Abstract
Rationale
Targeted genetic engineering using programmable nucleases such as transcription activator–like effector nucleases (TALENs) is a valuable tool for precise, site-specific genetic modification in the human genome.
Objective
The emergence of novel technologies such as human induced pluripotent stem cells (iPSCs) and nuclease-mediated genome editing represent a unique opportunity for studying cardiovascular diseases in vitro.
Methods and Results
By incorporating extensive literature and database searches, we designed a collection of TALEN constructs to knockout (KO) eighty-eight human genes that are associated with cardiomyopathies and congenital heart diseases. The TALEN pairs were designed to induce double-strand DNA break near the starting codon of each gene that either disrupted the start codon or introduced a frameshift mutation in the early coding region, ensuring faithful gene KO. We observed that all the constructs were active and disrupted the target locus at high frequencies. To illustrate the general utility of the TALEN-mediated KO technique, six individual genes (TNNT2, LMNA/C, TBX5, MYH7, ANKRD1, and NKX2.5) were knocked out with high efficiency and specificity in human iPSCs. By selectively targeting a dilated cardiomyopathy (DCM)-causing mutation (TNNT2 p.R173W) in patient-specific iPSC-derived cardiac myocytes (iPSC-CMs), we demonstrated that the KO strategy ameliorates the DCM phenotype in vitro. In addition, we modeled the Holt-Oram syndrome (HOS) in iPSC-CMs in vitro and uncovered novel pathways regulated by TBX5 in human cardiac myocyte development.
Conclusion
Collectively, our study illustrates the powerful combination of iPSCs and genome editing technology for understanding the biological function of genes and the pathological significance of genetic variants in human cardiovascular diseases. The methods, strategies, constructs and iPSC lines developed in this study provide a validated, readily available resource for cardiovascular research.
Keywords: Genome editing, iPSCs, gene knockout, dilated cardiomyopathy, Holt-Oram syndrome, stem cell, cardiac, gene targeting, disease modeling
Subject Terms: Basic Science Research, Cardiomyopathy, Congenital Heart Disease, Genetically Altered and Transgenic Models
INTRODUCTION
Cardiovascular disease is a major cause of morbidity and mortality around the world. In recent years, exciting progress has been made in defining the etiology of congenital heart disease (CHD)1 and inherited cardiomyopathies,2 including hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), left ventricular non-compaction (LVNC), and arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D). Recent advances in genomics and molecular medicine have identified genetic mutations in plethora of genes that are implicated in the pathogenesis of inherited cardiomyopathies. Although the molecular analysis efforts have revealed important insights regarding the role of genetics in cardiomyopathies, the underlying molecular mechanisms remain poorly understood and the genotype-phenotype relationship from the ever-growing number of disease-associated gene mutations remains to be established.
Recent advances in technologies for genome editing using site-specific nucleases,3 such as zinc finger nucleases (ZFNs), transcription activator–like effector nucleases (TALENs), and the clustered regularly interspaced short palindromic repeat/CRISPR-associated protein 9 (CRISPR/Cas9) system, offer a powerful tool for reverse genetics, genome engineering, and targeted transgene integration experiments to be performed in a precise and predictable manner. The use of engineered nucleases to make targeted, permanent changes to genes have revolutionized molecular genetics and present an alternative to the more established method of RNA interference (RNAi)-mediated knockdown using short hairpin RNA (shRNA) or short interfering RNA (siRNA). However, the RNAi-mediated post-transcriptional down-regulation of gene expression without changing the genetic code does not completely shut off the gene of interest.4 In most cases, functional RNA or protein remains and is translated albeit at lower levels. Thus, the gene function is reduced, but not eliminated. By contrast, genome editing changes the genetic code and typically causes a functional “knockout” (KO), or complete elimination of the gene function. The nucleases cut both DNA strands of the targeted locus generating a double-strand break (DSB) in the chromosome, which is then repaired by the non-homologous end joining (NHEJ) mechanism that re-ligates the two free chromosome ends. However, NHEJ is error-prone, often resulting in small insertions or deletions that can disrupt, or knockout, the gene of interest.
Over the past decade, the advent of the human induced pluripotent stem cell (iPSC) technology, and the improvements in the differentiation method of iPSCs into specific cell types, such as cardiac myocytes (iPSC-CMs)5, endothelial cells (iPSC-ECs),6 and smooth muscle cells (iPSC-SMCs),7 provide an unprecedented opportunity for the generation of patient-specific in vitro models for disease modeling. Combining genome editing and iPSC technologies can successfully create human-based cell knockout models in vitro. Such models could improve our understanding of the underlying pathological mechanisms, and potentially lead to novel therapies.8
In this study, we describe the design, construction, and validation of a cardiomyopathy TALEN-based (cTAL) panel to knock out a comprehensive set of genes associated with cardiovascular diseases. We demonstrated the utility of this panel, and presented two case studies that provided novel insights into the pathogenesis of genetic cardiovascular disease. The readily available cTAL panel will allow researchers to fast-track projects by providing a validated panel of TALEN constructs for gene KO genome editing. This approach could provide novel insights into gene function, disease mechanisms, and ultimately disease pathogenesis.
METHODS
TALEN construction
TALEN genomic binding sites were designed using the TAL Effector Nucleotide Targeter 2.0,9 with the following constraints: (i) having a repeat array length of 15 repeat variable di-residue domains, and (ii) having a spacer length of 14–18 nucleotides. A preceding T base in position “0” anchored each binding site as has been shown to be optimal for naturally occurring TAL proteins.10,11 Each custom TALEN was generated from a library of 832 plasmids through a five-piece subcloning ligation: three sequence-specific tetramer-recognition pieces, one trimer-recognition piece, and an expression vector backbone (pTAL) as previously described.12 Briefly, the tetramer or trimer TAL repeats were digested out of library plasmids with the restriction enzyme BsmBI (NEB), gel purified, and subcloned into the pTAL vectors. The forward and reverse TALENs were subcloned into the pTAL_GFP and pTAL_RFP backbones, respectively. The sequences of all constructs used in this study are provided in the Supplemental Information. The TALEN plasmids will be available from Addgene. The cell lines are available upon request from the Stanford CVI iPSC Biobank (http://med.stanford.edu/scvibiobank.html).
Culture and cardiac differentiation of iPSCs
The human iPSC lines (SCVI-15, SCVI-114, and SCVI-19) were obtained from the Stanford CVI iPSC Biobank. The iPSCs were maintained under feeder-free conditions in defined E8 media (Life Technologies) on tissue culture plates coated with hESC-qualified Matrigel (BD Biosciences) in 5% CO2/5% O2/90% N2 environment at 37°C. Human iPSCs were differentiated toward cardiac myocytes using a small molecule mediated directed differentiation protocol.13 Briefly, cardiac differentiation was initiated by treatment with recombinant BMP4 and Activin A (Day 0–3), followed by treatment with 5 μM IWR-1 for 72 hr (day 4 to day 6).
TALEN transfection
Human iPSCs were enzymatically dissociated with Accutase (Sigma) and plated on Matrigel coated dishes at 1:3 ratio in E8 supplemented with 10 um Y-27632 (Selleck Chemicals). 24 – 48 hr later, human iPSCs were dissociated with Accutase into single cells. ~2×106 cells were transfected with a pair of TALENs (1.0 μg of each TALEN) by nucleofection using the Amaxa 4D Nucleofector system (Lonza) with the P3 Primary Cell Nucleofector Kit and program CM-150 per manufacturer’s instructions (Lonza). Following nucleofection, iPSCs were re-suspended in 1 ml pre-warmed E8 supplemented with 5 μM Thiazovivin and then plated in 6-well plates pre-coated with Matrigel and allowed to recover for 48 hr.
SMRT sequencing
Genome-editing outcomes at the endogenous loci were quantified using single-molecule real-time (SMRT) DNA sequencing as previously described.14 Genomic DNA was extracted from TALEN-transfected iPSCs at 72 hr post-nucleofection without enrichment for transfected cells, and used as a template for PCR amplification using primer pairs designed to amplify a ~500 bp fragment surrounding the TALEN targeted loci. The PCR amplicons were purified using the nucleotide removal kit (Qiagen) and the sequencing libraries were constructed using the DNA Template Prep Kit 1.0 (Pacific Biosciences). SMRTbell libraries contained amplicons that were pooled together with different barcodes appended to allow multiplex analysis. Purified, closed circular SMRTbell libraries were annealed with a sequencing primer complementary to a portion of the single-stranded region of the hairpin. For all SMRTbell libraries, annealing was performed at a final template concentration between 30 and 60 nM, with a 20-fold molar excess of sequencing primer. All annealing reactions were carried out at 80°C for 2 min, with a slow cool to 25°C at a rate of 0.1°C/s. Annealed templates were stored at −20°C until polymerase binding. DNA polymerase enzymes were stably bound to the primed sites of the annealed SMRTbell templates using the DNA Polymerase Binding Kit 2.0 (Pacific Biosciences). SMRTbell templates (3 nM) were incubated with 6 nM of polymerase in the presence of phospholinked nucleotides at 30°C for 2 hr. Following incubation, samples were stored at 4°C. Sequencing was performed within 72 hr of binding using a final concentration of 0.3 nM. Each sample was sequenced using the DNA Sequencing Kit 2.0 (Pacific Biosciences). Sequencing data collection was performed on the PacBio RS (Pacific Biosciences) using C2/C2 chemistry and movies of 55 min in each case. The SMRT Sequencing Analysis pipeline was implemented in Strawberry Perl and utilizes the NCBI BLAST software as well as the mEmboss Needleman-Wunsch pairwise alignment algorithm.
Isolation of targeted clonal cell populations
TALEN-transfected iPSCs were washed once with PBS and enzymatically dissociated with Accutase for 3–5 min at 37°C followed by gently pipetting to ensure single cell suspension. The cells were washed once in PBS and re-suspended in E8 supplemented with Y-27632 (10 um). Double GFP+/RFP+ cells were then sorted by fluorescence activated cell sorter (FACSAria II; BD Biosciences), plated on 6-well plates at a clonal density of 1,000 cells/well and allowed to recover. After 7–10 days, putative single cell-derived clones were manually picked, expanded, and maintained in standard conditions.
RNA-sequencing
Total RNA was isolated with the RNeasy Isolation kit with on-column DNase I treatment (Qiagen), and the quality of the RNA samples was assessed using the Agilent Bioanalyzer 2100 (Agilent). ERCC spike-in synthetic transcripts were added at manufacturer’s recommended amounts (Life Technologies) and 1 μg of each RNA was enriched for poly-A RNA using the Dynabeads® mRNA Direct Kit (Life Technologies) per manufacturer’s protocol. Whole transcriptome library preparation was performed using 5–10 ng of fragmented enriched poly-A RNA according to the manufacturer’s protocol (Ion Total RNA-Seq Kit V2 protocol; Life Technologies), followed by purification with AMPure beads (Beckman-Coulter Genomics). The quality and quantity of the libraries was assessed using the Agilent Bioanalyzer High Sensitivity Chip (Agilent). Each library concentration was adjusted to 100 pM and 70 ul were used for Ion Template preparation in the automated Ion Chef system and loaded on the Ion PI Chip Kit v2 (Life Technologies). Sequencing was performed in the Ion Proton sequencing platform using the Ion PI™ Sequencing 200 Kit v3 per manufacturer’s protocol (Life Technologies). Base calls were collected with Ion Torrent Suite software (Life Technologies).
Allelic discrimination by digital PCR
Total RNA was extracted from iPSC-CMs at day 30 post-differentiation using RNeasy Mini Kit (Qiagen), and complementary DNA (cDNA) preparation was carried out using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). The concentration of cDNA was reduced to about 0.2 ng/μl RNA equivalent and 1 ng (5 μl of 0.2 ng/μl) of RNA-equivalent cDNA was mixed with primers, probes and ddPCR Supermix reaction (total volume 20 μl). The final concentrations of the primers and the probe were 900 nM and 500 nM, respectively. The following primers and probes were used for discriminating the expression of the R173W and the WT TNNT2 alleles; Fw: GAGGAGGAGAACAGGAG and Rv: GCATCATGTTGGACAAAGCC. wt-probe: [FAM]AGGATGAGGCCCGGAAGAAGA[BHQ] and mt-probe [HEX]AGGATGAGGCCTGGAAGAAGA[BHQ]. Droplet formation was carried out using a QX100 droplet generator. A rubber gasket is placed over the cartridge and loaded into the droplet generator. The emulsion (35 μL in volume) was then slowly transferred using a multichannel pipette to a 96-Well twintec™ PCR Plates (Eppendorf). The plate was heat-sealed with foil and the emulsion was cycled to end point per the manufacturer’s protocol with an annealing temperature at 61°C. Finally, the samples were analyzed using a BioRad QX100 reader. The expression of TNNT2 was quantified by Real-Time qPCR (Applied Biosystems) using a custom TaqMan probe designed to detect the wild type transcript after TALEN-mediated KO (Fw: AGACGCCTCCAGGATCTGT, Rv: GCTTCTTCCTGCTCCTCCTC, Probe: [FAM]CAGACATGGTCTCTGCTCTCCCTC[BHQ].
TALEN off-target detection
Genomic DNA was extracted from genome edited iPSC clones using the DNeasy Blood & Tissue Kit (Qiagen). The potential TALEN off-target sites were predicted in silico based on sequence homology using the bioinformatics tool PROGNOS.15 The primers designed by PROGNOS were used to amplify the genomic regions of putative off-target sites by PCR. The PCR products were analyzed by Sanger sequencing.
ChIP-seq analysis
The raw Fastq files of ChIP-seq were aligned to human genome (hg19) by TMAP (https://github.com/iontorrent/TS/tree/master/Analysis/TMAP), and then all duplicate reads aligned to same loci were removed.16 Peak calling was applied by HOMER,17 and the parameters are: style “factor”, genome “hg19”, fold-change cutoff 4.0 of DNA input, fold-change cutoff of peak calling 2.0, and p-value cutoff 0.0001. Peaks were annotated by HOMER, and the nearest genes were assigned as the genes of the peaks. All sequences around coding promoters (upstream 400 bp, downstream 100 bp) were extracted and motif enrichment analysis was performed using HOMER. Then KEGG enrichment analysis was performed using the GeneAnswers package (http://www.bioconductor.org/packages/release/bioc/html/GeneAnswers.html), and adjusted p-value cutoff was 0.1. All alignment bam files were processed by IGVTools, and loaded to IGV genome browser18 for the visualization of specific genes, all tracks normalized to 1 million reads.
Whole-cell patch-clamp recordings
Contracting monolayer iPSC-CMs were enzymatically dispersed (Accutase, Sigma) and attached to Matrigel-coated glass coverslips (Warner, USA) for whole-cell patch clamp recordings. These recordings were conducted using an EPC-10 patch clamp amplifier (HEKA, Germany). 3–4 MΩ glass pipettes were prepared using thin-wall borosilicate glass (A-M System, USA) with a micropipette puller (Sutter Instrument, P-97, USA). Action potentials (APs) were recorded from iPSC-CMs suffused with Tyrode’s solution at 37°C. The Tyrode’s solution consisted of NaCl (140 mM), KCl (5.4 mM), CaCl2 (1.8 mM), MgCl2 (1 mM), HEPES (10 mM), and glucose (10 mM); pH was adjusted to 7.4 with NaOH. The pipette solution consisted of KCl (120 mM), MgCl2 (1 mM), Mg-ATP (3 mM), HEPES (10 mM), and EGTA (10 mM); pH was adjusted to 7.2 with KOH. Data were acquired using PatchMaster software (HEKA, Germany) and digitized at 1.0 kHz. Data were analyzed using a custom-written MATLAB program.
Statistical analysis
Unpaired two-tailed Student’s t tests were used to determine the significance between two groups, assuming significance at P < 0.05. The one-way analysis of variance (ANOVA) was used to determine whether there are any statistically significant differences among the means of three or more groups, with P < 0.05 considered statistically significant. All values are expressed as the mean ± SEM.
RESULTS
Design, construction, and characterization of TALEN constructs
We selected 88 genes associated with cardiomyopathies and congenital heart diseases (Figure 1a and Online Table I), including genes implicated in syndromes for which clinical diagnosis may be challenging, such as CHARGE syndrome (chromodomain helicase DNA binding protein 7 (CHD7) mutation), Leigh syndrome (SURF1 mutations), Holt-Oram syndrome (TBX5 mutations), Noonan syndrome, LEOPARD syndrome, Raf-1 proto-oncogene, serine/threonine kinase (RAF1) and protein tyrosine phosphatase, non-receptor type 11 (PTPN11) mutations. To knock out these genes in the human genome, we designed TALENs that target sequences located around the start codon, ATG, of each gene (Figure 1b). We constructed one TALEN pair construct for each gene using a library of pre-assembled tetramers/trimers through a five-piece subcloning ligation.12 The details of the TALEN design for each gene and the respective target site are shown in Online Table I. To validate the genome editing activities of the TALEN library in human iPSCs, we quantified the level of NHEJ using the SMRT technology.14 Every TALEN pair tested was active and efficiently induced small deletions, insertions, or both at the target sites (Online Table II). The individual TALEN pairs induced mutations with a frequency ranging from 0.5% to 50% (Figure 1c), and the majority of the TALEN-mediated NHEJ outcomes were deletions of variable lengths within the spacer region, while insertion mutations were only observed in a few instances (Online Table II).
Figure 1. The cTAL-KO panel.
a) Genes associated with cardiomyopathies and congenital heart diseases included in the panel. b) Schematic representation of the gene KO strategy. c) Frequency distribution of the TALEN-mediated mutagenesis in human iPSCs as assessed by single-molecule real-time (SMRT) technology. The DNA fragments surrounding the TALEN target site was amplified and sequenced by PacBio RS as described in the “Materials and Methods” section. The mutation frequency of each TALEN pair was calculated as follows: mutation frequency (%) = number of reads containing a different length of deletion mutations/total number of reads harboring deletion mutation in the target locus × 100. HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; LVNC, left ventricular non-compaction; ARVD, arrhythmogenic right ventricular dysplasia; RC, restrictive cardiomyopathy.
To illustrate the general utility of the TALEN-mediated NHEJ technique, we next targeted six individual genes (TNNT2, TBX5, lamin A/C (LMNA/C), myosin, heavy chain 7, cardiac muscle, beta (MYH7), ankyrin repeat domain 1 (cardiac muscle) (ANKRD1), and NK2 homeobox 5 (NKX2.5)) in human iPSCs. After TALEN transfection and FACS sorting, we screened single cell-derived clones for NHEJ events. We observed that the targeted loci were disrupted at high efficiency, with indels occurring in 33% to 100% of the clones screened (Table 1). These results indicate that all of our TALEN constructs are highly active and can be used for gene KO experiments.
Table 1.
Efficiency of TALEN-Mediated Gene KO in iPSCs
| Targeted Gene | NHEJ (%) | Clones Screened | MutantsClones | Efficiency (%) |
|---|---|---|---|---|
| TNNT2 | 13.1 | 22 | 11 | 50.0 |
| LMNA | 12.5 | 12 | 8 | 66.7 |
| MYH7 | 50.2 | 24 | 24 | 100 |
| ANKRD1 | 6.7 | 24 | 11 | 45.8 |
| TBX5 | 48.5 | 32 | 26 | 81.3 |
| NKX2.5 | 9.4 | 26 | 20 | 76.9 |
Targeted disruption of the cardiac troponin T gene causes sarcomere disassembly
Mutations associated with cardiomyopathies are commonly inherited in an autosomal dominant manner. Mutant proteins are thought to act through a dominant-negative mode that either interfere with normal function or assume a new function. In some instances, the mutant allele is inactivated, resulting in haploinsufficiency whereby a single functional copy of the gene is insufficient to maintain the normal phenotype. Although mutations in the cardiac troponin T (TNNT2) gene are commonly implicated in familial HCM, distinct mutations can also lead to DCM.19 To address whether haploinsufficiency of TNNT2 is responsible for HCM or DCM, we ablated either one or both TNNT2 alleles in human iPSCs by TALEN-mediated gene KO in a single round of TALEN targeting. We generated both monoallelic (heterozygous) KO (TNNT2+/−) and biallelic (homozygous) KO (TNNT2−/−) iPSC lines (Figure 2a). These TNNT2-KO iPSC lines retained their pluripotency as assessed by immunostainining and gene expression assays of pluripotency markers (Online Figure I). Upon differentiation, the cardiac Troponin T protein (cTnT) was not detected in TNNT2−/− iPSC-CMs, while comparable levels of cTnT were observed in wild-type and TNNT2+/− iPSC-CMs (Figure 2b). At the mRNA level, TNNT2+/− iPSC-CMs had reduced expression of the non-targeted transcript compared to the parental iPSC-CMs (Figure 2c), suggesting that the cTnT protein levels are not regulated at the transcription level. Most likely a post-transcriptional mechanism, such as an increase in ribosome translational kinetics or lower protein turnover rates, is responsible for the comparable levels of cTnT protein expression in the TNNT2+/− and WT iPSC-CMs. At the functional level, we observed that TNNT2−/− iPSC-CMs displayed severe sarcomeric disarray (Figure 2d) and exhibited impaired intracellular Ca2+ cycling (Online Figure II). In contrast, TNNT2+/− iPSC-CMs showed no functional or structural abnormalities, suggesting that one TNNT2 allele is sufficient to maintain normal cTnT protein expression and cardiac myocyte structure and function (Figure 2 and Online Figure II). These results suggest that haploinsufficiency is unlikely to explain the pathogenesis of cardiomyopathies associated with TNNT2 mutations.
Figure 2. Generation of TNNT2 knockout iPSC clones.
a) Schematic representation of TNNT2 gene structure. TALENs were designed to target the translation initiation site (ATG) at exon 2 of TNNT2 gene. Boxes indicate the TALEN binding sites. Deletions in the two alleles of each clone are indicated. b) Expression of cardiac troponin-T protein in isogenic wild-type (WT), heterozygous (TNNT2+/−), and homozygous (TNNT2−/−) knockout iPSC-CMs. Representative blots of the protein expression and densitometric analysis of TNNT2 protein expression levels normalized to α-sarcomeric actinin (ACTN2) in isogenic iPSC-CMs as indicated. Data represent mean ± SEM of three independent differentiation experiments, * P < 0.05. c) mRNA expression of the WT allele in the TNNT2+/− and WT iPSC lines. A qPCR probe was designed to distinguish between the non-edited (WT) and the TALEN-mutated mRNA of the TNNT2+/− iPSC-CMs. Gene expression levels were normalized to cardiac specific gene ACTN2. Data represent mean ± SEM of three independent differentiation experiments, *P < 0.05. d) Representative immunofluorescence images of iPSC-CMs stained for the cardiac myocyte-specific markers cardiac troponin-T (TNNT2, red) and α-sarcomeric actinin (ACTN2, green). DNA was counterstained with DAPI (blue). Scale bar = 20 μm. All the assays were performed at 30 days post-differentiation with one isogenic pair.
Phenotypic rescue of DCM by targeted allelic-specific KO in vitro
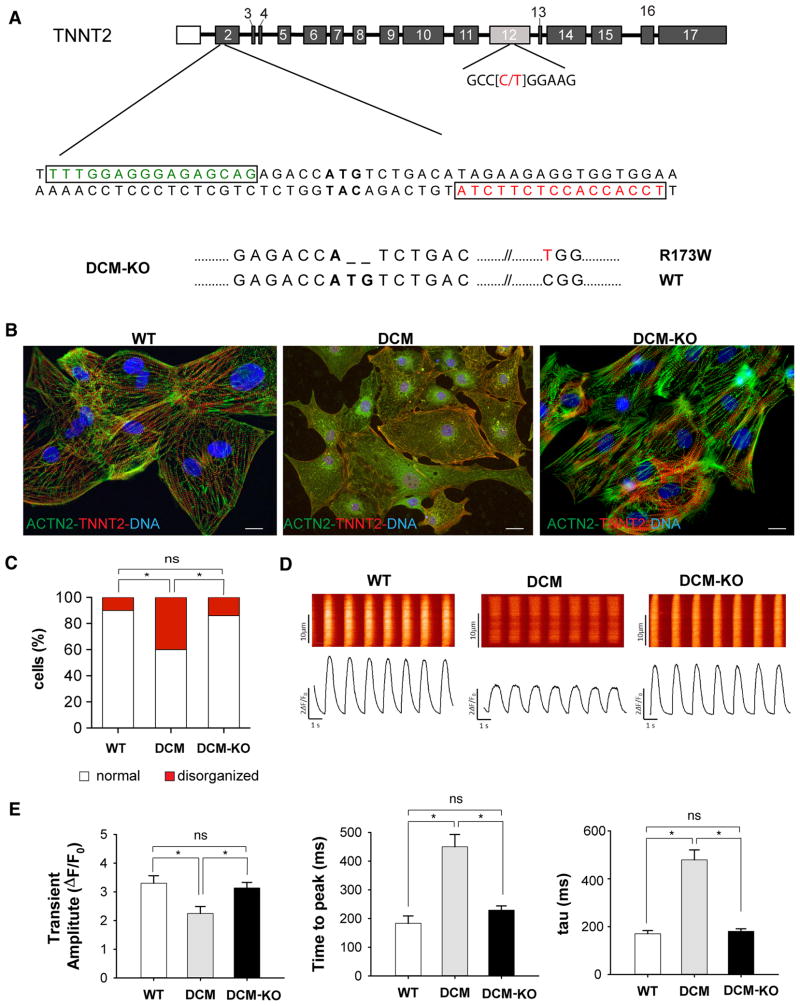
To test this hypothesis, we next disrupted the starting codon of TNNT2 gene in a patient-specific iPSC line harboring a missense mutation in exon 12 of the TNNT2 gene (NM_001001430.2: c.517 C>T; p.R173W) (Figure 3a).20 We screened the TALEN-targeted clones for NHEJ events, and identified an iPSC clone with a disruption of the starting codon of the mutant TNNT2 p.R173W allele (hereafter referred to as DCM-KO) and without any detectable off-target mutations (Figure 3a and Online Table III). This isogenic KO line retained pluripotency as assessed by both immunostaining and gene expression assays of pluripotency markers (Online Figure III). We differentiated the isogenic iPSC lines to iPSC-CMs and observed that the DCM-KO iPSC-CMs had undetectable (<10%) mRNA expression of the mutant TNNT2 allele when compared to the parental line, consistent with the activation of the nonsense-mediated mRNA decay mechanism following the NHEJ repair process (Online Figure IV).21 In addition, we observed that the loss of the mutant allele ameliorated the DCM phenotype in vitro, including sarcomere disarray (Figure 3b–c) and Ca+2 cycling parameters (Figure 3d–e). Taken together, these data suggest that the TNNT2 p.R173W is a dominant negative mutation, and allelic-specific KO could ameliorate the DCM phenotype in vitro.
Figure 3. TNNT2 R173W is a dominant, causal DCM mutation.
a) Generation of allelic-specific TNNT2 knockout iPSC clones. TALENs were designed to target the translation initiation site (ATG) at exon 2 of TNNT2 gene. Boxes indicate the TALEN binding sites. The nucleotide in red indicates the missense mutation for R173W. A deletion in the TNNT2 allele (R173W) allele is indicated. b) Representative immunofluorescence images of iPSC-CMs stained for the cardiac myocyte-specific markers cardiac troponin-T (TNNT2, red) and α-sarcomeric actinin (ACTN2, green). DNA was counterstained with DAPI (blue). Scale bar = 20 μm. c) Quantification of disorganized sarcomeric staining pattern in WT, isogenic DCM, and DCM-KO iPSC-CMs. Data represent mean ± SEM (n=150 iPSC-CMs per iPSC line), *P < 0.05. d) Representative Ca2+ transients of iPSC-CMs as indicated. e) Quantification of calcium handling parameters in WT, isogenic DCM, and DCM-KO iPSC-CMs. Data represent mean ± SEM (n=30 iPSC-CMs per line), *P < 0.05. All the assays were performed at 30 days post-differentiation with one isogenic pair.
Modeling Holt-Oram syndrome in vitro
Cardiac development is a critical and complex embryologic process requiring the integration of cell commitment, growth, looping, septation, and chamber specification.22 Multiple transcription factors, including NKX2.5, GATA4, and TBX5 play important roles in cardiac development, and genetic studies have implicated dominant mutations in these genes in human CHD. TBX5 is a T-box-containing transcription factor, which like other T-box family members, has been implicated in vertebrate tissue patterning and differentiation.23–25 TBX5 represents one of the few genes which, when mutated, is known to cause CHD.23 TBX5 haploinsufficiency is associated with Holt-Oram syndrome (HOS), a congenital disorder characterized by structural cardiac and limb abnormalities.26 Tbx5 heterozygous null (Tbx5−/+) mice recapitulated the CHD seen in HOS patients, whereas homozygous null mice (Tbx5−/−) are growth arrested at E9.0 and die in utero by E10.5 due to severe heart defects.26 Although the expression of many genes such as NPPA, GJA5, IRX4, MYL2, GATA4, NKX2.5, and HEY2 was reduced in TBX5-null hearts,26 little is known about their downstream targets and hence the molecular basis of HOS is poorly understood.
As a proof-of-principle experiment for creating CHD models, we generated a human cell-based HOS in vitro model by utilizing TALEN-mediated NHEJ to knockout the TBX5 gene in iPSCs. In humans, TBX5 is highly regulated through alternative splicing and several transcript variants encoding different isoforms have been described for TBX5. Based on RNA-seq data of iPSC-CMs, the transcript variant 4 (NM_181486) is the predominant TBX5 isoform that is expressed in iPSC-CMs. Of note, the presence of this transcript was also reported in the initial identification of TBX5 as the HOS gene.27 The isoforms 1 (NM_000192) and 3 (NM_080717) were also detected in iPSC-CMs, albeit at very low levels (Online Figure V). Hence, we designed a TALEN pair and targeted the starting codon at exon 1 of the major isoform 4 and isoform 1 (Figure 4a). We identified an iPSC clone carrying a homozygous deletion, which resulted in frameshift mutations and an early termination of the TBX5 gene (hereafter referred to as TBX5-KO) (Figure 4b). The isogenic TBX5-KO iPSCs retained their pluripotency as assessed by immunostaining and gene expression assays of pluripotency markers (Online Figure VI). In order to check the specificity, we assessed potential off-target cutting sites in the edited clones using in silico prediction algorithms and did not detect any mutations in the 25 most likely off-target sites, suggesting a high specificity of the TBX5 TALEN pair (Online Table IV). We then differentiated the isogenic iPSC clones into iPSC-CMs and confirmed that the TBX5 (isoforms 1 and 4) was not expressed at the protein level (Figure 4c). The directed differentiation protocol yielded cultures enriched (70%–85%) in cTnT (+) beating iPSC-CMs in both WT and TBX5-KO iPSC lines at day 15 post-differentiation (Online Figure VII) that displayed a typical sarcomeric morphology (Figure 4b). As HOS is associated with electrophysiological abnormalities,26,28 we next characterized the action potential (APs) of the isogenic iPSC-CMs. Both TBX5-KO and WT iPSC-CMs displayed typical AP morphologies, including ventricular-, atrial-, and nodal-like subtypes (Figure 4d and Online Table V). However, we observed that 35% of TBX5-KO iPSC-CMs exhibited marked proarrhythmic activity characterized by the development of depolarizing humps during phase 2 and 3 of the action AP that resemble early after-depolarizations (EADs) when compared to the parental iPSC-CMs (Figure 4e).
Figure 4. Modeling HOS in human iPSCs.
a) Schematic representation of TBX5 gene structure. TALENs were designed to target the translation initiation site (ATG) at exon 1 of TBX5 gene. Boxes indicate the TALEN binding sites. The TALEN-mediated deletions in the two alleles of the iPSC clone are shown. b) Representative immunofluorescence images of iPSC-CMs stained for the cardiac myocyte-specific marker cardiac troponin-T (cTnT). DNA was counterstained with DAPI (blue). c) Assessment of TBX5 protein expression in isogenic iPSC-CMs by western blot analysis; cTnT was used as a loading control. d) AP characterization in isogenic iPSC-CMs. d) TBX5-KO iPSC-CMs exhibit a proarrhythmia phenotype manifested as early after-depolarizations (EADs) during phase 2 and 3 of the AP waveform.
Identification of novel TBX5 target genes
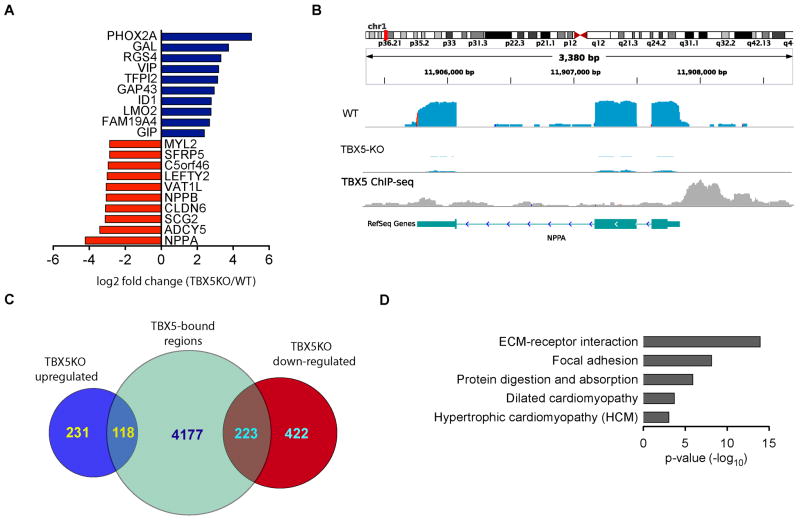
To identify downstream targets and TBX5-dependent molecular networks, we next performed chromatin immunoprecipitation coupled to massively parallel sequencing (ChIP-seq) together with RNA-seq analyses. RNA-seq analysis of isogenic iPSC-CMs revealed profound changes in global gene expression. We identified 349 up-regulated and 645 down-regulated gene transcripts in TBX5-KO when compared to the parental WT iPSC-CMs at a false discovery rate (FDR) of 5%. Analysis of a representative subset of these genes by qRT-PCR in independent experiments validated our findings (Online Figure VIII). Of note, the most significant down-regulated gene was NPPA (Figure 5a and Figure 5b), a known direct target of TBX5.29 As available antibodies for TBX5 are not suitable for genome-wide ChIP-seq, we used a lentivirus to express a FLAG-tagged TBX5 in WT iPSC-CMs. We performed FLAG-mediated ChIP-seq to define the binding sites of TBX5 genome-wide. We identified 4,518 TBX5-bound peaks that were significantly enriched in the TBX5-FLAG sample compared with the control sample (FDR < 0.01). To validate the ChIP-seq peaks, we next performed de novo motif analysis to investigate the predominant motifs enriched in TBX5 binding sites. As expected, the identified peaks were highly enriched for the previously experimentally discovered motif of TBX5 (Online Figure XI).29
Figure 5. TBX5 regulates extracellular matrix (ECM) genes in iPSC-CMs.
a) Top 20 differentially expressed genes between isogenic TBX5-KO and WT iPSC-CMs as assessed by RNA-seq. Blue bars represent up-regulated genes; red bars represent down-regulated genes. b) Representative browser tracks of NPPA gene expression in isogenic WT and TBX5-KO iPSC-CMs, and ChIP-seq footprint shows that TBX5 binds to the TSS of the NPPA gene. c) Intersection with ChiP-seq and transcriptional profiling identified 341 candidate TBX5 direct target genes. Blue circles represent up-regulated genes and red circles represent down-regulated genes (TBX5KO/WT); green circle represent TBX5-bound regions. d) A significant enrichment of extracellular matrix (ECM) components were observed in TBX5 direct target genes. The extracellular matrix (ECM)-receptor interaction and focal adhesion were the two most significant gene-sets over-represented among the 223 down-regulated (TBX5KO/WT) TBX5-bound genes.
Next, to define the direct TBX5 gene regulatory networks, we correlated TBX5 binding and TBX5-mediated gene regulation by combining the gene set containing TBX5 peaks with the genes differentially expressed between the TBX5KO and WT iPSC-CMs. We annotated the TBX5-bound regions to the nearest transcription-starting site (TSS) and identified 341 candidate TBX5 direct target genes (118 up- and 223 down-regulated genes) (Figure 5c). To further refine the identification of TBX5 target genes, we analyzed the 223 downregulated gene set andrevealed important genes associated with cardiac myocyte function, such as cardiac myosin-binding protein C (MYBPC3), titin (TTN), calsequestrin (CASQ2), natriuretic peptide type A (NPPA), connexin 43 (GJA5), and sodium voltage-gated channel alpha subunit 5 (SCN5A). Remarkably, we found that 40% of the TBX5 candidate target genes were enriched in unexpected pathways ostensibly unrelated to processes associated with heart function. These pathways included extracellular matrix (ECM)-receptor interaction, focal adhesion, and protein digestion and absorption (Figure 5d). We found that the TBX5 was bound to promoter regions of key components of the embryonic provisional matrix, including perlecan (HSPG2),30 fibronectin (FN1),31,32 fibulin-1 (FBLN1),33 collagen XIV (COL14A3),34 versican (VCAN),35–37 and versican-degrading protease ADMTS9.34 These ECM components play essential roles in cardiac development and are indispensable for normal heart development by regulating heart tube segmentation, chamber specification, endocardial cushion formation, interventricular septal formation, and cardiac myocyte differentiation.38 Taken together, these data suggest that genes encoding embryonic ECM components are direct TBX5 targets and represent potential novel candidate genes associated with HOS and CHD.
DISCUSSION
In the past decade, advances in cardiovascular genetics have uncovered a plethora of genes associated with inherited cardiomyopathies. Delineating the role of cardiomyopathy-associated genes and variants could provide a better understanding to the underlying pathogenic mechanisms, and provide potential targets for therapeutic interventions. The advent of new technologies, including iPSC and genome editing with designer nucleases, has provided an unprecedented opportunity for disease modeling in vitro. Since the development of a highly active TALEN architecture39 and simplified engineering platforms12, TALEN-mediated genome editing has been demonstrated in diverse cell types, including pluripotent stem cells.12,40–42 The relatively unconstrained target site requirements43 and the high degree of specificity of TALENs, provide a valuable tool for genome editing.
In principle, a TALEN pair can be targeted to any site in a genome, allowing more freedom and flexibility in target site selection with minimal off-target mutagenesis when compared to newer technologies such as CRISPR/Cas9.44–46 In this study, we designed, constructed, and validated TALEN vectors as an effective tool for gene KO in human iPSCs. The cTAL panel consists of 88 TALEN pairs that are designed to knockout genes that are associated with cardiomyopathies and CHD. Every TALEN pair was individually validated in human iPSCs and found to be active at the targeted locus. Furthermore, we have established that the target sites needs to be carefully chosen as TALEN pairs that target either the start codon (ATG) or regions immediately after are more effective in disrupting the open reading frame of the targeted gene. In contrast, indels at the 5-end UTR are inefficient in modifying the open reading frame. It should also be noted that even though the start codon is deleted, there might be a downstream translation starting sites that could function alternatively.
An important issue in cardiovascular genetics is determining whether putative mutations are causative of the disease, and establishing causality for putative disease causing variants is becoming increasingly clinically relevant. As a proof-of-concept, we showed that the DCM phenotype in iPSC-CMs was ameliorated by selectively disrupting the starting codon of the DCM-causing TNNT2 allele in a patient-specific iPSCs. In addition, using a similar strategy, we created a CHD model of HOS in vitro and identified a number of novel genes that are associated with TBX5 haploinsufficiency, providing an entry point to understanding the complex phenotypes caused by TBX5 haploinsufficiency and the pathogenesis of HOS. Taken together, these results demonstrated that TALEN-mediated gene KO strategies in iPSCs could be used to interrogate disease-causing mutations in a wide range of diseases and cell types as well as to model complex diseases in vitro.
In summary, combining iPSC and genome editing technologies holds great promise for advancing fundamental knowledge of the pathogenesis of inherited cardiomyopathies and CHD. The methods, strategies, and constructs developed in this study provide a validated, readily available resource for cardiovascular research that simplifies the custom generation of iPSC knock-out cell lines, and will therefore have a broad applicability for the generation of iPSC-based disease models and functional studies.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Advances in cardiovascular genetics have uncovered many genes associated with inherited cardiomyopathies.
The use of human induced pluripotent stem cell-derived cardiac myocytes (iPSC-CMs) provides an unprecedented opportunity for the generation of human cell-based disease models to study genetic cardiomyopathies.
What New Information Does This Article Contribute?
Transcription activator–like effector nucleases (TALENs) facilitate gene knockout (KO) with high efficiency, precision and accuracy.
Successful creation of human-based KO cell models in vitro by combining genome editing and iPSC-CM technologies.
TALEN-mediated allele-specific KO ameliorate dilated cardiomyopathy (DCM)-associated phenotypes in iPSC-CMs in vitro.
Modeling Holt-Oram syndrome (HOS) in iPSC-CMs in vitro uncovered novel genes and pathways regulated by TBX5.
The advent of human iPSC technology and an increasingly refined capacity to differentiate iPSCs into disease-relevant cell types, such as iPSC-CMs, provide an unprecedented opportunity for the generation of human cell-based disease models to study genetic cardiomyopathies. Genome editing can be used to change the DNA in iPSCs to aid the understanding of the biology of cardiomyopathy-associated genes and how they work. We can now make changes (or ‘edits’) to the DNA in specific location in the genome using an ‘engineered nuclease’, an enzyme that can be tailored to cut the genome in a specific place. Here we harnessed this technology to generate iPSC-based KO models of genetic cardiomyopathies to study the underlying pathogenic phenotypes and mechanisms, as well as to genetically correct the disease in vitro. Implementation of this unique and clinically relevant model system presents a significant advantage in cardiovascular research as it can circumvent complications in translating data from models across different species and biological characteristics. Ultimately, a better understanding of molecular mechanism(s) of genetic cardiomyopathies could provide opportunities for diagnosis and prognosis as well as enable the development of personalized therapeutic interventions.
Acknowledgments
SOURCES OF FUNDING
This study was funded by the NIH 4R00 HL104002-03, AHA 15BGIA22730027, and Stanford CVI Seed Grant (I.K.); Prince Mahidol Award Foundation, Thailand (V.T.); the German Research Foundation (T.S.); U54 DH068158-01 (Pilot Project) (VS), California Institute of Regenerative Medicine (CIRM) RB3-2209 (VS); and AHA 13EIA14420025, NIH R01 HL126527, NIH R01 HL130020, NIH R01 HL123968, NIH R01 HL132875, NIH R01 HL133272, and NIH R24 HL117756 (JCW).
Nonstandard Abbreviations and Acronyms
- cTAL
cardiomyopathy TALEN-based
- DSB
Double-strand break
- ECM
Extracellular matrix
- HOS
Holt-Oram syndrome
- iPSCs
induced pluripotent stem cells
- iPSC-CMs
iPSC-derived cardiac myocytes
- NHEJ
non-homologous end joining
- SMRT
single-molecule real-time
- TALENs
transcription activator–like effector nucleases
- TSS
transcription starting sites
- EADs
Early after depolarizations
- SCVI
Stanford Cardiovascular Institute
Footnotes
DISCLOSURE
None.
References
- 1.Fahed AC, Gelb BD, Seidman JG, Seidman CE. Genetics of congenital heart disease: The glass half empty. Circ Res. 2013;112:707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins H, Ashrafian H, Redwood C. Inherited cardiomyopathies. N Engl J Med. 2011;364:1643–1656. doi: 10.1056/NEJMra0902923. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 4.Mittal V. Improving the efficiency of rna interference in mammals. Nat Rev Genet. 2004;5:355–365. doi: 10.1038/nrg1323. [DOI] [PubMed] [Google Scholar]
- 5.Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiac myocytes: Insights into molecular, cellular, and functional phenotypes. Circ Res. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams WJ, Zhang Y, Cloutier J, Kuchimanchi P, Newton G, Sehrawat S, Aird WC, Mayadas TN, Luscinskas FW, Garcia-Cardena G. Functional vascular endothelium derived from human induced pluripotent stem cells. Stem Cell Reports. 2013;1:105–113. doi: 10.1016/j.stemcr.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee TH, Song SH, Kim KL, Yi JY, Shin GH, Kim JY, Kim J, Han YM, Lee SH, Lee SH, Shim SH, Suh W. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ Res. 2010;106:120–128. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- 8.Hockemeyer D, Jaenisch R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell. 2016;18:573–586. doi: 10.1016/j.stem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, Vandyk JK, Bogdanove AJ. Tal effector-nucleotide targeter (tale-nt) 2.0: Tools for tal effector design and target prediction. Nucleic Acids Res. 2012;40:W117–122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by tal effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 11.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of tal-type iii effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 12.Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT, Trevisan M, Gupta RM, Moisan A, Banks E, Friesen M, Schinzel RT, Xia F, Tang A, Xia Y, Figueroa E, Wann A, Ahfeldt T, Daheron L, Zhang F, Rubin LL, Peng LF, Chung RT, Musunuru K, Cowan CA. A talen genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karakikes I, Senyei GD, Hansen J, Kong CW, Azeloglu EU, Stillitano F, Lieu DK, Wang J, Ren L, Hulot JS, Iyengar R, Li RA, Hajjar RJ. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiac myocytes. Stem Cells Transl Med. 2014;3:18–31. doi: 10.5966/sctm.2013-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendel A, Kildebeck EJ, Fine EJ, Clark JT, Punjya N, Sebastiano V, Bao G, Porteus MH. Quantifying genome-editing outcomes at endogenous loci with smrt sequencing. Cell Rep. 2014;7:293–305. doi: 10.1016/j.celrep.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine EJ, Cradick TJ, Zhao CL, Lin Y, Bao G. An online bioinformatics tool predicts zinc finger and tale nuclease off-target cleavage. Nucleic Acids Res. 2014;42:e42. doi: 10.1093/nar/gkt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maze I, Shen L, Zhang B, Garcia BA, Shao N, Mitchell A, Sun H, Akbarian S, Allis CD, Nestler EJ. Analytical tools and current challenges in the modern era of neuroepigenomics. Nat Neurosci. 2014;17:1476–1490. doi: 10.1038/nn.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and b cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershberger RE, Pinto JR, Parks SB, Kushner JD, Li D, Ludwigsen S, Cowan J, Morales A, Parvatiyar MS, Potter JD. Clinical and functional characterization of tnnt2 mutations identified in patients with dilated cardiomyopathy. Circ Cardiovasc Genet. 2009;2:306–313. doi: 10.1161/CIRCGENETICS.108.846733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen J, Brogna S. Nonsense-mediated mrna decay. Biochem Soc Trans. 2008;36:514–516. doi: 10.1042/BST0360514. [DOI] [PubMed] [Google Scholar]
- 22.Epstein JA, Franklin h. Epstein lecture. Cardiac development and implications for heart disease. N Engl J Med. 2010;363:1638–1647. doi: 10.1056/NEJMra1003941. [DOI] [PubMed] [Google Scholar]
- 23.Papaioannou VE, Silver LM. The t-box gene family. Bioessays. 1998;20:9–19. doi: 10.1002/(SICI)1521-1878(199801)20:1<9::AID-BIES4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of holt-oram syndrome defines roles of the t-box transcription factor tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisua Belmonte JC. The t-box genes tbx4 and tbx5 regulate limb outgrowth and identity. Nature. 1999;398:814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- 26.Basson CT, Cowley GS, Solomon SD, Weissman B, Poznanski AK, Traill TA, Seidman JG, Seidman CE. The clinical and genetic spectrum of the holt-oram syndrome (heart-hand syndrome) N Engl J Med. 1994;330:885–891. doi: 10.1056/NEJM199403313301302. [DOI] [PubMed] [Google Scholar]
- 27.Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE. Mutations in human tbx5 [corrected] cause limb and cardiac malformation in holt-oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 28.Nadadur RD, Broman MT, Boukens B, Mazurek SR, Yang X, van den Boogaard M, Bekeny J, Gadek M, Ward T, Zhang M, Qiao Y, Martin JF, Seidman CE, Seidman J, Christoffels V, Efimov IR, McNally EM, Weber CR, Moskowitz IP. Pitx2 modulates a tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci Transl Med. 2016;8:354ra115. doi: 10.1126/scitranslmed.aaf4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori AD, Zhu Y, Vahora I, Nieman B, Koshiba-Takeuchi K, Davidson L, Pizard A, Seidman JG, Seidman CE, Chen XJ, Henkelman RM, Bruneau BG. Tbx5-dependent rheostatic control of cardiac gene expression and morphogenesis. Dev Biol. 2006;297:566–586. doi: 10.1016/j.ydbio.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Costell M, Carmona R, Gustafsson E, Gonzalez-Iriarte M, Fassler R, Munoz-Chapuli R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ Res. 2002;91:158–164. doi: 10.1161/01.res.0000026056.81424.da. [DOI] [PubMed] [Google Scholar]
- 31.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 32.Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207:145–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Cooley MA, Kern CB, Fresco VM, Wessels A, Thompson RP, McQuinn TC, Twal WO, Mjaatvedt CH, Drake CJ, Argraves WS. Fibulin-1 is required for morphogenesis of neural crest-derived structures. Dev Biol. 2008;319:336–345. doi: 10.1016/j.ydbio.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao G, Levay AK, Peacock JD, Huk DJ, Both SN, Purcell NH, Pinto JR, Galantowicz ML, Koch M, Lucchesi PA, Birk DE, Lincoln J. Collagen xiv is important for growth and structural integrity of the myocardium. J Mol Cell Cardiol. 2012;53:626–638. doi: 10.1016/j.yjmcc.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- 36.Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- 37.Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ Res. 1998;83:523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- 38.Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S. Myocardial extracellular matrix: An ever-changing and diverse entity. Circ Res. 2014;114:872–888. doi: 10.1161/CIRCRESAHA.114.302533. [DOI] [PubMed] [Google Scholar]
- 39.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, Dulay GP, Hua KL, Ankoudinova I, Cost GJ, Urnov FD, Zhang HS, Holmes MC, Zhang L, Gregory PD, Rebar EJ. A tale nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 40.Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, Zeitler B, Cherone JM, Meng X, Hinkley SJ, Rebar EJ, Gregory PD, Urnov FD, Jaenisch R. Genetic engineering of human pluripotent cells using tale nucleases. Nat Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karakikes I, Stillitano F, Nonnenmacher M, Tzimas C, Sanoudou D, Termglinchan V, Kong CW, Rushing S, Hansen J, Ceholski D, Kolokathis F, Kremastinos D, Katoulis A, Ren L, Cohen N, Gho JM, Tsiapras D, Vink A, Wu JC, Asselbergs FW, Li RA, Hulot JS, Kranias EG, Hajjar RJ. Correction of human phospholamban r14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat Commun. 2015;6:6955. doi: 10.1038/ncomms7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park CY, Kim J, Kweon J, Son JS, Lee JS, Yoo JE, Cho SR, Kim JH, Kim JS, Kim DW. Targeted inversion and reversion of the blood coagulation factor 8 gene in human ips cells using talens. Proc Natl Acad Sci U S A. 2014;111:9253–9258. doi: 10.1073/pnas.1323941111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. Flash assembly of talens for high-throughput genome editing. Nat Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuscu C, Arslan S, Singh R, Thorpe J, Adli M. Genome-wide analysis reveals characteristics of off-target sites bound by the cas9 endonuclease. Nat Biotechnol. 2014;32:677–683. doi: 10.1038/nbt.2916. [DOI] [PubMed] [Google Scholar]
- 45.Guilinger JP, Thompson DB, Liu DR. Fusion of catalytically inactive cas9 to foki nuclease improves the specificity of genome modification. Nat Biotechnol. 2014;32:577–582. doi: 10.1038/nbt.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, Joung JK. Dimeric crispr rna-guided foki nucleases for highly specific genome editing. Nat Biotechnol. 2014;32:569–576. doi: 10.1038/nbt.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.