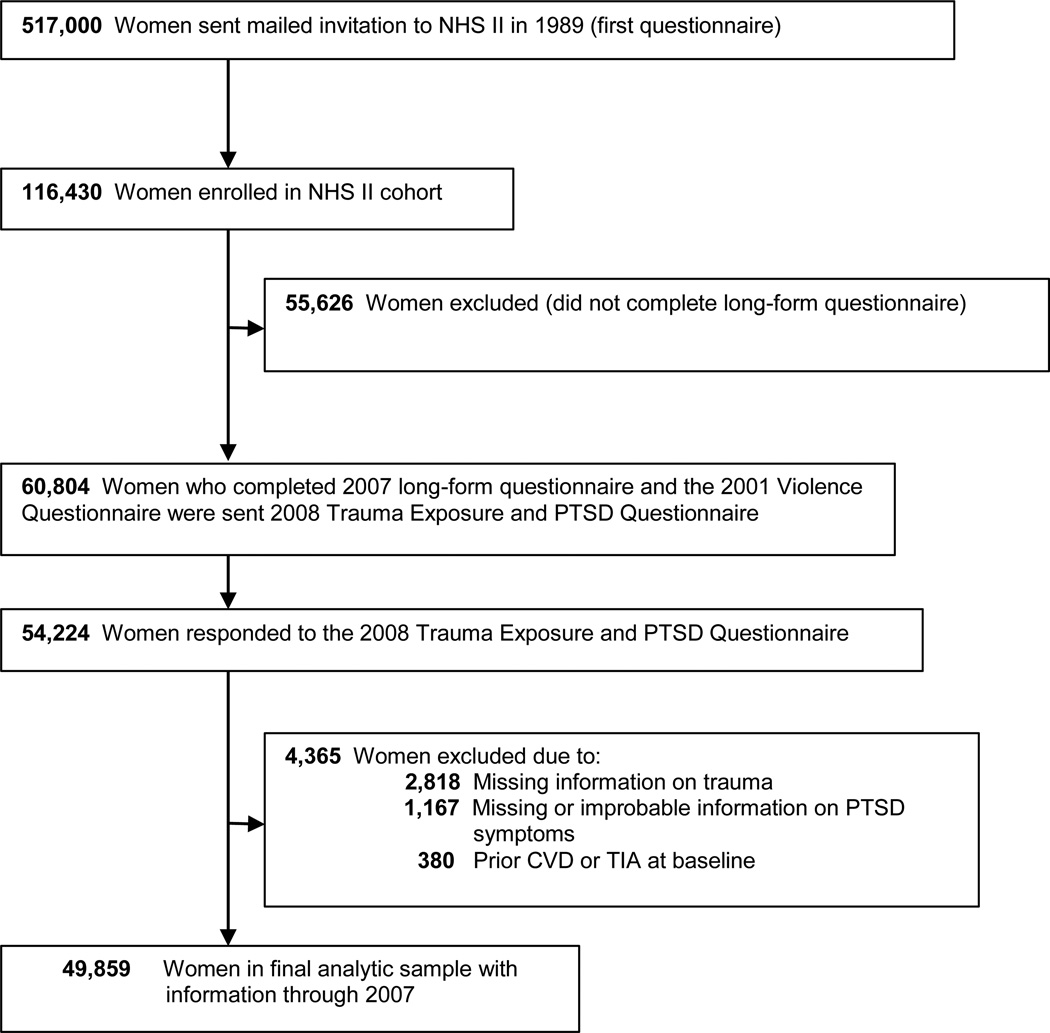
Figure 1.
Flowchart of exclusions for deriving the final analytic sample.
Research previously conducted in the Nurses’ Health Study II (NHS II) (Sumner et al., 2015) found CVD risk was elevated for women reporting trauma exposure without PTSD symptoms (HR=1.45, 95% CI, 1.15, 1.83) and for those reporting at least 4 PTSD symptoms (HR=1.60, 95% 1.20, 2.13) compared to women with no trauma exposure. Health behaviors and medical risk factors accounted for 47% (95% CI: 23, 70%) of the elevated CVD risk associated with 4+ PTSD symptoms. Using the same sample, we expand upon these findings by examining the association of CVD risk with PTSD symptom duration and remission. We also implement inverse probability weighting to adjust for potentially confounding effects of demographics factors, childhood socioeconomic status, family history of CVD, and health behaviors. We expected higher PTSD symptoms would be associated with higher risk of CVD and hypothesized that among individuals whose symptoms remit, the risk of CVD would be more similar to risk associated with low levels of PTSD symptoms or trauma exposure alone. Lastly, we hypothesized that PTSD symptom duration would be positively associated with CVD risk, independent of severity.
METHODS
Study population
The NHS II is a cohort of 116,430 civilian female nurses ages 25–42 at enrollment in 1989 and followed with biennial questionnaires inquiring about risk factors and health conditions. In 2008, 60,804 women who completed an earlier supplemental questionnaire on violence as well as the 2007 biennial questionnaire were mailed a supplemental questionnaire assessing trauma exposure and PTSD symptom onset, remission, and severity. 54,224 women completed the supplemental PTSD questionnaire (89% response rate). This study was approved by the Institutional Review Board of Brigham and Women’s Hospital. Return of the questionnaire via U.S. mail constituted implied consent.
The final analytic sample was comprised of 49,859 women after excluding 380 women who reported CVD or transient ischemic attack (TIA) at or before baseline, 2,818 women who did not report age at worst trauma, and 1,167 women with missing or improbable information on PTSD symptoms (Figure 1). Participants excluded from the sample were on average older, less educated, and more likely to identify as a racial or ethnic minority, but did not differ by baseline values of neighborhood mean income, smoking, physical activity, alcohol intake, diet quality, parental home ownership at time of birth, family history of stroke or family history of MI.
Trauma and PTSD symptom exposure
Trauma exposure was assessed by a modified version of the Brief Trauma Questionnaire (BTQ), which includes 16 potentially traumatic events (Koenen et al., 2009) and additional questions capturing age at first and worst events. PTSD symptoms in relation to the worst trauma were assessed using an adaptation of the 7-item Short Screening Scale for DSM-IV PTSD (Breslau et al., 1999, Koenen et al., 2009). For women reporting any symptoms, the screener assessed whether symptoms lasted at least one month and the age at which women last experienced symptoms. A symptom score of 4 or greater identifies cases of PTSD with a sensitivity of 85% and specificity of 93% (Breslau et al., 1999). Exposure to trauma and PTSD symptoms was ascertained for each study period year using age at worst trauma (i.e. start of PTSD symptom exposure) and age of last PTSD symptom (i.e. end of PTSD symptom exposure). PTSD symptom levels and duration were assigned when they persisted longer than one month. Reliability of self-reported age-of-onset of trauma and PTSD has been found to be excellent in this sample (ICC=.95) (Lee et al., 2016). At each year, women were classified as: a) no trauma exposure, b) trauma exposure but no PTSD symptoms, c) trauma exposure with mild (1–3) ongoing PTSD symptom levels, d) trauma exposure with moderate (4–5) ongoing PTSD symptoms, e) trauma exposure with elevated (6–7) ongoing PTSD symptoms, f) trauma exposure with no current PTSD symptoms but a history of 1–3 PTSD symptoms (i.e., remitted mild symptoms), or g) trauma exposure with no current PTSD symptoms but a history of 4–7 symptoms (i.e., remitted moderate/severe symptoms).
For women with current PTSD symptoms, duration of PTSD symptoms was operationalized as number of years since worst trauma until current age. For women whose symptoms had remitted, duration equaled the number of years since worst trauma until age of last reported symptom. Women with symptoms lasting at least one month but remitting the same year as the worst trauma were assigned a duration of 0.5 years. Duration of symptoms ranged from 0.5 to 60 years since some women reported PTSD symptoms starting in early childhood.
To illustrate derivation of duration and remission status variables, we provide the following example: A woman reported experiencing her worst trauma in 1991 at age 28 and indicated that this trauma was associated with 6 PTSD symptoms. She also reported that she last experienced these PTSD symptoms at age 42 in 2006. For the purposes of the current analyses this woman is classified as having no trauma exposure for 1989 and 1990, as having trauma/severe symptoms from 1991 until 2005, and as having remitted moderate/severe symptoms from 2006 until 2008. The duration of symptoms increases each year starting in 1991 until it reaches its maximum of 14 years in 2005.
CVD outcomes
We defined CVD cases as incident myocardial infarction (MI) or stroke events (not including TIA). Cases were captured at each biennial interview through self-report of a doctor diagnosis of stroke or MI. Permission was requested from participants (or next of kin in the case of death) to review medical records after report of CVD events. Medical records review was conducted by a physician blinded to trauma and PTSD exposure status. Confirmed MI was defined as meeting the World Health Organization criteria based on symptoms plus diagnostic ECG changes or elevated cardiac enzyme concentrations (Rose and Blackburn, 2002). Confirmed strokes were defined according to the National Survey of Stroke criteria as a neurologic defect of sudden or rapid onset that lasted >24 hours or until death (Walker et al., 1981). Fatal MIs and strokes were detected through information provided by the next of kin, postal authorities, and the National Death Index searches and confirmed through medical record review to the extent this information was available (Adebamowo et al., 2015, Ferraro et al., 2013).
Covariates
Time-invariant and time-varying confounders were considered. Time-invariant variables included baseline age (linear and quadratic functional forms) (Brewin et al., 2000), race/ethnicity (Brewin et al., 2000, Graham-Garcia et al., 2001), childhood socioeconomic status (SES) (Koenen et al., 2007, Nandi et al., 2012), and family history of MI or stroke. Childhood SES was evaluated through items reported in the 2005 questionnaire related to parental home ownership at time of birth (yes/no) and highest level of education attained by either parent (high school or less, some college, college or beyond). Time-varying confounders included age (linear and quadratic forms) and adult SES measured biennially by census tract median family income. Census tract median income was first collected in 1989 thus 1989 values were used for 1987. We also considered confounding by the following health behaviors: cigarette smoking, physical activity, alcohol consumption, and diet quality. Current smoking was assessed every two years and participants were classified as never smokers, former smokers, or current smokers of 1–14, 15–24, or 25+ cigarettes/day. Alcohol consumption (i.e. beer, wine, and liquor) was measured in 1989, 1991, 1995, 1999, and 2003 and categorized as 0, 1–4, 5–9, 10–19, or 20+ grams/day. Diet was measured every four years starting in 1991, with responses ranked into quintiles of least to most healthy diet using the Alternative Healthy Eating Index (Chiuve et al., 2012). Physical activity was measured in 1989, 1991, 1997, 2001, and 2005 and categorized as <3, 3–8.9, 9–17.9, 18–26.9, or 27+ metabolic equivalent (MET) hours/week. In 2001, lifetime depressive symptoms were self-reported with the following item: “In your lifetime, have you ever had two weeks or longer when nearly every day you felt sad, blue, or depressed for most of the day?” Women were then asked about depressive symptom in the prior two years in the 2003 and 2005 questionnaire cycles using the following item, “In the past two years, have you ever had two weeks or longer when nearly every day you felt sad, blue, or depressed for most of the day?” Missing indicators were used for measured confounders on which women were missing information.
Statistical analyses
We conducted descriptive analyses and examined the distribution of trauma/PTSD symptoms and covariates at each biennial questionnaire wave. In primary analyses we estimated the odds of incident CVD associated with trauma exposure, PTSD severity, and PTSD remission using marginal structural pooled logistic regression models (MSMs) through the use of inverse probability weights (IPW). Odds ratios estimated by pooled logistic regressions approximate hazard ratios when the outcome is rare and the time-intervals are small, as in this study (D'Agostino et al., 1990). We used a tall data structure with each observation corresponding to a biennial questionnaire when CVD was assessed (i.e. an outcome wave), so the effect estimates refer to odds for incident CVD within the 2-year window for each questionnaire. We do not include CVD occurring after 2007, enabling us to link all outcome waves to time-varying trauma/PTSD exposure status (from the year preceding the outcome wave) and to lagged time-varying confounders (using assessments from the year prior to trauma/PTSD exposure). For example, 1990 trauma/PTSD exposures were linked with CVD outcomes during the next questionnaire cycle (1991 to 1993) and 1989 time-varying confounders. Participants were censored at first reported CVD event, death, or last interview.
Person- and time-specific IPWs for exposure to trauma/PTSD symptoms were estimated with multinomial logistic regression using the 7 categories of trauma/PTSD exposure status as the outcome. Time-varying and -invariant confounders were included as predictors for models estimating denominator values. Time-varying confounders were added to the denominator models in two sets: time-varying socio-demographics followed by health behaviours. To obtain narrower confidence intervals, we stabilized the weights by including time-invariant variables in models estimating the numerator (Hernán et al., 2000). To reduce the influence of outliers, stabilized inverse probability weights (SIPWs) were truncated at the 99th percentile. Further details regarding construction of time- and person-specific SIPWs can be found elsewhere (Robins et al., 2000, Hernán et al., 2000). See Web Table 1 for the distribution of the original and truncated mean values of SIPW including and excluding health behaviors.
We conducted two sensitivity analyses related to PTSD status and severity. First, we further adjusted for self-reported history of depressive symptoms as reported in 2001 through direct inclusion in the MSM using closest following interview in cases where 2001 values are missing. Second, we repeated the primary analyses excluding from the MSM individuals who indicated an illness was their worst trauma.
MSMs were also used to estimate the effect of each additional year of PTSD duration (in increments of 5 years) on CVD incidence among women ever reporting at least one PTSD symptom. Initial models adjusted only for demographic factors, childhood SES, and family history of CVD. To assess whether duration might be independent of effects of severity, a separate set of models additionally adjusted for PTSD symptom severity (mild, moderate, or severe symptom levels). Lastly, possible effect modification of the association between duration of PTSD symptoms and CVD by PTSD severity level was tested by including the appropriate interaction terms in the models. All analyses were replicated using conventional models. We accounted for the non-independence of the outcome in the variance of our estimate by implementing the marginal structural pooled logistic regression models using the SAS command proc genmod with an option specifying repeated measures on individuals, which corrects the variance estimates based on the correlation between repeated measures. As recommended for models implementing inverse probability weights, we use an independent working correlation structure (Tchetgen Tchetgen et al., 2012).
RESULTS
Participants were a mean age of 34.75 years at baseline (Table 1). There were 552 incident cases of CVD (276 MI and 276 stroke events) during follow-up. Participant characteristics by trauma exposure and PTSD symptom status at 1989 baseline assessment are in Web Table 2.
Table 1.
Participant characteristics at the NHS II 1989 baseline assessment (N=49,859 women)
| Characteristic | n or mean | % or SD |
|---|---|---|
| Trauma/PTSD exposure | ||
| No trauma | 15,316 | 30.72 |
| Trauma/no PTSD | 25,085 | 50.31 |
| Trauma/mild PTSD | 3,120 | 6.26 |
| Trauma/moderate PTSD | 2,303 | 4.62 |
| Trauma/severe PTSD | 1,682 | 3.37 |
| Remitted mild PTSD | 1,606 | 3.22 |
| Remitted moderate or severe PTSD | 747 | 1.5 |
| Age in 1989 (years, mean) | 34.75 | 4.64 |
| Maternal history of MI | 5,493 | 11.02 |
| Paternal history of MI | 14,734 | 29.55 |
| Maternal history of stroke | 4,410 | 8.84 |
| Paternal history of stroke | 6,520 | 13.08 |
| Neighborhood median family income ($, mean) | 61,120 | 22,559 |
| Parents’ education at birth, ≥ college | 11,427 | 22.92 |
| Missing | 2,207 | 4.43 |
| Parents own a home at birth | 25,611 | 51.37 |
| Missing | 2,388 | 4.79 |
| Race | ||
| Non-Hispanic white | 46,783 | 93.8306 |
| Minority | 2,441 | 4.89581 |
| Missing | 635 | 1.27359 |
| Alcohol intake, (grams/day, mean) | 3 | 5.47 |
| Physical activity | ||
| <3 MET hrs/wk | 7,124 | 14.29 |
| 3–8.9 MET hrs/wk | 11,398 | 22.86 |
| 9–17.9 MET hrs/wk | 10,606 | 21.27 |
| 18–26.9 MET hrs/wk | 6,712 | 13.46 |
| 27+ MET hrs/wk | 13,879 | 27.84 |
| Missing | 140 | 0.28 |
| Worst diet (1st quintile) on the Alternative Healthy Eating Index* |
9,198 | 18.45 |
| Missing | 217 | 0.44 |
| Cigarette smoking | ||
| Never | 33,264 | 66.71 |
| Former | 10,880 | 21.82 |
| Current, 1–14 | 2,262 | 4.54 |
| Current, 15–24 | 2,257 | 4.53 |
| Current, 25+ | 1,031 | 2.07 |
| Missing | 165 | 0.33 |
Abbreviations: %=percent; $=US dollar; MET hrs/wk=metabolic equivalent hours per week; MI=myocardial infarction; NHS II=Nurses’ Health Study II; SD=standard deviation
First assessed in 1991.
In marginal structural models with women unexposed to trauma as the reference group, women with trauma/no PTSD (odds ratio (OR)=1.30; 95% CI: 1.03, 1.65) or trauma/severe symptoms (OR=1.69; 95% CI: 1.08, 2.63) had elevated odds of CVD (Table 2). Odds of CVD decreased when PTSD symptoms remitted: neither remitted mild nor remitted moderate/severe symptoms were significantly associated with CVD incidence. Moreover, when compared to remitted moderate/severe symptoms, trauma/severe symptoms were associated with significantly elevated odds of CVD (OR=1.95; 95% CI: 1.05, 3.63). Adjusting for lifetime depression in 2001 only slightly attenuated the hazard ratio for women with trauma and moderate or severe PTSD symptoms. Analyses further adjusting for health behaviors as possible time-varying confounders found that only trauma/severe PTSD was significantly associated with elevated odds of CVD onset (OR=2.28; 95% CI: 1.30, 4.02) compared to women without trauma exposure. Results from conventional models, without SIPW weighting were qualitatively similar (Web Tables 3) with the exception that trauma/no PTSD remained associated with elevated risk of CVD in models adjusting for health behaviors (OR=1.36; 95% CI: 1.08, 1.72).
Table 2.
Marginal structural models evaluating odds ratios for the association of PTSD severity and remission with incident CVD
| Model 1* | Model 2** | |||||||
|---|---|---|---|---|---|---|---|---|
| Obs (cases) |
OR | 95% CI | Obs (cases) |
OR | 95% CI | |||
| No trauma (ref) | 125,283 (100) |
1.00 | (Reference) | 109,945 (95) |
1.00 | (Reference) | ||
| Trauma no PTSD | 249,370 (295) |
1.30 | 1.03 | 1.65 | 224,174 (291) |
1.31 | 0.97 | 1.78 |
| Mild ongoing PTSD symptoms |
35,471 (40) |
1.10 | 0.75 | 1.62 | 32,342 (40) |
1.01 | 0.64 | 1.60 |
| Moderate ongoing PTSD symptoms |
27,485 (39) |
1.41 | 0.95 | 2.09 | 25,145 (38) |
1.21 | 0.74 | 1.97 |
| Severe ongoing PTSD symptoms |
19,631 (35) |
1.69 | 1.08 | 2.63 | 17,932 (35) |
2.28 | 1.30 | 4.02 |
| Remitted mild PTSD symptoms |
25,024 (19) |
0.70 | 0.41 | 1.21 | 23,418 (19) |
0.70 | 0.39 | 1.25 |
| Remitted moderate/severe PTSD symptoms |
14,880 (24) |
0.86 | 0.51 | 1.46 | 14,114 (24) |
1.07 | 0.55 | 2.08 |
Abbreviations: CI=confidence interval; CVD=cardiovascular disease;Obs=observations; OR=odds ratio; PTSD=posttraumatic stress disorder
Women contributed an observation each time they completed a questionnaire cycle; 49,859 women contributed these 497,144 observations. Models were adjusted for the following confounders: baseline age, race/ethnicity (minority vs not minority status), parental education, home ownership, maternal history of MI or stroke, paternal history of MI or stroke, age, and neighborhood income.
Model 2 included 49,826 women who contributed 447,070 observations over the follow up period and adjusted for all potential confounders present in Model 1 as well as physical activity, alcohol, smoking, and diet
In an MSM excluding 2,850 women who reported an illness as their worst trauma, women with trauma exposure but no PTSD symptoms had somewhat elevated odds of CVD (OR=1.29; 95% CI: 0.97, 1.71), although the OR did not reach statistical significance. Compared to women without trauma or PTSD symptoms, neither women with remitted mild PTSD symptoms (OR=0.90; 95% CI: 0.50, 1.60) nor women with remitted moderate or severe PTSD symptoms (OR=1.23; 95% CI: 0.72, 2.10) had significantly elevated odds of CVD. In contrast, compared to women without trauma or PTSD, women with moderate (OR=1.88; 95% CI: 1.22, 2.90) and severe PTSD symptoms (OR= 2.15; 95% CI: 1.34, 3.45) showed elevated odds of developing CVD even in analyses excluding women who reported an illness as their worst trauma.
Among women whose PTSD symptoms lasted at least one month, the mean duration was 17.5 years (standard deviation (SD)=16.1 years) (Web Table 4) at the time of censoring. As expected, severe PTSD symptoms had the longest duration (mean=21.2 years; SD=16.7 years), followed by moderate (mean=18.1 years; SD=16.2 years) and mild symptoms (mean=15.6 years; SD=15.6 years). In unadjusted conventional models, each additional 5 years of PTSD symptom duration was marginally associated with elevated odds of CVD (OR=1.06; 95% CI: 1.00, 1.12), but the association was attenuated after adjusting for time-invariant and time-varying confounders (Table 3). In contrast, in MSMs, each additional 5 years of PTSD symptoms was associated with 9% increased odds of CVD (OR=1.09; 95% CI: 1.02, 1.16) in adjusted models, and the odds remained significant after controlling for PTSD severity (OR=1.08; 95% CI: 1.01, 1.15). There was no evidence of effect modification by PTSD symptom severity in either type of model.
Table 3.
Conventional and marginal structural models estimating odds ratios for the association of PTSD duration (in 5 year increments) with incident CVD
| Conventional model |
Marginal structural model |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Duration (in 5 year increments) of at least 1 PTSD symptoms |
1.02 | 0.97 | 1.07 | 1.09 | 1.02 | 1.16 |
| Duration (in 5 year increments) of at least 1 PTSD symptoms further adjusting for PTSD severity |
1.01 | 0.96 | 1.06 | 1.08 | 1.01 | 1.15 |
Abbreviations: CI=confidence interval; CVD=cardiovascular disease; OR=odds ratio; PTSD=posttraumatic stress disorder.
Note: These analyses include 15,824 women who were trauma exposed and contributed 122,491 observations. All models adjusted for the following confounders: baseline age, race/ethnicity (minority vs not minority status), parental education, home ownership, maternal history of MI or stroke, paternal history of MI or stroke, age, and neighborhood income. PTSD severity was categorized as mild, moderate, or severe.
DISCUSSION
In a large longitudinal study of women with up to 17 years of follow-up adjusted MSMs showed that trauma exposure not resulting in PTSD symptoms was associated with approximately a 30% increase in odds of CVD incidence. Trauma exposure that resulted in severe PTSD symptoms was associated with an almost 70% increase in odds of CVD. The increase in odds of CVD associated with severe ongoing symptoms was attenuated among women whose PTSD symptoms had remitted. The association between PTSD symptoms and CVD risk was even stronger when excluding women with illness as their worst trauma; risk in odds of CVD was doubled among women with trauma and severe PTSD symptoms and increased by 88% in women with trauma and moderate levels of PTSD symptoms. The link between trauma exposure and CVD risk is consistent with prior studies in civilian (Sumner et al., 2015, Kubzansky et al., 2009, Edmondson et al., 2013) and military populations (Edmondson et al., 2013). This study is one of the first to evaluate effects of both PTSD symptom and duration. While it is perhaps impossible to fully disentangle severity from duration, we considered these as separate features of the PTSD experience. In more rigorous MSM models taking account of potential time-varying confounding and exploring the effect of PTSD symptom duration among women ever experiencing PTSD symptoms, every additional five years of experiencing symptoms was associated with an 8% increased risk of CVD controlling for PTSD severity.
Overall our findings consistently show that more severe PTSD symptoms are associated with increased risk of CVD and that this risk is no longer significant when symptoms remit. Individuals with severe ongoing PTSD symptoms may have dysregulated biological responses or engage in unhealthy coping strategies (D’Andrea et al., 2011) that generate negative health outcomes. Additionally, individuals previously exposed to trauma who do not develop PTSD may be experiencing posttraumatic psychopathology other than PTSD which might also lead to dysregulated biological or behavioral responses that increase risk of CVD. We hypothesize that individuals whose PTSD symptoms remit have adapted healthier coping mechanisms than trauma-exposed individuals who do not develop PTSD symptoms or who develop severe PTSD symptoms, reducing the risk of negative cardiovascular outcomes. The lack of an association between remitted PTSD symptoms and CVD risk is particularly intriguing since a recent meta-analysis found that in 44% of individuals with PTSD, symptoms eventually remitted (Morina et al., 2014). These findings have exciting implications if replicated, suggesting if PTSD symptoms can be effectively treated, the apparent cardiotoxic sequelae may be mitigated. Ideally, such effects would be tested with studies designed more explicitly to look at effects of PTSD treatment over the long-term, perhaps using biomarkers or conditions linked with cardiovascular risk to expedite research.
To our knowledge, this is the first study to implement inverse probability weights to examine the association between PTSD and CVD, permitting the most refined possible adjustment for time-varying confounders such as income and health behaviors when using observational data. With one exception (i.e., estimates of remitted moderate/severe symptoms), effect estimates produced by the MSM models were similar to those from the standard regression model. The association between PTSD symptoms and incident CVD risk was not explained by lifetime depression and was strengthened when excluding women reporting an illness as their worst trauma. Prior work in this cohort suggests depression is neither a strong confounder nor an effect modifier (Sumner et al., 2015).
Several studies have demonstrated a link between PTSD and cardiotoxic behaviors such as smoking (Fu et al., 2007), drinking (Brown and Wolfe, 1994), and diet (Hall et al., 2015), but prior work rarely suggests that such behaviors fully explain the associations (Sumner et al., 2015). Physiological alterations that occur with PTSD symptoms are hypothesized to play an important role in the link between PTSD and CVD. Such alterations include dysregulation of the hypothalamic pituitary adrenal (HPA) axis, which may lead to inflammation (Boscarino, 2011) and hypertension (O'Toole and Catts, 2008, Coughlin, 2011). Elevated levels of circulating catecholamines also occur and can increase platelet aggregation (Coughlin, 2011). Changes in the frequency and levels of neuropeptide Y in response to stress associated with PTSD have also been identified as contributing to the risk of developing metabolic syndrome or obesity, both risk factors for CVD (Levine et al., 2014). Future research examining the association between PTSD and relevant biological processes could help elucidate the mechanisms linking PTSD to CVD risk.
This study has several limitations. Information regarding trauma and PTSD symptoms was retrospectively collected, which could lead to measurement error and possible survivor bias. Additionally, PTSD symptoms were captured through a screener that did not include all PTSD symptoms and could not capture change in symptoms over time. Information regarding health behaviors was only collected consistently after 1989; thus we could not model whether health behaviors predicted PTSD onset prior to 1989. Absent life course data, we cannot definitively disentangle whether PTSD precedes or follows initiation (and maintenance) of health behaviors. However, prior work suggests that PTSD precedes uptake of unhealthy behavior (Zen et al., 2012, Yehuda and LeDoux, 2007, Hall et al., 2015) and more rapid weight gain (Kubzansky et al., 2014). Thus, it is unlikely that health behaviors are purely confounders; they are more likely to be on the causal pathway (or perhaps not strongly relevant). Given the observational nature of the data, the possibility of unmeasured confounding still remains, and generalizability may be limited given the sample is comprised primarily of white women. However, a number of strengths are also noteworthy. Data are from a large, population-based sample of civilian women with repeated measures of a wide range of potential confounders. These data make it possible to estimate MSMs to appropriately account for time-invariant and time-varying confounders. Additionally, CVD cases were confirmed by medical records or national death index searches, reducing concerns about potential self-report or recall bias.
Findings from this study suggest that not only severity but also duration and remission of PTSD may contribute to risk of CVD, even among what is likely to be an unusually healthy population of women with PTSD (given that they are all able to maintain a professional career). It is encouraging to find that this risk may be alleviated if PTSD symptoms remit. Future research should examine if the reduction in risk differs if the PTSD symptoms were treated or if they naturally resolved. Additionally, research should examine whether effective treatment of PTSD can reduce the adverse physical health effects. If so, treatments should be provided shortly after PTSD symptom onset to limit CVD (and potentially other disease-related) risk. Future research identifying possible mediating pathways linking trauma, PTSD symptoms, and CVD may also provide insight into whether and how cardiotoxic biological processes associated with PTSD onset and severity may be reversed or mitigated.
Supplementary Material
Acknowledgments
Funding: This study was supported by the National Institutes of Health grants R01MH078928 (to KCK), R01MH101269-01A1 (to KCK, LDK, AW), T32MH017119 (to PG), K01HL130650 (to JAS), and UM1CA176726 (for NHS II infrastructure), as well as the Yerby Postdoctoral Fellowship Program (to PG).
Footnotes
Conflict of interest: None.
References
- Adebamowo SN, Spiegelman D, Willett WC, Rexrode KM. Association between intakes of magnesium, potassium, and calcium and risk of stroke: 2 cohorts of US women and updated meta-analyses. The American Journal of Clinical Nutrition. 2015;101:1269–1277. doi: 10.3945/ajcn.114.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, D.C.: Author; 1994. [Google Scholar]
- Beristianos MH, Yaffe K, Cohen B, Byers AL. PTSD and risk of incident cardiovascular disease in aging veterans. The American Journal of Geriatric Psychiatry. 24:192–200. doi: 10.1016/j.jagp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Post-Traumatic Stress Disorder and cardiovascular disease link: Time to identify specific pathways and interventions. The American Journal of Cardiology. 2011;108:1052–1053. doi: 10.1016/j.amjcard.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson E, Kessler R, Schultz L. Short screening scale for DSM-IV posttruamatic stress disorder. American Journal of Psychiatry. 1999;156:908–911. doi: 10.1176/ajp.156.6.908. [DOI] [PubMed] [Google Scholar]
- Brewin C, Andrews B, Valentine J. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:317–336. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Wolfe J. Substance abuse and post-traumatic stress disorder comorbidity. Drug and Alcohol Dependence. 1994;35:51–59. doi: 10.1016/0376-8716(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Burns DM. Epidemiology of smoking-induced cardiovascular disease. Progress in Cardiovascular Diseases. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, Mccullough ML, Wang M, Stampfer MJ, Willett WC. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. The Journal of Nutrition. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SS. Post-traumatic stress disorder and cardiovascular disease. Open Cardiovascular Medicine Journal. 2011;5:164–170. doi: 10.2174/1874192401105010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Statistics in Medicine. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- D’andrea W, Sharma R, Zelechoski AD, Spinazzola J. Physical health problems after single trauma exposure: When stress takes root in the body. Journal of the American Psychiatric Nurses Association. 2011;17:378–392. doi: 10.1177/1078390311425187. [DOI] [PubMed] [Google Scholar]
- Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Progress in Cardiovascular Diseases. 2013;55:548–556. doi: 10.1016/j.pcad.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: A meta-analytic review. American Heart Journal. 2013;166:806–814. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro P, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC. History of kidney stones and the risk of coronary heart disease. Journal of American Medical Association. 2013;310:408–415. doi: 10.1001/jama.2013.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SS, Mcfall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, Joseph AM. Post-traumatic stress disorder and smoking: A systematic review. Nicotine & Tobacco Research. 2007;9:1071–1084. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- Graham-Garcia J, Raines TL, Andrews JO, Mensah GA. Race, ethnicity, and geography: Disparities in heart disease in women of color. Journal of Transcultural Nursing. 2001;12:56–67. doi: 10.1177/104365960101200108. [DOI] [PubMed] [Google Scholar]
- Hall KS, Hoerster KD, Yancy WS. Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiologic Reviews. 2015;37:103–115. doi: 10.1093/epirev/mxu011. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- Koenen K, De Vivo I, Rich-Edwards J, Smoller J, Wright R, Purcell S. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses' Health Study II. BMC Psychiatry. 2009;9:29. doi: 10.1186/1471-244X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen K, Moffitt T, Poulton R, Martin J, Caspi A. Early childhood factors associated with posttraumatic stress disorder: Results from a longitudinal birth cohort. Psychological Medicine. 2007;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Bordelois P, Jun H, Roberts AL, Cerda M, Bluestone N, Koenen KC. The weight of traumatic stress: A prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry. 2014;71:44–51. doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychology. 2009;28:125–130. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A, 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Archives of General Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- Lee YC, Agnew-Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, Roberts AL, Koenen KC, Karlson EW. Post-traumatic stress disorder and risk for incident rheumatoid arthritis. Arthritis Care & Research. 2016;68:292–298. doi: 10.1002/acr.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127:1–19. doi: 10.1159/000354910. [DOI] [PubMed] [Google Scholar]
- Lowe SR, Galea S, Uddin M, Koenen KC. Trajectories of posttraumatic stress among urban residents. American Journal of Community Psychology. 2014;53:159–172. doi: 10.1007/s10464-014-9634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: A systematic review and meta-analysis of long term outcome studies. Clinical Psychology Review. 2014;34:249–255. doi: 10.1016/j.cpr.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Nandi A, Glymour MM, Kawachi I, Vanderweele TJ. Using marginal structural models to estimate the direct effect of adverse childhood social conditions on onset of heart disease, diabetes, and stroke. Epidemiology. 2012;23:223–232. doi: 10.1097/EDE.0b013e31824570bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'toole BI, Catts SV. Trauma, PTSD, and physical health: An epidemiological study of Australian Vietnam veterans. Journal of Psychosomatic Research. 2008;64:33–40. doi: 10.1016/j.jpsychores.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Robins JM. Association, causation, and marginal structural models. Synthese. 1999;121:151–179. [Google Scholar]
- Robins JM, Hernán MÁ, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Rose G, Blackburn H. Cardiovascular survey methods. Geneva: World Health Organization; 2002. [PubMed] [Google Scholar]
- Scherrer JF, Chrusciel T, Zeringue A, Garfield LD, Hauptman PJ, Lustman PJ, Freedland KE, Carney RM, Bucholz KK, Owen R. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed Veterans Administration patients. American Heart Journal. 2010;159:772–779. doi: 10.1016/j.ahj.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biological Psychiatry. 2013;73:1103–1110. doi: 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Elkind MSV, Roberts AL, Agnew-Blais J, Chen Q, Cerdá M, Rexrode KM, Rich-Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchetgen Tchetgen EJ, Glymour MM, Weuve J, Robins J. Specifying the Correlation Structure in Inverse-Probability- Weighting Estimation for Repeated Measures. Epidemiology. 2012;23:644–646. doi: 10.1097/EDE.0b013e31825727b5. [DOI] [PubMed] [Google Scholar]
- Turner JH, Neylan TC, Schiller NB, Li Y, Cohen BE. Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biological Psychiatry. 2013;74:861–866. doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States, and Center for Chronic Disease Prevention and Health Promotion. The health benefits of smoking cessation: a report of the Surgeon General. Betheseda: US Public Health Service, Office on Smoking and Health; 1990. [Google Scholar]
- Van Der Velden PG, Kleber RJ, Koenen KC. Smoking predicts posttraumatic stress symptoms among rescue workers: a prospective study of ambulance personnel involved in the Enschede Fireworks Disaster. Drug and Alcohol Dependence. 2008;94:267–271. doi: 10.1016/j.drugalcdep.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12:I13–I44. [PubMed] [Google Scholar]
- Yehuda R, Ledoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zen AL, Zhao S, Whooley MA, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: Findings from the Heart and Soul Study. Health Psychology. 2012;31:194–201. doi: 10.1037/a0025989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.