Abstract
DNA vaccines offer many advantages over other anti-tumor vaccine approaches due to their simplicity, ease of manufacturing, and safety. Results from several clinical trials in patients with cancer have demonstrated that DNA vaccines are safe and can elicit immune responses. However, to date few DNA vaccines have progressed beyond phase I clinical trial evaluation. Studies into the mechanism of action of DNA vaccines in terms of antigen-presenting cell types able to directly present or cross-present DNA-encoded antigens, and the activation of innate immune responses due to DNA itself, have suggested opportunities to increase the immunogenicity of these vaccines. In addition, studies into the mechanisms of tumor resistance to anti-tumor vaccination have suggested combination approaches that can increase the antitumor effect of DNA vaccines. This review focuses on these mechanisms of action and mechanisms of resistance using DNA vaccines, and how this information is being used to improve the anti-tumor effect of DNA vaccines. These approaches are then specifically discussed in the context of human prostate cancer, a disease for which DNA vaccines have been and continue to be explored as treatments.
Keywords: Prostate cancer, immunotherapy, DNA vaccines, mechanism of action
1. INTRODUCTION – DNA VACCINES
DNA vaccines are closed circular DNA plasmids designed to encode an antigen or epitope(s) of interest under a strong mammalian promoter (Liu, 2011). Plasmid DNA was initially examined as a gene therapy tool to introduce a functional gene in vivo, and quickly emerged as a promising therapeutic after surprising observations that simple injection of naked plasmid DNA led to profound transgene expression in vivo (Wolff et al., 1990). Shortly thereafter, other investigators reported the generation of cross-reactive humoral immunity against influenza nucleoproteins encoded by a plasmid (Donnelly et al., 1995; Ulmer et al., 1993). Multiple reports of antigen-specific T-cell immunity also followed, laying the foundation for plasmid DNA as a simple and promising method to induce strong antigen-specific adaptive immunity (Fu et al., 1997; Xiang et al., 1994). These latter findings were of significant interest, as plasmid DNA closely mimicked viral infections in that there is endogenous production of the antigen and presentation by MHC-I molecules to potentially elicit strong cellular immunity. In contrast, existing vaccination regimens at that time mostly consisted of inactivated whole pathogens or recombinant polypeptides that were predominantly endocytosed and presented on MHC-II molecules to generate Th2-biased cellular and humoral immunity. DNA vaccines were also quickly recognized for harboring several distinguishing characteristics and advantages (including low cost, ease and rapidity of manufacturing, and stability) making them a method of choice to address future global health threats.
DNA vaccination has been extensively explored in multiple animal models of both infectious and malignant diseases. Results in preclinical models have been extremely encouraging, with strong immunity and therapeutic efficacy generally observed in rats, mice, and non-human primates (Liu, 2011). DNA vaccines have recently received USDA approval for treatment of West Nile virus infection in horses, melanoma in dogs, and infectious necrosis virus in fish. The first human DNA vaccine trial was reported in 1998 for the treatment of human immunodeficiency virus type I infection, and demonstrated safety and immunogenicity (MacGregor et al., 1998). However, this and subsequent human trials of DNA vaccines only produced modest immune responses, and no DNA vaccine has yet been tested for, or met, the efficacy standard for FDA approval. Initial investigational efforts interpreted the low immunogenicity of DNA vaccines in humans as a result of low antigen/body mass ratio in humans when compared to less massive preclinical models, setting the stage for enhancing antigen expression through 1) improvement of transfection efficiency and 2) optimization of plasmid vectors. While these methods have yielded significant improvements in preclinical models, they have only moderately improved immunogenicity in humans. For example, while electroporation of plasmid DNA has been frequently reported to improve immunogenicity up to a 1000 fold in mice, translation of this technology to human trials has resulted in a modest 2–3 fold enhancement over delivery of naked DNA by simple injection (Saade & Petrovsky, 2012). Efforts to leverage chemokine and cytokine adjuvants have been similarly less successful. However, recent insights into innate immunity and the importance of direct antigen presentation, as well as the role of subverting immune regulation in augmenting adaptive immunity, have created several novel exciting avenues of investigation that might herald a new generation of DNA vaccine-based treatment modalities (Barber, 2011; Iwasaki & Medzhitov, 2010; Kirkwood et al., 2012; Pardoll, 2012).
In this review, we will review the modes of action by which DNA vaccines are capable of generating cellular immunity, and within that context discuss current novel strategies and combinations to enhance DNA vaccination immunogenicity based on these new findings. We will then focus on prostate cancer, and the application of DNA vaccines specifically to the treatment of human prostate cancer, given that it is a model disease for which several clinical trials using DNA vaccines have already been conducted, many are underway, and for which another vaccine approach has already been FDA approved. Possible future directions and approaches using DNA vaccines for prostate cancer will then be discussed.
2. DNA VACCINES – MECHANISMS OF ACTION; INCREASING IMMUNOGENICITY BY TARGETING ANTIGEN PRESENTATION
2A. Innate immune signaling from bacterial DNA
Recent research has highlighted the link between innate and adaptive immunity and the importance of strong innate signaling in establishing a broad adaptive response (Desmet & Ishii, 2012; Iwasaki & Medzhitov, 2010). One of the primary advantages of DNA vaccination is the “built-in” adjuvant effect, or the ability of bacterial DNA to itself stimulate innate immune responses (Kobiyama et al., 2013). Mammalian cells have evolved to sequester DNA within the nuclear compartment and its presence in the cytoplasm is sufficient to activate several innate immune signaling cascades (Barber, 2011; Nie & Wang, 2013; Sharma & Fitzgerald, 2011). Toll-like receptor 9 (TLR9) mediates recognition of unmethylated CpG islands, which are typically enriched in bacterial DNA, including many plasmids (Hemmi et al., 2000). Binding of unmethylated CpG DNA to TLR9 results in the release of type I IFNs and in some contexts proinflammatory cytokines (Sasai et al.,2010; Wagner, 2004). Studies examining the role of TLR9 signaling in DNA vaccine immunogenicity suggested that it may play a role in the enhancement of adaptive immune responses, but is not necessary for their induction (Babiuk et al., 2004; Chen et al., 2015; Li et al., 2015; Mitsui et al., 2009; Schneeberger et al., 2004; Stan et al., 2001). In our own preclinical and clinical studies with DNA vaccines encoding tumor antigens, we have enhanced TLR9 signaling by using a plasmid vector (pTVG4) whose backbone was modified to contain CpG-rich sequences (Becker et al., 2010a; McNeel et al., 2009a; Rekoske et al., 2015; Smith & McNeel, 2011). However, TLR9 is only one of many innate DNA sensors.
Cyclic GMP-AMP synthase (cGAS) is the most recently identified DNA sensor (Sun et al., 2012). Upon binding to DNA, it causes the production of cGAMP from ATP and GTP. cGAMP then serves as a second messenger to cause dimerization and translocation of STING to the perinuclear region (Ishikawa et al., 2009; Wu et al., 2012). In the context of DNA vaccines, TBK1 and immunogenicity. Tbk−/− and IFNar2−/− mice failed to elicit antigen-specific T-and B-cell responses after vaccination, establishing this pathway as a critical component of successful DNA vaccination. Similar results were obtained with STING−/− mice, where a several-fold reduction in antigen-specific antibody titers and secretion of IFNγ was observed (Ishikawa et al., 2009). However, a recent report has found that cGAS is dispensable for DNA vaccine-induced immunity in vivo, suggesting that even cGAS might be a redundant DNA sensor upstream of STING. Further bolstering the evidence for the requirement of type I IFN release in DNA vaccine-induced immunogenicity, this report highlights the necessity of IRF7 in the generation of an adaptive immune response (Suschak et al., 2016). Interestingly, multiple reports described the necessity of IRF3 in eliciting T cell immunity, but not antibody responses, after plasmid DNA vaccination (Shirotav et al., 2009; Suschak et al., 2016).
AIM2 (absent in melanoma 2) is an interferon-inducible pyrin domain-containing DNA sensor initially identified in a melanoma cell line, where its absence caused greater cell proliferation and oncogenic potential (Lee et al., 2012; Ratsimandresy et al., 2013). AIM2 binds cytoplasmic self and pathogen dsDNA to undergo dimerization that allows the recruitment of the ASC adaptor protein through interactions in the pyrin domain (Fernandes-Alnemri, et al., 2009; Hornung et al., 2009). The binding of AIM2 to ASC leads to the formation of the pyroptosome via interactions of its CARD domain, which subsequently induces inflammatory cell death in cells via the activation of caspase-1. Caspase-1 activation by this pathway further leads to the maturation and release of pro-inflammatory cytokines IL-1β and IL-18. By causing inflammatory cell death in a type I IFN inducible manner, the AIM2 cascade can reduce the production of type I IFN by abrogation of signaling through the cGAS-STING and/or TLR9 pathways. Indeed, a recent report found that AIM2 inhibits autophagy and IFNβ production during Mycobacterium bovis infection (Liu et al., 2016). The significance of this pathway in DNA vaccine sensing has been recently evaluated, and the authors found that Aim2−/− deficient mice have impaired T and B cell immunity, in an IL-1β and IL-18-dependent manner (Suschak et al., 2014). Altering innate DNA sensing mechanisms is one possible approach to increasing the immunogenicity of DNA vaccines, either by using agents to activate different sensors, or potentially targeting DNA vaccines to individual DNA sensors.
2B. DNA uptake and antigen presentation
2B1. Direct presentation and cross-presentation
DNA vaccines are thought to work through both endogenous and exogenous pathways of antigen presentation (Figure 1). Classically, the primary pathway for antigen presentation of exogenous proteins is that extracellular protein antigens, such as those from bacteria, are endocytosed, lysosomally degraded, and presented on MHC molecules to prime T cells. This was the basis for the earliest infectious disease and anti-tumor vaccine approaches using protein vaccines. However, this pathway favors production of a Th2 type of immune response and a subsequent humoral response that results in secretion of protective antibodies, making very effective when protection from an antigen bearing pathogen is the goal. On the other hand, intracellular antigens, such as those from tumors or viruses, are usually degraded in the proteasome, and enter the endogenous presentation pathway, with epitopes being presented on MHC-I molecules such that they may elicit cytotoxic T lymphocytes (CTL) capable of lysing antigen-expressing diseased cells (Blum et al., 2013). This pathway is the most beneficial for cancer immunotherapy in which removal of antigen-expressing cells is the goal. Subsequently, it was discovered that there exists a third pathway of antigen presentation, whereby endocytosed antigen can escape into the cytosol to be processed as an intracellular antigen and presented to CD8+ T cells. This process was named cross-presentation, and cross-priming, the result of which is similar to the intrinsic pathway in that CTL capable of lysing antigen-expressing diseased cells are generated (Allan, 2008; Joffre et al., 2012).
Figure 1. Model for DNA vaccine mechanism of action.
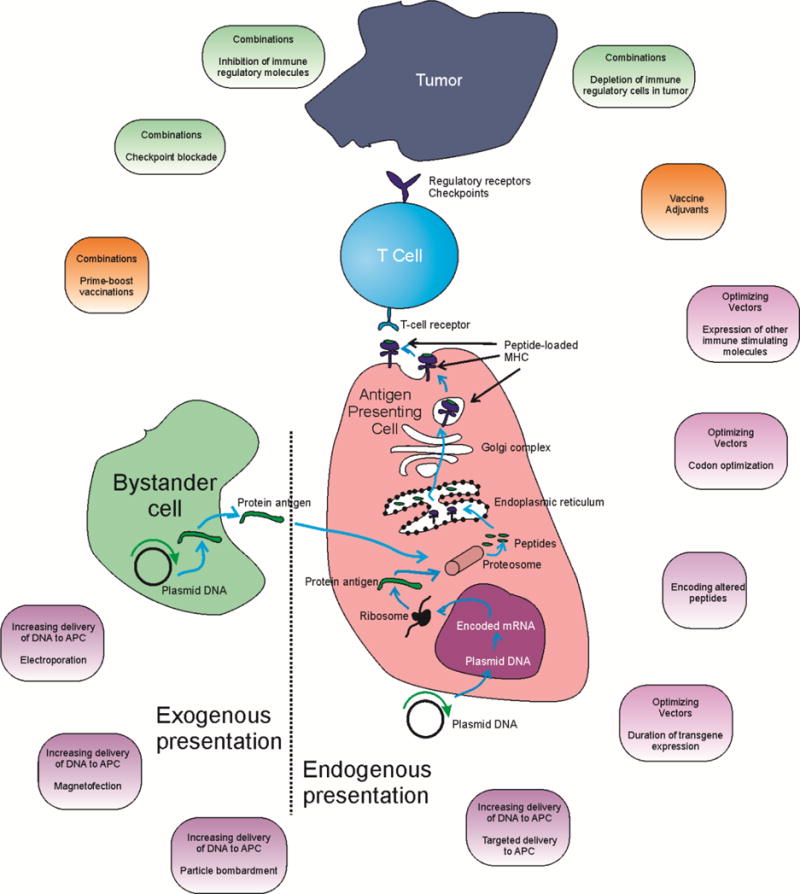
Shown is a schema of the presumed mechanism of action of DNA vaccines acting by either direct antigen presentation or crosspresentation following cellular uptake. Highlighted are areas of potential intervention, discussed in this review, to increase the resulting cellular immune response.
A lot of the initial enthusiasm surrounding DNA vaccines arose from their proposed ability to result in the production of intracellular antigens that are processed endogenously and able to elicit CTL responses (Fu et al., 1997). However, preliminary investigations into the mechanism regarding DNA vaccine action revealed that at least a fraction of the CTL response arose as the result of presentation by cells other than those that were producing the encoded antigen. For example, strong CTL responses were induced following intramuscular injection of a DNA plasmid encoding influenza virus nucleoprotein (NP) or transplantation of myoblasts stably transfected with the NP gene (Ulmer et al., 1993). Using bone marrow chimaeras, the same group showed that the CTL response against NP peptides could not be generated unless the peptides were presented in the context of MHC molecules present on professional antigen-presenting cells (APCs) (Fu et al., 1997). This was the first conclusive demonstration that antigens produced in non-APCs are able to activate a CTL response via cross-presentation to professional APCs (Fu et al., 1997; Ulmer & Otten, 2000). More work in this direction led to the discovery that muscle- or skin-specific promoters in the plasmid vector did not result in a reduction of antigen-specific immunity when compared to plasmids employing ubiquitous promoters (Cho et al., 2001; Corr et al., 1999; Hon et al., 2005). For example, Hon et al showed that using a keratinocyte promoter, incapable of expressing an antigen in a professional APC, was no worse than a ubiquitous cytomegalovirus (CMV) promoter in the generation of a CTL response (Hon et al., 2005). Together, these data established that antigen priming after DNA vaccination occurs predominantly by cross-presentation and not through the traditional endogenous pathway. More recently, insights into innate immunity have revealed that direct presentation and associated cell intrinsic activation is superior to cross-presentation in eliciting strong adaptive immunity (Iwasaki & Medzhitov, 2010). Direct presentation involves the transfection, activation, and endogenous production of plasmid-encoded antigen in a professional APC rather than a skin or muscle cell. Several investigators have reported the presence of professional APCs, most commonly in draining lymph nodes, which express plasmid encoded antigen and have in vivo priming capacity (Akbari et al., 1999; Bot et al., 2000; Bouloc et al., 1999; Casares et al., 1997; Porgador et al., 1998). For example, Porgador et al demonstrated that after gene gun mediated inoculation of plasmid DNA they were able to detect antigen-expressing DCs in the draining lymph nodes (Porgador et al., 1998). Similar results were obtained by Akbari et al (Akbari et al., 1999). While these data argue that dendritic cells are able to directly present plasmid DNA encoded antigen, efforts to use dendritic or myeloid cell promoters have given mixed results (Cho et al., 2001; Corr et al., 1999; Hon et al., 2005; Moulin et al., 2012; Ni et al., 2009). Corr et al and Cho et al demonstrated that use of CD11b or MHC-II promoters resulted in a loss of adaptive immunity, in spite of detectable antigen expression (Cho et al., 2001; Corr et al., 1999). Hon et al similarly showed that employing a CD11c promoter resulted in a loss of humoral or cellular immunity in spite of detectable antigen expression in DCs from draining lymph nodes. On the other hand, Ni et al showed that inoculation with a plasmid harboring a truncated version of the CD11c promoter, along with electroporation, resulted in antigen expression in DCs and immune responses similar to those obtained using a ubiquitous viral promoter (Ni et al., 2009). These findings suggest that DCs may be capable of directly presenting antigens encoded by DNA but also indicate that forcing this pathway in DCs may provide little or no benefit to the generation of CTL.
2B2. Improving direct antigen-presentation
How are these insights into DNA uptake by different cell types, and the resulting antigenpresentation, being used to guide the development of improved vaccine strategies? One approach has been to evaluate the optimal type of professional APC that might mediate both direct antigen presentation and cross-presentation. A benefit to the use of some bacterial vaccines, including Listeria monocytogenes vectors, is that they can directly infect DCs and macrophages leading to direct antigen presentation (Kapadia et al., 2011). As described above, it is clear that DCs are required for DNA vaccine immunogenicity, but that effect is predominantly mediated by cross-presentation. However, B cells have been shown to directly express and present plasmid-encoded antigens. That is, B cells from draining lymph nodes and bone marrow have been found to express plasmid encoded antigen after intramuscular DNA vaccination (Coelho-Castelo et al., 2003). Filaci et al further demonstrated that human peripheral B lymphocytes undergo “spontaneous transgenesis” when co-incubated with naked plasmid DNA, and B lymphocytes and B cell lines were able to exhibit plasmid uptake, encode antigen mRNA, and express the encoded protein (Filaci et al., 2004). Notably, this ability to spontaneously encode the antigen was observed with both IgG (B-cell specific) and CMV promoters (Gerloni et al., 2004). This observation also held true in CD11c-DTR DC-deficient mice, suggesting that B lymphocytes are able to encode and present plasmid antigen without DC help (Gerloni et al., 2004). We have recently found that B cells are, in fact, preferred direct APCs for DNA following passive transfer. In murine and human systems, passive delivery of DNA to B cells, and not DC, resulted in presentation of the encoded antigen to expand cognate CD8+ T cells and elicit anti-tumor responses (Colluru et al., 2016). Meanwhile, DNA delivered by passive transfer to DC or other myeloid cells was predominantly degraded prior to expression (Colluru et al., 2016). These findings suggest that efforts to specifically recruit B cells or target DNA vaccines to B cells may be a means to increase direct antigen presentation, or using methods to increase lysosomal escape of DNA in DC and other myeloid cell types (Garu et al., 2015; Porgador et al., 1998).
2B3. Improving delivery to all cells at site of immunization
Other approaches to improve antigen uptake and presentation by professional APCs have relied on methods to improve DNA transfection of all cells at the site of immunization, including bystander cells, and thus take advantage of increased cross-presentation. Many of these methods, including electroporation, particle-mediated delivery by gene-gun, and needle free delivery, have been reviewed in greater detail by us and others (Colluru et al., 2013; Liu, 2011; Saade & Petrovsky, 2012). Briefly, electroporation is the most popular method and has increased DNA vaccine-induced cellular immunity in multiple models, most notably that of human papilloma virus (HPV)-induced carcinoma (Lee et al., 2011). It has also been evaluated as a DNA vaccine delivery approach in multiple human trials, including for prostate cancer (Table 1). The enhancement of vaccine efficacy by electroporation is thought to be mediated both by an increase in antigen dose and the adjuvant effect of electroporation itself, which leads to expression of inflammatory cytokines by bystander cells as well as copious immune infiltration of electroporated tissue (Roos et al., 2009; Lee et al., 2011). Interestingly, electroporation seems to primarily increase the quantum of antigen expression, and not the duration of expression (Roos et al., 2009; Lee et al., 2011). However, it has several drawbacks, including pain at the vaccination site, portability, cost, and scalability; as such many other methods to increase DNA uptake in vivo are being explored.
Table 1. DNA vaccines in clinical evaluation for prostate cancer.
Shown is a list of DNA vaccines that have entered clinical trials as treatments for prostate cancer. The phase of development, ClinicalTrials.gov identification number, stage of disease, method, and brief results are given. Current ongoing trials are highlighted in grey.
| Antigen | Phase of Trial | NCT ID | Disease stage | Method to increase efficacy/immunogenicity | Results | Reference |
|---|---|---|---|---|---|---|
| PSMA | I/II | N/A | Mixed | Delivered with adenoviral vector +/−CD86 coexpression, and GM-CSF protein adjuvant | DTH responses observed | (Mincheff et al., 2000) |
| I | N/A | Metastatic | Prime-boost with DNA encoding xenoantigen (mouse) or native protein | Not reported | (Slovin et al., 2007) | |
| I/II | N/A | Non-metastatic, non-castrate | DNA fusion encoding HLA-A2 epitope and tetanus DOM protein, with or without electroporation | Antibody responses to DOM protein, and CD8 responses to PSMA epitopes | (Chudley et al., 2012; Low et al., 2009) | |
| I | NCT02616185 | Multiple stages | DNA vaccine delivered with electroporation, tremelimumab and sunitinib | Pending | ||
| PSA | I | N/A | Castration-resistant | Administered with GM-CSF and IL-2 | 900 mcg dose elicited PSA-specific IFNγ secretion and PSA-specific antibodies | (M Pavlenko et al., 2004) |
| I | NCT00859729 | Non-metastatic | Intradermal delivery with electroporation, and using rhesus xenoantigen | Pre-existing immune responses to PSA | (Eriksson et al., 2013) | |
| PAP | I/IIa | NCT00582140 | Non-metastatic, non-castrate | Six intradermal immunizations and coadministered with GM-CSF | CD8+ immune responses elicited, IFNγ-secreting responses associated with increased PSA doubling time | (Becker et al., 2010; McNeel et al., 2009) |
| I/II | NCT00849121 | Non-metastatic, castrate | Immunization over 2 years with schedule determined by immune monitoring | Thl-biased immune responses elicited | (McNeel et al., 2014) | |
| II | NCT01341652 | Non-metastatic, non-castrate | Randomized to vaccine + GM-CSF versus GM-CSF only over 2 years | Pending | ||
| Pilot | NCT02499835 | Castration-resistant, metastatic | Administered in combination or in sequence with pembrolizumab | Pending | ||
| Pilot | NCT01706458 | Castration-resistant, metastatic | Administered following sipuleucel-T as prime-boost | Pending | ||
| AR | I | NCT02411786 | Metastatic | Administered with or without GM-CSF and with two different schedules of immunization | Pending | |
| NY-ESO-1 | I | NCT00199849 | Advanced disease | Administered by particle-mediated delivery | Immune responses to NY-ESO-1 elicited | (Gnjatic et al., 2009) |
| Combination | I | NCT02514213 | Non-metastatic, non-castrate | DNA encoding PSA and PSMA fragments, delivered with DNA encoding IL-12 and with electroporation | Pending |
Several newer technologies have been reported to enhance the transfection of different primary cells and might be adapted for in vivo DNA transfer into immune cell types. Sonoporation, for example, involves the use of ultrasound to cause a temporary increase in cell membrane permeability through the creation of pores, allowing the intracellular diffusion of macromolecules such as DNA (Fan et al., 2014). Sonoporation has recently been described for high-efficiency transfection of B cells (Ling Yong et al., 2014) and has been employed to increase in vivo delivery of a DNA vaccines to APCs. Using ultrasound (US)-responsive and APC-selective lipolex capsules to carry plasmid DNA, this targeted delivery approach was found to generate potent and sustained effects against solid and metastatic melanomas (Un et al., 2011). Another method that has been used both in vitro and in vivo to increase cellular uptake of DNA plasmids, and thus direct presentation, is magnetofection. This rapidly maturing technology was designed specifically for the efficient intracellular delivery of nucleic acids. Magnetofection requires the association of nucleic acid vectors with magnetic nanoparticles followed by the use of a magnetic field to move the complex across cellular membranes (Plank et al., 2011). The formulation typically consists of paramagnetic iron oxide nanoparticles that are coated with a cationic lipoplex that forms complexes with the DNA (Scherer et al., 2002). Cells, tissues, or laboratory animals are then subjected to a localized magnetic field that causes movement of the magnetic nanoparticles and their cargo across biological membranes. This method was first described for non-viral gene delivery in 2002 and has been applied for the ex vivo transfection of immune cells (Muthana et al., 2008). Furthermore, Xiang and colleagues demonstrated that using magnetic nanoparticles to increase the uptake of plasmid DNA vaccine after intramuscular injection led to greater adaptive immune responses in vivo (L. Xiang et al., 2007).
2B4. Chemo-attraction of APC
Chemo-attraction of immune cells has long been employed as an adjuvant for DNA vaccination, most commonly through encoding of a chemokine along with the antigen of interest in a plasmid vector (“genetic” adjuvant), or supplying a chemoattractant as a protein adjuvant. Several reports have described the use of chemokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage inflammatory proteins (MIP) for enhancing DNA vaccination in infectious disease of HIV, HSV2, and Hepatitis C (Disis et al., 2003). These approaches have been reviewed recently (Flingai et al., 2013). In addition to these cytokines, chemokines of the C-C cytokine family, including CCL19, CCL20, and CCL21, have been evaluated as DNA vaccine chemo-attractants. Notably, the migration of antigen-engaged B cells and DCs to secondary lymphoid tissues is mediated by CCL19. In a model of Her2/neu expressing breast cancer, CCL19 was found to enhance the immunogenicity of a DNA vaccine expressing Her2/neu and increase prophylactic efficacy from 22% to 56% when included along with the antigen as part of a polycistronic transcript (Nguyen-Hoai et al., 2012). In the same model, the investigators later reported that the enhancing effects of CCL19 co-administration were B-cell dependent and that the increase in prophylactic efficacy was lost in a B-cell deficient mouse (Nguyen-Hoai et al., 2012). CCL20 and CCL21 co-administration similarly reduced tumor growth rate in a therapeutic model of melanoma (Igoucheva et al., 2013). Another group demonstrated that injection of a CCL21-encoding plasmid up to 24 hours prior to vaccine administration at the same site induced better anti-tumor responses both therapeutically and prophylactically (Yamano et al., 2007; Tomoki et al., 2006). Co-administration, or administration 1–3 days later, did not improve immune responses in this model. These findings suggest that the presence of APCs at the site and time of vaccination might be necessary for enhancement of immunogenicity by CCL21 and that using chemokines to attract B cells and DC to the site of vaccination might generally be useful to improve vaccine efficacy.
2B5. Targeted delivery of DNA to APC
As suggested above in discussing direct antigen presentation, another potential method to induce greater immunogenicity with DNA vaccines would be to target them specifically to APCs in vivo, rather than relying on cross-presentation from antigen produced by bystander cells. In its simplest approach this could be accomplished by transfecting APC ex vivo with DNA and then transferring these cells, similar to the approach of the approved sipuleucel-T vaccine. Alternatively, this could be done using agents to target DNA directly to APC subsets in vivo. This approach has usually involved a DNA-complexing agent linked to a targeting moiety, such as a peptide or protein ligand to a receptor of choice. In recently published studies, engineered cell-derived exosomes and synthetic nanoparticle complexes are among the most promising methods for such delivery.
Exosomes are extracellular vesicles typically defined as being ~50 − 200nm in diameter and of endocytic origin (Théry et al., 2002). The first observation suggesting that exosomes might be useful for plasmid DNA delivery came from Valadi et al, who demonstrated that exosomes from human and mouse mast cell lines contained RNA from ~1300 genes, many of which were not even expressed in the original host cell (Valadi et al., 2007). The transfer of this mRNA into recipient cells led to the expression of their associated proteins, proving that exosome mRNA can be translated in the recipient cell. Exosomes have been shown to play a key role in eliciting adaptive immunity and maintaining immune homoeostasis. In fact, the renewed interest in exosome biology was heralded by the finding that Epstein Barr Virus (EBV)-transformed B-cell lines secreted exosomes enriched for MHC-II molecules that are able to prime CD4+ T cell clones in vitro (Raposo et al., 1996). Soon thereafter it was demonstrated that DC-derived exosomes could be pulsed with tumor antigen peptides to control tumor growth in vivo (Zitvogel et al., 1998). Since that time, exosomes have been described in direct or indirect presentation of antigens to T cells and regulation of immunity through transfer of signaling molecules like heat shock protein 60 (HSP60), PDL1 or ICOS (inducible T cell co-stimulator) (Robbins & Morelli, 2014). These observations of functional nucleic acid delivery and the role of exosomes in antigen transfer are extremely encouraging for exosomes as prospective plasmid DNA delivery vehicles. One report recently highlighted that tumor cell line-derived exosomes could, in fact, be used as prophylactic vaccines (Zhang et al., 2014). In this report, the authors found that this protective immunity depended on DNA content of the exosome and resultant STING signaling, suggesting that delivery of DNA using exosomes is inherently immunogenic. In addition, recent advances in transfection technology have demonstrated that exosomes can be transfected with plasmid DNA, indicating that they may be useful in the targeted delivery of DNA vaccines to professional APCs (Salama et al., 2014).
Nanoparticles can be employed to allow for controlled, cell-targeted, or even site-specific release of plasmid DNA cargo in vivo. They include polymer-, lipid-, liposome-, and peptide-based delivery modalities. The mechanism of action and characteristics of each of these techniques have been reviewed in detail elsewhere (Dong et al., 2016; Farris et al., 2016; Shah et al., 2015). Nanoparticle delivery methods have been used to specifically increase direct presentation of plasmid DNA by professional APCs as briefly discussed above. DCs and macrophages have been the major targets of plasmid DNA delivery, most commonly through specific targeting of the macrophage mannose receptor (MMR), a C-type lectin receptor that acts as a pattern recognition receptor (PRR) on professional APCs.
Chitosan is a partially acetylated form of chitin, a polysaccharide of N-acetylglucosamine found in the shells of common crustaceans. It has been extensively investigated for delivery of DNA in vivo and is particularly suitable due to its inherent positive charge which enables facile nanoscale complexing of plasmid DNA (Farris et al., 2016). Layek et al used mannosylated L-phenylalanine grafted chitosan (Man-CS-Phe) for delivery of plasmid DNA to DCs and macrophages (Layek et al., 2015). Specifically, they observed greater transfection and subsequent cellular and humoral immunity against Hepatitis B in vitro when compared to a commercial lipid-based reagent. With a liposome-based approach utilizing a similar principle, Garu et al demonstrated that a liposomal complex containing plasmid DNA was able to specifically cause transgene expression in DCs from draining lymph nodes of mice immunized subcutaneously (Garu et al., 2015). They further demonstrated that this liposomal carrier complex was able to induce strong prophylactic and therapeutic responses in a mouse model of melanoma, although no comparison with naked DNA immunization or dependence of anti-tumor efficacy on the presence of DCs was made (Gam et al., 2015).
Other methods have also been described to specifically target plasmid DNA to certain cell types (Dürrbach et al., 1999; Ye et al., 2014). Ye et al described the use of a small peptide to target DNA vaccines to DCs. Specifically, a peptide with known tropism to the acetylcholine receptor expressed by macrophages/DCs was fused to a DNA binding protamine residue to create a fusion peptide “RVG-P” capable of binding DNA and specific delivering it to DCs. They demonstrated transgene expression in, and maturation of, bone marrow-derived DCs, along with superior cellular and humoral immunity against vaccinia and West Nile Virus antigens when compared to a non-DC targeting protamine fusion peptide (Ye et al., 2014). Durrbach et al demonstrated the ability of antibody-mediated targeting to specifically deliver plasmid DNA to a tumor cell line of interest, a method they termed “antifection.” A biotinylated monoclonal antibody against G250, a surface protein expressed on a renal cell carcinoma line, and biotinylated histone H3 to bind the DNA, were complexed to create a delivery vector guided by the specificity of the antibody (Dürrbach et al., 1999). These findings indicate that strategies to target DNA vaccines to DCs could be utilized to increase the immunogenicity of DNA vaccines. After uptake the antigen encoded in the DNA plasmid must be expressed.
2C. Changes to the DNA vector construct
2C1. Optimizing/increasing antigen expression
Optimization of transgene expression has been major avenue of investigation with DNA vaccines, with the goal of increasing the expression of the antigen within the APC after uptake. Optimizing the plasmid vector has traditionally involved the use of optimal promoters, introns, codon optimization of viral/bacterial antigens, and terminators among others (Choi et al., 1991; Melcher et al., 2002; Papadakis et al., 2004; Vandermeulen et al., 2009; Morrissey et al., 2013; Garmory et al., 2003). Reviewed elsewhere (Williams, 2013), each of these methods has similarly been reported to cause an incremental increase in antigen expression in vivo, and corresponding increases in immunogenicity. DNA vaccines can also be optimized to include only those codons that are most commonly used in humans which leads to increased transcription. This comes into play primarily when the target of the DNA vaccine is of nonhuman origin. Codon-optimized DNA vaccines targeting HPV’s E6, E7, or L1 and hepatitis C virus NS3 have all shown improved immunogenicity over their native counterparts (Frelin et al., 2004; Lin et al., 2006; Lorenz et al., 2015; Mossadegh et al., 2004). However, targets of DNA vaccines for most cancers have targeted non-viral “self” proteins and codon-optimizing approaches have not generally been pursued. Rather, methods of antigen optimization are focused on increasing the presentation of the antigen to the T cells.
More recent advances in the gene therapy field have given rise to novel vector configurations with reduced extra-genic spacer lengths that are able to enhance and sustain transgene expression both in vitro and in vivo. These novel gene therapy vectors include minicircle and mini-intronic plasmids. Initial observations examining the effect of bacterial DNA (from the plasmid backbone) on transgene expression in vivo led to the creation of minicircle vectors without the bacterial backbone by employing a phage ψ31 integrase-mediated recombination technology (Chen et al., 2003). When used to deliver a therapeutic gene, minicircle vectors demonstrated sustained high-level expression for over 2 months, whereas plasmids were rapidly silenced after ~1 week (Osborn et al., 2011). Further mechanistic studies revealed that conventional plasmid DNA is transcriptionally silenced by deposition of repressive heterochromatin on its promoter sequences in a manner that depends on the extragenic length of the plasmid backbone (Riu et al., 2007; Maniar et al., 2012; Lu et al., 2012). Interestingly, the nature or origin of the DNA used to create the extragenic spacer did not influence transgene expression, as long as it was 1kb or greater in length. This suggested the involvement of transcriptional machinery interactions at the promoter and terminator sites that determine transcriptional activity or “gene looping” (Lu et al., 2012). While minicircles induced robust transgene expression, they were hard to produce in a pure form and involved the use of inefficient recombination techniques that were not amenable to large scale production (Kay et al., 2010). In order to overcome these drawbacks, Lu et al designed a vector with a bacterial origin of replication and a resistance gene incorporated within an intron downstream of the promoter sequence (Luv et al., 2013). This allowed the propagation of “mini-intronic” plasmids in bacteria using standard methods, while maintaining the benefits of sustained expression achieved with minicircle plasmids. In fact, mini-intronic plasmids mediated even greater transgene expression in vivo than minicircles (Lu et al., 2013). As genetic vaccines, minicircles encoding ovalbumin were shown to elicit greater frequencies of CD8+ T cells when compared to a traditional plasmid vector upon DNA tattooing (Dietz et al., 2013). This report further described greater protection upon challenge with a Listeria monocytogenes strain expressing ovalbumin using prophylactic minicircle immunization. Similarly, Wang et al demonstrated that electroporation of a minicircle encoding a codon-optimized HIV1 gag gene elicited greater frequencies of IFNγ-secreting CD8+ T cells (Wang et al., 2013). These data would suggest that use of minicircle or mini-intronic plasmids should similarly yield greater cell mediated immunity and resultant anti-tumor benefit in tumor models. Surprisingly, we recently found that a mini-intronic plasmid encoding synovial sarcoma, X breakpoint 2 (SSX2), a tumor-associated antigen, mediated an inferior anti-tumor effect despite eliciting greater antigen expression in vivo and increased frequencies of antigen-specific CD8+ T cells (Colluru et al., 2016a). We found that increasing antigen dose or duration of antigen expression by DNA immunization increased the expression of LAG3 on CD8+ T cells and rendered them ineffective against an SSX2-expressing tumor. These data suggest that increased gene expression per se may not confer additional benefit in the absence of methods to block tumor mechanisms of resistance (Colluru et al., 2016b). However, other approaches to increase gene expression are being explored.
2C2. Optimizing/increasing antigen presentation
Antigen sequence optimization techniques have aimed to increase the immunogenicity of selfantigens by increasing the presentation or recognition of the antigen. In order to maintain selftolerance, self-antigens are often low affinity for MHC-I and cognate T-cells of high affinity are generally eliminated by central tolerance. As a result, most native self-proteins are not presented or recognized efficiently. There are currently three main antigen-altering approaches that have attempted to increase the immunogenicity of tumor-specific self-antigens by altering their presentation on APCs and/or recognition by the cognate T cells via changes made to the amino acids encoded by the DNA plasmid.
The first of these approaches has been to make epitope-specific changes encoded within the DNA (altered peptide ligand, APL) to modify MHC-I affinity as a means to improve antigen presentation (Hoppes et al., 2014; Lazoura et al., 2006; Ma & Kapp, 2001). We recently assessed the ability of an MHC-I-optimized DNA vaccine targeting SSX2 by making sequence-specific modifications to anchor residues of HLA-A2 epitopes to enhance their HLA-A2 binding. We found that increasing the affinity of the APL for HLA-A2 elicited increased frequencies of antigen-specific, Th1-biased multifunctional T cells following immunization (Smith et al., 2014), however, these same T cells had a surprisingly inferior antitumor effect relative to the native vaccine (Rekoske et al., 2015). Both native and optimized vaccines led to increased expression of PD-L1 on tumor cells and antigen-specific CD8+ T cells from mice immunized with the optimized construct cells expressed higher programmed death 1 (PD-1). PD-1 blockade was able to restore the anti-tumor efficacy of the high affinity vaccine (Rekoske et al., 2015). This suggests that vaccines incorporating epitopes with increased MHC-I affinity may be less useful as anti-tumor vaccines in the absence of PD-1/PD-L1 blockade.
Another approach has been to make epitope-specific changes to increase the affinity of the antigenic peptide-MHC-I complex for the TCR (Corse et al., 2010; McMahan et al., 2006; Simon et al., 2015). Slansky and colleagues showed immunization directly with a tumor-associated modified peptide that bound to the TCR with high affinity resulted in T-cell proliferation but did not control tumor growth. Alternately, APLs of moderate affinity for the TCR were capable of preventing tumor growth. The authors found that TIL resulting from high affinity vaccination produced less IFNγ in response to ex vivo stimulation with their antigen (McMahan et al., 2006). The mechanism for these effects is not known, but may be related to differences in expression of checkpoint receptors on resulting T cells, similar to our findings above (Rekoske et al., 2016).
A third approach has been to use DNA encoding xenogeneic antigens, without prior knowledge of the specific epitopes that are modified, as this general approach has been reported to be able to overcome immunologic tolerance and increase cytolytic T cell (CTL) responses (Bowne et al., 1999; Hawkins et al., 2000; Johnson et al., 2012a; Naftzger et al., 1996; Slovin et al., 1999). In fact, this approach is the basis of the USDA-approved vaccine for canine melanoma, a DNA vaccine that encodes human tyrosinase, with the purported mechanism of eliciting a crossreactive T-cell response to canine tyrosinase expressed by melanomas (Grosenbaugh et al., 2011). In support of this mechanism, Naftzer and colleagues reported that immunization of mice with syngeneic gp75 failed to elicit antibody or CTL. However, mice immunized with xenogeneic gp75 from insect cells or human gp75 rejected metastatic melanomas. Gregor and colleagues have explored this approach using vaccines encoding prostate-specific membrane antigen (PSMA) in preclinical prostate cancer models (Gregor et al., 2004). This approach has also been evaluated in human trials for prostate cancer, as further described below. However, a recent study from our group in which Lewis rats were immunized with DNA encoding human prostatic acid phosphatase (PAP), found that immunization with the DNA encoding the human xenoantigen led to an immune response specific to a foreign epitope without cross-reactive response to the native antigen, suggesting that this approach may not be uniformly applicable in every circumstance (Johnson et al., 2012b).
3. DNA VACCINES – ADJUVANTS AND PRIME-BOOST VACCINATION
3A. Adjuvants for DNA vaccines
Adjuvants are typically used with vaccines to modify or increase the resulting immune response from vaccination. Many adjuvants, including traditional adjuvants such alum and mineral oil, as well as cytokine and chemokine adjuvants like GM-CSF, have been explored when delivered with DNA vaccines, as described above and elsewhere (Disis et al., 2003). In this section we review novel DNA vaccine adjuvants that have been more recently identified based on the mechanisms of action described above.
3A1. Toll-like receptor (TLR) agonist adjuvants
Based on studies that plasmid DNA itself may stimulate TLR9, TLR agonists have been studied as adjuvants for DNA vaccines. The use of TLR agonists in vaccine development and cancer immunotherapy have been reviewed elsewhere (Dowling & Mansell, 2016; Kaczanowska et al., 2013). TLRs are a family of pattern recognition receptors known to stimulate the innate immune system. Currently ten human TLRs have been identified (TLR1–TLR10) and 12 in mouse (TLR1–9, TLR11–13) (Dowling & Mansell, 2016). The binding of ligands to TLRs expressed by APCs can lead to their maturation, induction of inflammatory cytokines, and the priming of naïve T cells. Therefore, activation of TLRs promotes both innate inflammatory responses and the induction of adaptive immunity. Stimulating TLR signaling during DNA vaccination can enhance the induction of vaccine-specific responses (Gableh et al., 2016; Pavlenko et al., 2007; Sajadian et al., 2014). As described above, plasmid DNA vaccines naturally stimulate TLR9, which interacts with unmethylated CpG DNA from bacteria, some viruses and plasmid DNA. Sajadian and colleagues also demonstrated that the use of either TLR3 and/or TLR7 agonists in combination with a DNA vaccine encoding the HPV E7 antigen enhanced the anti-tumor capabilities of the vaccine to eliminate HPV-induced tumors in mice. Specifically, they showed that DNA vaccine alone resulted in no significant inhibitory effects on TC-1 cells in C57BL/6 mice, but the addition of poly (I:C) (TLR3 agonist) or resiquimod (TLR7 agonist) resulted in significant tumor regression (Sajadian et al., 2014). More recently, Gableh and colleagues found that stimulation of TLR4 with monophophoryl lipid A (MPL) significantly increased lymphocyte proliferation, CTL activity, IFN-γ, IL-4 and IL-12 responses, and tumor protection against TC-1 cells (Gableh et al., 2016). Combinations of TLR agonists with DNA vaccines is consequently an interesting approach, although the mechanisms of potential synergy using different TLR agonists are currently poorly understood.
3A2. Non-TLR molecular adjuvants
The identification of cytoplasmic DNA sensors, and the signaling pathways involved to promote adaptive immune responses, has led to the evaluation of specific pathway signals as adjuvants for DNA vaccines. Interferon regulatory factors (IRF)-1, −3 and −7 have been evaluated as genetic adjuvants for DNA vaccines against influenza. Specifically, co-transfection of DNA plasmids encoding IRF3 and IRF7 with plasmids encoding influenza hemagglutinin and nucleoprotein increased cellular immune responses upon intramuscular injection by 10-fold (Sasaki et al., 2002). Similarly, Bramson et al showed that plasmids encoding constitutively active forms of IRF3 and IRF7 increased both cellular and humoral immunity, resulting in greater protection against challenge with a recombinant vaccinia virus (Bramson et al., 2003). In other studies, coexpression of TBK1 in a DNA vaccine enhanced humoral immune responses (Coban et al., 2011). New agonists for the STING pathway have been recently described, and have already demonstrated robust adjuvant effects. For example, STING agonistic cyclic dinucleotides coadministered with a GM-CSF-secreting cellular vaccine led to the regression of poorly immunogenic tumors resistant to PD1 blockade (Fu et al., 2015). This adjuvant effect was STING-dependent and led to the direct activation of DCs in vivo. Alternatives to cyclic dinucleotides that could be similarly evaluated include interferon stimulatory DNA (ISD), a 45bp non-CpG oligomer from the Listeria monocytogenes genome that induces potent expression of IFNP upon cellular uptake (Ishikawa et al., 2009), or HSV-60, a 60bp oligonucleotide containing viral DNA motifs that also has a similar mechanism of action as ISD (Unterholzner et al., 2010). Use of such synthetic molecular adjuvants in combination with DNA vaccines to enhance type I IFN signaling is likely to enhance immunogenicity and are areas of current and future exploration.
3B. DNA vaccines with other vaccines -Prime-boost
As a means of increasing an antigen-specific immune response, the prime-boost strategy uses DNA vaccines in combination with other vaccination approaches. Prime-boost has been demonstrated in multiple models to generate greater immune responses to a target antigen when compared to DNA alone (Srivastava et al., 2012). Typical prime-boost strategies have primed with DNA followed by a boost with a different type of vaccine encoding the same or a different antigen. For example, intramuscular electroporation of carcinoembryonic antigen (CEA) DNA vaccine, followed by CEA encoded by adenovirus as a boost, induced the most antigen-specific CD4+ and CD8+ T-cell responses in wild-type mice. In a tolerized CEA transgenic mouse model, repeated injection of this prime-boost scheme increased antigen-specific CD8+ T-cell responses (Mennuni et al., 2005). However, a phase 1 study evaluating a similar HER2/CEA DNA vaccine prime with adenoviral boost containing the same antigens was not found to augment detectable cell-mediated immune responses in adult cancer patients (Diaz et al., 2013). Currently successful prime-boost regimens in the clinic have included vaccines targeting foreign antigens such as those of influenza, malaria, and HIV; with one trial targeting Ap42 for the treatment of Alzheimer disease (Chuang et al., 2013; Churchyard et al., 2011; Lambracht-Washington et al., 2013; Ledgerwood et al., 2011).
Prime-boost strategies with DNA vaccines specifically focused on prostate cancer have also been reported in preclinical models. Garcia-Hernandez and colleagues assessed a heterologous prime/boost strategy in the transgenic adenocarcinoma mouse prostate (TRAMP) mouse model. Their prime-boost strategy, focused on prostate stem cell antigen (PSCA), used a DNA plasmid encoding prostate-specific antigen (PSA), delivered by gene gun, followed by Venezuelan equine encephalitis virus replicons encoding PSCA. Immune responses were primarily CD8 mediated and vaccinated TRAMP mice had a 90% survival rate at 12 months of age. In contrast, all control mice had succumbed to prostate cancer or had heavy tumor loads (Garcia-Hernandez et al., 2008). Another group has evaluated a prime-boost approach using recombinant DNA and modified vaccinia (MVA) vectors, both encoding either PSCA or six transmembrane epithelial antigen of the prostate 1 (STEAP1). Antitumor activity was assessed in the TRAMP-C1 subcutaneous syngeneic tumor model. DNA prime/MVA boost immunization against either PSCA or STEAP1 delayed tumor growth. Furthermore, simultaneous vaccination with both antigens produced a stronger anti-tumor effect than vaccination with either PSCA or STEAP1 alone. Most importantly, concurrent DNA prime/MVA boost vaccination regimen with those antigens significantly decreased primary tumor burden in TRAMP mice without producing any apparent adverse effects (Krupa et al., 2011).
4. DNA VACCINES – MECHANISMS OF RESISTANCE; COMBINATION THERAPIES TO PREVENT TUMOR EVASION
The discussion above has focused on how the understanding of mechanisms of action of DNA vaccines have led to approaches to improve on their immunogenicity. These approaches are primarily intrinsic to DNA vaccination. However, common to all anti-tumor vaccines, the development of an anti-tumor immune response can be met with tumor resistance mechanisms and evasion of immune-mediated destruction. Hence there has been much effort to combine vaccines, including DNA vaccines, with methods to prevent tumor immune evasion. In this section we discuss these approaches, focusing on combinations with immune checkpoint inhibitors, and focusing on methods to decrease regulatory cell populations and the regulatory molecules secreted by these cell populations, as methods to increase the anti-tumor efficacy of DNA vaccines.
4A. Immune checkpoints
Immune checkpoints function to shape the expansion and efficacy of T cells following antigen encounter and activation. Normally these checkpoints prevent auto-immunity but they can also interfere with a productive anti-tumor immune response. Among the many immune checkpoint pathways that have recently been discovered, the two major targets that have shown clinical efficacy following blockade in clinical trials are the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and PD-1 pathways. CTLA-4 was the first to be described and evaluated, and an antibody blocking CTLA-4, ipilimumab, was granted FDA approval in 2011 after it demonstrated the ability to improve survival in patients with metastatic melanoma (Hodi et al., 2010; Robert et al., 2011). However, as a monotherapy for prostate cancer it has been shown to be less ineffective. Despite individual patients having evidence of treatment response, and despite an increase in progression-free survival, a randomized phase III that evaluated ipiplimumab in patients with castration-resistant prostate cancer (CRPC) following chemotherapy and radiation therapy failed to demonstrate an improvement in overall survival (Kwon et al., 2014). A second phase III trial, conducted in CRCP patients prior to treatment with chemotherapy, has completed accrual, but results have not yet been reported at the time of this writing (NCT01057810). However when combined with prostate cancer vaccines, CTLA-4 blocking antibodies have shown efficacy in preclinical models (Curran & Allison, 2009; Hurwitz et al., 2000) and a human clinical trial (Eertwegh et al., 2012), suggesting that DNA vaccination targeting a prostate cancer antigen could benefit from the combination with anti-CTLA-4 antibodies.
There are currently two anti-PD-1 antibodies that have been FDA approved. Nivolumab was approved for the treatment of advanced melanoma, non-small cell lung cancer, and renal cell carcinoma, and pembrolizumab has been approved for the treatment of advanced melanoma and non-small cell lung cancer (Borghaei et al., 2015; Garon et al., 2015; Krupa et al., 2011; Motzer et al., 2015; Robert et al., 2015; Robert et al., 2015; Weber et al., 2015). Neither agent led to objective responses in patients with advanced prostate cancer treated in phase I trials (Brahmer et al., 2010; Topalian et al., 2012). In preclinical studies using tumors expressing the SSX2 tumor antigen we have found that vaccination, and DNA vaccination in particular, can lead to expression of PD-1 on antigen-specific CD8+ T cells (Rekoske et al., 2015). Combining PD-1 or PD-L1 blockade with DNA vaccination led to greater anti-tumor responses than either treatment alone (Rekoske et al., 2015). Similarly, we have demonstrated that modifications to DNA vaccines that led to increased antigen expression resulted in increased expression of another checkpoint inhibitor, LAG-3, on antigen-specific CD8+ T cells. Combining LAG-3 blockade with DNA vaccination similarly elicited greater anti-tumor activity than either treatment alone (Colluru et al., 2016b). We have also found that patients with prostate cancer, treated with a DNA vaccine, developed PD-1-regulated antigen-specific T-cell responses, and increases in PD-L1 on circulating tumor cells (Rekoske et al., 2016). Consequently, the combination of checkpoint blockade with DNA vaccines is a rational approach for clinical trials.
4B. Depletion of regulatory immune cells and immunosuppressive molecules
In addition to regulation via the immune checkpoints, there are regulatory immune cell populations that can be recruited into the tumor microenvironment and function to suppress CTL. As such removal of these regulatory immune cell populations is an attractive mechanism by which one might increase the anti-tumor efficacy of DNA vaccines. The two major classes of tumor-infiltrating regulatory immune cells are regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSC). Tregs are a subpopulation of T cells, typically CD4+CD25hiFoxP3+ cells, which modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. On the other hand, increased numbers of Tregs in cancer patients have been associated with negative outcomes (Adeegbe & Nishikawa, 2013). Similarly, MDSCs are a heterogeneous group of immune cells comprised of undifferentiated myeloid cells. MDSCs strongly expand in pathological situations such as chronic infections and cancer as a result of an altered hematopoiesis. Unlike differentiated myeloid cells (macrophages, dendritic cells), MDSCs possess strong immunosuppressive activities (Gabrilovich et al., 2012).
Tregs have been shown to suppress the response to anti-cancer DNA vaccines and play an important role in the regulation of prostate cancer growth in both people and mice (Akins et al., 2010; Jacob et al., 2009; Kiniwa et al., 2007; Klyushnenkova et al., 2014; Kursar et al., 2002; Miller et al., 2006; Niri et al., 2016; Qin et al., 2015; Rolla et al., 2010; Tang et al., 2012). The classic method of Treg depletion has been to use an anti-CD25 antibody. Interestingly, in a murine prostate cancer model, the use of systemic anti-CD25 treatment alone was shown to leave intratumoral Tregs unaffected (Akins et al., 2010). More recently, Niri et al. demonstrated the ability of a DNA vaccine targeting Foxp3 to efficiently decrease these regulatory T cells systemically in mice (Niri et al., 2016), an approach which may be useful in combination with tumor antigen-specific DNA vaccines. Others have focused on Treg-specific chemokines and their receptors to prevent Tregs from accumulating in the tumor microenvironment. The best understood chemokines are the CXCR and CCR families (Debnath et al., 2013; Highfill et al., 2014; Katoh et al., 2013; Peled et al., 2014; Weitzenfeld & Ben-Baruch, 2014). CXCR4 has been associated with the accumulation of Tregs and poor prognosis (Yan et al., 2011), while CCR5 on T cells supports the accumulation of Tregs and the progenitors for tumor-associated macrophages (TAMs) and MDSCs (Weitzenfeld & Ben-Baruch, 2014). Maraviroc is a small-molecule CCR5 antagonist that has been studied extensively in clinical trials and was approved in 2007 for the treatment of HIV. While it has itself been evaluated in the treatment of colorectal cancer (NCT01736813), studies combining maraviroc with vaccines have not yet been reported.
The effects of MDSCs, their function, and targeting in clinical cancer therapy has recently been reviewed elsewhere (Baniyash, 2016). Within the scope of prostate cancer, the presence of MDSCs within the tumor microenvironment has been shown to contribute to disease progression and potentially resistance to immunotherapy (Pal & Kortylewski, 2015; Santegoets et al., 2014). In addition, the suppression or elimination of MDSCs has been shown to enhance the response to DNA vaccination (Diniz et al., 2016; Sakamaki et al., 2014; Yan et al., 2014; Yu et al., 2015). There are many methods to reduce the presence of MDSCs in the tumor microenvironment: blockade of IL-4 receptor-α (IL4Rα), tasquinimod, iNOS inhibitors, and multiple kinase inhibitors have all been explored. IL4Rα is critical for MDSCs suppressive function and in tumor-bearing mice an anti-IL4Rα aptamer preferentially targeted MDSCs for apoptosis and promoted their elimination. This effect that was associated with an increased number of tumor-infiltrating T cells and a reduction in tumor growth (Roth et al., 2012). Tasquinimod is a S100 calcium-binding protein A9 (S100A9) inhibitor and has been shown to reduce the infiltration of MDSCs into the tumor microenvironment. Preclinical studies showed that tasquinimod can suppress the cross-talk between cancer and tumor-infiltrating host cells such as MDSCs and macrophages resulting in anti-angiogenesis, immunomodulation and inhibition of metastasis (Dalrymple et al., 2007; Isaacs et al., 2013; Olsson et al., 2010; Shen et al., 2015). Given that tasquinimod has already been evaluated alone as an anti-cancer therapy, specifically in the treatment of CRPC, it is a logical choice for combination therapies (Mehta & Armstrong, 2016).
MDSCs also produce high levels of inducible nitric oxide synthase (iNOS) with immunosuppressive function. Studies in which tumor-bearing mice were treated with AT38, an iNOS inhibitor to block intratumoral reactive nitrogen species, demonstrated a large influx of T lymphocytes into tumors. Combining AT38 with an adoptive cell transfer of antigen-specific CD8+ T cells increased the magnitude and duration of T-cell infiltration into tumors (Molon et al., 2011). The use of agents to block the production of iNOS, and other immunosuppressive molecules, with DNA vaccines is an area of active investigation.
Multiple other kinase inhibitors have also been shown to reduce MDSC accumulation in tumors. Sorafenib, an inhibitor of several tyrosine protein kinases, such as VEGFR (vascular endothelial growth factor receptor), PDGFR (platelet-derived growth factor receptor) and Raf family kinases was demonstrated to reduce the number of MDSCs in a murine liver cancer model (Cao et al., 2011). Sunitinib similarly has been demonstrated to reduce the accumulation of MDSC in tumors of patients with renal cell cancer (Ko et al., 2009). Clinical studies targeting the BRAF signaling pathway have also revealed decreased levels of circulating MDSCs and decreased IL-1-driven immunosuppression in patients with melanoma treated with vemurafenib (Khalili et al., 2012; Schilling et al., 2013) along with enhanced antigen presentation and an increase in CD8+ TIL in patients with metastatic melanoma treated with either vemurafenib (BRAF inhibitor) or the combination of dabrafenib (BRAF and MEK inhibitor) plus trametinib (MEK inhibitor) (Frederick et al., 2013). Taken together these data provide a compelling argument for the combination of anti-tumor DNA vaccines with tyrosine kinase inhibitors already in common use for cancers, but strategically aimed at reducing the number of, or suppressing the function of, Tregs and/or MDSCs.
5. PROSTATE CANCER – A MODEL DISEASE FOR DNA VACCINES
Prostate cancer is a significant worldwide health problem. In the United States it is the most commonly diagnosed malignancy in men, and the second leading cause of cancer-related death in men (Siegel et al., 2016). While the majority of newly diagnosed, organ-confined prostate cancer can be cured with surgery and/or radiation therapy, approximately one third of patients will have recurrent disease. At present there is no approved adjuvant therapy that prevents recurrence following definitive local therapy. The first evidence of recurrence is usually detected by a rise in the serum PSA blood test, the so-called D0/M0 stage of disease. The majority of patients in this setting have no symptoms from their disease and enter surveillance with serial PSA blood tests and periodic radiographic staging studies. Ultimately, with a median of 8 years, metastases can be detected, predominantly in the axial skeleton or pelvic/retroperitoneal lymph nodes (Pound et al., 1999). For recurrent prostate cancer, usually once radiographic metastases are detected but sometimes with PSA recurrence alone, androgen deprivation therapy is used. While androgen deprivation causes tumor regression in the vast majority of patients, unfortunately the disease becomes refractory to this therapy, typically within 3 years. The median survival of patients with metastatic, castration-resistant prostate cancer (mCRPC) is less than 3 years.
Over the last decade, several agents have been approved as treatments for prostate cancer. These have included chemotherapy agents docetaxel and cabazitaxel (Bono et al., 2010; Petrylak et al., 2004), androgen receptor targeted agents abiraterone and enzalutamide (Bono et al., 2011; Scher et al., 2012), bone-targeted radiation therapy with radium-223 (Parker et al., 2013), and an autologous cellular vaccine sipuleucel-T (Kantoff et al., 2010). All of these have been evaluated and approved for patients with advanced mCRPC given the current paradigm that increased survival has been the major metric for new drug approval. Unfortunately, with each of these therapies the median survival benefit has been only a few weeks to a few months. Hence there is a need for new therapies, and therapies in earlier stages of disease that might prevent or significantly delay the establishment of metastases. This is particularly important for prostate cancer because the development of metastases is a major turning point in the disease where quality of life may be impacted by either symptoms from the disease or by the use of androgen deprivation therapy.
Immune-based treatments, and vaccines in particular, have been of interest as therapies for prostate cancer for many reasons. First, as described above, prostate cancer typically has a long natural history. The slower growth of the disease makes it more amenable to treatments that require time to develop an anti-tumor response, such as therapeutic vaccination (Madan et al., 2010). Moreover, prostate cancer is a disease typically of older men keen to avoid side effects from androgen deprivation or chemotherapy, and who may otherwise have no symptoms from the disease. The safety that has been observed with vaccines in clinical trials has been particularly appealing in this situation. In addition, prostate cancer can be detected at a minimal residual disease setting by serum PSA, before the disease is apparent with radiographically detectable metastases. Studies in animal models suggest that vaccines may have their greatest anti-tumor efficacy when employed with small tumor volumes, and hence these may be more appropriate settings for anti-tumor vaccination (Wen et al., 2012). Moreover, the prostate is an expendable organ. As such there is less of a concern for autoimmune toxicity to normal prostate tissue, particularly since most patients undergo extirpative therapy at the time of diagnosis. Finally, many prostate-tissue specific proteins have been identified, and many other proteins are known to be overexpressed in prostate cancer. As such there are many known candidate targets for anti-tumor vaccines.
Several different vaccine approaches have been explored in preclinical models and clinical trials for patients with prostate cancer. We have previously reviewed these different vaccine approaches(McNeel & Disis, 2000; McNeel, 2007; McNeel & Malkovsky, 2005). Three separate vaccine approaches have been or are being explored in phase III clinical trials for patients with advanced, mCRPC. These have included the whole tumor cell vaccine approach known as GVAX (Ward & McNeel, 2007), and antigen-specific vaccine approaches, including a poxviral vaccine (Prostvac-VF) targeting PSA (Madan et al., 2009a). As described above, an autologous cellular vaccine targeting the prostate-specific protein prostatic acid phosphatase (PAP, sipuleucel-T) was FDA approved in 2010 on the basis of an improved survival in patients with mCRPC treated with sipuleucel-T compared to placebo (Kantoff et al., 2010). Collectively, these findings demonstrate that anti-tumor vaccines can have an impact on the treatment of prostate cancer, and may have greater impact still in patients with earlier stages of disease, following debulking therapies, or in combination with other immune-targeted therapies or other conventional therapies (Madan et al., 2009b). In this regard, using DNA vaccines as a simple methods of antigen delivery has been a particularly attractive approach.
6. CLINICAL TRIALS WITH DNA VACCINES FOR PROSTATE CANCER
We have previously reviewed clinical trials using DNA vaccines as treatments for prostate cancer (McNeel et al., 2012). These completed and ongoing clinical trials are summarized in Table 1. These trials are briefly described here, highlighting how the methods described above to improve the immunogenicity of DNA vaccines are being incorporated into clinical trials.
The first clinical trial using a DNA vaccine encoding a prostate cancer antigen opened in 1998. This phase I/II toxicity-dose escalation study assessed the ability of DNA encoding PSMA, in various combinations with a plasmid encoding CD86 and soluble GM-CSF as adjuvants, to generate antigen-specific immune responses (Mincheff et al., 2000). Subsequent trials have also been conducted using DNA vaccines targeting the PSMA antigen. One trial evaluated DNA constructs encoding murine or human PSMA, delivered in prime-boost sequences as described above, in patients with advanced prostate cancer. No evidence of immunity elicited by this approach has been reported (S. Slovin et al., 2007). A third investigator group has evaluated the delivery of specific epitopes derived from PSMA fused to a domain of the tetanus toxin, to provide CD4+ T cell help, and delivered with or without electroporation in patients with early PSA-recurrent, non-metastatic prostate cancer. They have reported that this approach elicited immune responses to both moieties of the fusion construct, including cytolytic CD8+ T cells specific for the PSMA epitopes, and this was associated with increases in PSA doubling time after immunization, irrespective of whether electroporation was used (Chudley et al., 2012; Low et al., 2009).
The next antigen that was evaluated as a target for DNA vaccines was PSA. The first phase I trial opened in 2000, assessing the safety and immunological effect of a DNA vaccine encoding PSA in combination with recombinant GM-CSF and IL-2 protein administered systemically as adjuvants (M Pavlenko et al., 2004). This study found that two of three patients that received the highest DNA vaccine dose (900 μg) had a significant increase in IFNγ response after vaccination; no responses were obtained with other doses (Miller et al., 1997; Pavlenko et al., 2004). A subsequent trial, conducted by the same investigator group, evaluated a similar vaccine, encoding the rhesus PSA as a xenoantigen, and delivered by electroporation. While no safety concerns were identified, there was little evidence of induced immunity given that most subjects had pre-existing immunity to PSA (Eriksson et al., 2013).
Our group has investigated a DNA vaccine encoding human PAP (pTVG-HP). The first trial targeting this antigen opened in 2005. In that trial, conducted in patients with PSA-recurrent nonmetastatic prostate cancer, CD4+ and CD8+ T-cell responses to hPAP were detected, and the presence of persistent hPAP-specific IFNγ-secreting T cells detected by enzyme-linked immunoSpot (ELISPOT) was associated with prolonged PSA doubling time (Becker et al., 2010; McNeel et al., 2009). A subsequent trial, conducted in patients with castrate-resistant, nonmetastatic prostate cancer, demonstrated that immunization could be continued over many months with induction of Th1-biased antigen-specific immunity and similar changes in PSA doubling time (McNeel et al., 2014).
While not specific to prostate cancer, another group has evaluated a DNA vaccine encoding the cancer-testis antigen NY-ESO-1 in patients with non-small cell lung cancer, esophageal carcinoma, or prostate adenocarcinoma. Ten patients with prostate cancer were included. The vaccine induced both antigen-specific effector CD4+ and/or CD8+ T-cell responses in 93% (14 of 15) of patients who did not have detectable pre-vaccine immune responses, however little evidence of clinical activity was observed (Gnjatic et al., 2009).
To our knowledge, at the time of this writing there are currently six ongoing trials assessing DNA vaccines as treatments for prostate cancer (Table 1). The most advanced of these are three trials using pTVG-HP. The first of these (NCT 01341652), which opened in 2011, is a randomized phase II trial being conducted in patients with PSA-recurrent, non-metastatic prostate cancer with rapid PSA doubling time. The primary endpoint of that trial is 2-year metastasis-free survival, and patients receive either vaccine with GM-CSF protein as adjuvant, or just GM-CSF protein alone. This is the first DNA vaccine targeting a “self” tumor antigen to be evaluated in a randomized trial for a phase 2 clinical endpoint. A second trial (NCT 01706458), opened in 2012, is evaluating in a pilot trial the use of this vaccine in a prime-boost fashion with the sipuleucel-T vaccine, an FDA-approved vaccine that similarly targets the hPAP antigen. A third trial (NCT 02499835) is evaluating this DNA vaccine in combination or in sequence with the PD-1 blocking antibody pembrolizumab, and is being evaluated in patients with metastatic, castration-resistant prostate cancer (McNeel et al., 2016).
Other prostate cancer DNA vaccine trials currently underway are phase I trials. The first of these (NCT 02411786), sponsored by Madison Vaccines, Inc., is a DNA vaccine encoding the ligandbinding domain of the androgen receptor (pTVG-AR, MVI-118) and is being evaluated in patients with newly metastatic prostate cancer. This trial is focused on the evaluation of a new target vaccine antigen, the androgen receptor, the primary driver of prostate cancer, and is evaluating two different schedules of administration. Another trial (NCT02514213), sponsored by Inovio Pharmaceuticals, is testing a dual-antigen DNA vaccine (INO-5150) that contains partially xenogeneic sequences for PSMA and PSA. The consensus sequence for these antigens was designed based on human and macaque sequences with the idea that using a partially nonhuman version of the antigens will generate greater cross-reactive immune response than purely “self” or xenoantigen sequences. The vaccine is administered intramuscularly with and without a DNA-based IL-12 immune activator (INO-9012) followed by electroporation. Finally, another trial, sponsored by Pfizer, is a large phase 1 trial (NCT02616185) testing a multiple combination approach including a DNA vaccine encoding PSMA delivered by electroporation, with sunitinib, and an anti-CTLA-4 antibody (tremelimumab).
7. SUMMARY AND FUTURE DIRECTIONS – PROSTATE CANCER DNA VACCINES
In summary, plasmid DNA vaccines offer significant advantages over other anti-tumor vaccine approaches in terms of simplicity, manufacturing, and the absence of potentially infectious agents. The cost of DNA vaccines relative to autologous cellular vaccines suggests that they can be more feasibly integrated into the long-term management of prostate cancer, and other cancers, when used alone or with other therapies. Several clinical trials using DNA vaccines as treatments for prostate cancer have been conducted and these have uniformly demonstrated safety and immunogenicity; some have demonstrated possible clinical effects. Results from studies beyond phase I evaluation, where clinical benefit can be rigorously evaluated, are eagerly awaited. Notwithstanding, preclinical studies have shed new insights into mechanisms of action and mechanisms of resistance that have led to approaches that are just now entering clinical trial evaluation, and suggest logical directions for future exploration and future improvements. In particular, we believe that current efforts studying the APC populations able to directly present and cross-present DNA-encoded antigens will produce superior methods to target DNA vaccines, potentially to one or more APC types. In addition, we expect that studies of the immunogenicity of bacterial DNA itself will provide new molecular adjuvants, targeting innate signaling pathways, that will produce greater magnitude and quality of tumor-specific T cells. Finally, ongoing studies evaluating the means by which tumors avoid immune detection, and whether some of these mechanisms are used preferentially by prostate cancer, will provide the most rational combination treatments using DNA vaccines in combination with agents targeting specific mechanisms of resistance. Specifically, we believe that trials combing DNA vaccines with checkpoint inhibitors, including agents targeting CTLA-4, PD-1/PD-L1, and/or LAG3, will be most effective given recent results in murine studies (Rekoske et al., 2015; Colluru et al., 2016b). Moreover, a combination of a DNA vaccine with PD-1 blockade has recently demonstrated encouraging findings with objective clinical responses observed in patients with advanced stage prostate cancer, NCT 02499835 (McNeel et al., 2016).
Acknowledgments
CDZ, VTC and DGM were supported by the Department of Defense Prostate Cancer Research Program W81XWH-15-1-0492 and NIH P30 CA014520; CDZ was supported by NIH 5 T32 CA157322-05.
ABBREVIATIONS
- AIM2
absent in melanoma 2
- APC
antigen-presenting cell
- APL
altered peptide ligand
- CD
cluster of differentiation
- CEA
carcinoembryonic antigen
- cGAS
cyclic GMP-AMP synthase
- CpG
cytosine and guanine separated by only one phosphate - 5′—C—phosphate—G—3
- CRPC
castrate-resistant prostate cancer
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T lymphocyte-associated protein 4
- CXCR
CXC receptor
- DNA
deoxyribonucleic acid
- FDA
Food and Drug Administration
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HER2
human epidermal growth factor receptor 2
- HIV
human immunodeficiency virus
- HLA-A2
human leukocyte antigen A2
- hPAP
human prostatic acid phosphatase
- HPV
human papilloma virus
- HSV2
herpes simplex virus 2
- IFN
interferon
- IL
interleukin
- IRF
Interferon regulatory factor
- LAG3
lymphocyte-activation gene 3
- mCRPC
metastatic castration-resistant prostate cancer
- MDSC
myeloid-derived suppressor cells
- MHC
major histocompatibility complex
- mRNA
messenger ribonucleic acid
- MVA
modified vaccinia Ankara
- NCT
clinicalTrials.gov registry number
- PAP
prostatic acid phosphatase
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- PSCA
prostate stem cell antigen
- PSA
prostate-specific antigen
- PSMA
prostate-specific membrane antigen
- pTVG-AR (MVI-118)
DNA vaccine encoding the ligand-binding domain of the androgen receptor
- pTVG-HP
DNA vaccine encoding human prostatic acid phosphatase
- RNA
ribonucleic acid
- SSX2
synovial sarcoma, X breakpoint 2 protein
- STEAP1
six transmembrane epithelial antigen of the prostate 1 protein
- TAM
tumor-associated macrophages
- TCR
T-cell receptor
- Th2
type 2 helper T cell
- Th1
type 1 helper T cell
- TIL
tumor-infiltrating lymphocyte
- TLR
toll-like receptor
- TRAMP
transgenic adenocarcinoma of the mouse prostate Tregs - regulatory T cells
- USDA
United States Department of Agriculture
- US
ultrasound
Footnotes
CONFILCT OF INTEREST STATEMENT: DGM has ownership interest, receives research support, and serves as consultant to Madison Vaccines, Inc., that has licensed material described in this report. CDZ and VTC have no potential competing interests.
References
- Adeegbe DO, Nishikawa H. Natural and Induced T Regulatory Cells in Cancer. Front Immunol. 2013;4:190. doi: 10.3389/fimmu.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA Vaccination: Transfection and Activation of Dendritic Cells as Key Events for Immunity. J Exp Med. 1999;189:169–178. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins EJ, Moore ML, Tang S, Willingham MC, Tooze JA, Dubey P. In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castration-resistant tumor burden in prostate-specific pten knockout mice. Cancer Res. 2010;70:3473–3482. doi: 10.1158/0008-5472.CAN-09-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan S. Antigen presentation: Prime time: insights into cross-presentation. Nat Rev Immunol. 2008;8:322–323. [Google Scholar]
- Babiuk S, Mookherjee N, Pontarollo R, Griebel P, Van Drunen Littel-Van Den Hurk S, Hecker R, Babiuk L. TLR9−/− and TLR9+/+ mice display similar immune responses to a DNA vaccine. Immunology. 2004;113:114–120. doi: 10.1111/j.1365-2567.2004.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniyash M. Myeloid-derived suppressor cells as intruders and targets: clinical implications in cancer therapy. Cancer Immunol Immunother. 2016;65:857–867. doi: 10.1007/s00262-016-1849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33:639–647. doi: 10.1097/CJI.0b013e3181dda23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JS, Wearsch PA, Cresswell P. Pathways of Antigen Processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Saad F, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot A, Stan AC, Inaba K, Steinman R, Bona C. Dendritic cells at a DNA vaccination site express the encoded influenza nucleoprotein and prime MHC class I-restricted cytolytic lymphocytes upon adoptive transfer. Int Immunol. 2000;12:825–832. doi: 10.1093/intimm/12.6.825. [DOI] [PubMed] [Google Scholar]
- Bouloc A, Walker P, Grivel JC, Vogel JC, Katz SI. Immunization through dermal delivery of protein-encoding DNA: a role for migratory dendritic cells. Eur J Immunol. 1999;29:446–454. doi: 10.1002/(SICI)1521-4141(199902)29:02<446::AID-IMMU446>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bowne WB, Srinivasan R, Wolchok JD, Hawkins WG, Blachere NE, Dyall R, Lewis JJ, Houghton AN. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent antiprogrammed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramson JL, Dayball K, Hall JR, Millar JB, Miller M, Wan YH, Lin R, Hiscott J. Super-activated interferon-regulatory factors can enhance plasmid immunization. Vaccine. 2003;21:1363–1370. doi: 10.1016/s0264-410x(02)00694-1. [DOI] [PubMed] [Google Scholar]
- Cao M, Xu Y, Youn J, Cabrera R, Zhang X, Gabrilovich D, Nelson DR, Liu C. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest. 2011;91:598–608. doi: 10.1038/labinvest.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares S, Inaba K, Brumeanu TD, Steinman RM, Bona CA. Antigen Presentation by Dendritic Cells after Immunization with DNA Encoding a Major Histocompatibility Complex Class II-restricted Viral Epitope. J Exp Med. 1997;186:1481–1486. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SP, Peng RH, Chiou PP. Modulatory effect of CpG oligodeoxynucleotide on a DNA vaccine against nervous necrosis virus in orange-spotted grouper (Epinephelus coioides) Fish Shellfish Immunol. 2015;45:919–926. doi: 10.1016/j.fsi.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther J Am Soc Gene Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Cho JH, Youn JW, Sung YC. Cross-priming as a predominant mechanism for inducing CD8(+) T cell responses in gene gun DNA immunization. J Immunol. 2001;167:5549–5557. doi: 10.4049/jimmunol.167.10.5549. [DOI] [PubMed] [Google Scholar]
- Choi T, Huang M, Gorman C, Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol. 1991;11:3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One. 2013;8:e55571. doi: 10.1371/journal.pone.0055571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudley L, McCann K, Mander A, Tjelle T, Campos-Perez J, Godeseth R, Creak A, Dobbyn J, Johnson B, Bass P, et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol Immunother. 2012;61:2161–2170. doi: 10.1007/s00262-012-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchyard GJ, Morgan C, Adams E, Hural J, Graham BS, Moodie Z, Grove D, Gray G, Bekker LG, McElrath MJ, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS One. 2011;6:e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban C, Kobiyama K, Aoshi T, Takeshita F, Horii T, Akira S, Ishii KJ. Novel strategies to improve DNA vaccine immunogenicity. Curr Gene Ther. 2011;11:479–484. doi: 10.2174/156652311798192815. [DOI] [PubMed] [Google Scholar]
- Coelho-Castelo AAM, Santos RR, Bonato VLD, Jamur MC, Oliver C, Silva CL. B-Lymphocytes in Bone Marrow or Lymph Nodes Can Take Up Plasmid DNA After Intramuscular Delivery. Hum Gene Ther. 2003;14:1279–1285. doi: 10.1089/104303403767740812. [DOI] [PubMed] [Google Scholar]
- Colluru VT, Johnson LE, Olson BM, McNeel DG. Preclinical and clinical development of DNA vaccines for prostate cancer. Urol Oncol. 2013;34:193–204. doi: 10.1016/j.urolonc.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colluru VT, McNeel DG, Teja Colluru V, McNeel DG. B lymphocytes as direct antigen-presenting cells for anti-tumor DNA vaccines. Oncotarget. 2016a;5 doi: 10.18632/oncotarget.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colluru VT, Zahm CD, McNeel DG. Mini-intronic plasmid vaccination elicits tolerant LAG3+ CD8 T cells and inferior anti-tumor responses. Oncoimmunology. 2016b doi: 10.1080/2162402X.2016.1223002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr M, von Damm A, Lee DJ, Tighe H. In Vivo Priming by DNA Injection Occurs Predominantly by Antigen Transfer. J Immunol. 1999;163:4721–4727. [PubMed] [Google Scholar]
- Corse E, Gottschalk RA, Krogsgaard M, Allison JP. Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol. 2010;8:e1000481. doi: 10.1371/journal.pbio.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple SL, Becker RE, Isaacs JT. The quinoline-3-carboxamide antiangiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate. 2007;67:790–797. doi: 10.1002/pros.20573. [DOI] [PubMed] [Google Scholar]
- Debnath B, Xu S, Grande F, Garofalo A, Neamati N. Small molecule inhibitors of CXCR4. Theranostics. 2013;3:47–75. doi: 10.7150/thno.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- Diaz CM, Chiappori A, Aurisicchio L, Bagchi A, Clark J, Dubey S, Fridman A, Fabregas JC, Marshall J, Scarselli E, et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. J Transl Med. 2013;11:62. doi: 10.1186/1479-5876-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WM, Skinner NEB, Hamilton SE, Jund MD, Heitfeld SM, Litterman AJ, Hwu P, Chen ZY, Salazar AM, Ohlfest JR, et al. Minicircle DNA is Superior to Plasmid DNA in Eliciting Antigen-specific CD8+ T-cell Responses. Mol Ther. 2013;21:1526–1535. doi: 10.1038/mt.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz MO, Sales NS, Silva JR, Ferreira LCS. Protection against HPV-16-Associated Tumors Requires the Activation of CD8+ Effector Memory T Cells and the Control of Myeloid-Derived Suppressor Cells. Mol Cancer Ther. 2016;15:1920–1930. doi: 10.1158/1535-7163.MCT-15-0742. [DOI] [PubMed] [Google Scholar]
- Disis ML, Shiota FM, McNeel DG, Knutson KL. Soluble cytokines can act as effective adjuvants in plasmid DNA vaccines targeting self tumor antigens. Immunobiology. 2003;207:179–186. doi: 10.1078/0171-2985-00230. [DOI] [PubMed] [Google Scholar]
- Dong Y, Yang J, Zhang J, Zhang X. Nano-Delivery Vehicles/Adjuvants for DNA Vaccination Against HIV. J Nanosci Nanotechnol. 2016;16:2126–2133. doi: 10.1166/jnn.2016.10947. [DOI] [PubMed] [Google Scholar]
- Donnelly JJ, Friedman A, Martinez D, Montgomery DL, Shiver JW, Motzel SL, Ulmer JB, Liu MA. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- Dowling JK, Mansell A. Toll-like receptors: the swiss army knife of immunity and vaccine development. Clin Transl Immunol. 2016;5:e85. doi: 10.1038/cti.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrbach A, Angevin E, Poncet P, Rouleau M, Chavanel G, Chapel A, Thierry D, Gorter A, Hirsch R, Charpentier B, et al. Antibody-mediated endocytosis of G250 tumor-associated antigen allows targeted gene transfer to human renal cell carcinoma in vitro. Cancer Gene Ther. 1999;6:564–571. doi: 10.1038/sj.cgt.7700085. [DOI] [PubMed] [Google Scholar]
- van den Eertwegh AJM, Versluis J, van den Berg HP, Santegoets SJAM, van Moorselaar RJA, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- Eriksson F, Tötterman T, Maltais AK, Pisa P, Yachnin J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine. 2013;31:3843–3848. doi: 10.1016/j.vaccine.2013.06.063. [DOI] [PubMed] [Google Scholar]
- Fan Z, Kumon RE, Deng CX. Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery. Ther Deliv. 2014;5:467–486. doi: 10.4155/tde.14.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris E, Brown DM, Ramer-Tait AE, Pannier AK. Micro-and nanoparticulates for DNA vaccine delivery. Exp Biol Med. 2016;241:919–929. doi: 10.1177/1535370216643771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filaci G, Gerloni M, Rizzi M, Castiglioni P, Chang HD, Wheeler MC, Fiocca R, Zanetti M. Spontaneous transgenesis of human B lymphocytes. Gene Ther. 2004;11:42–51. doi: 10.1038/sj.gt.3302132. [DOI] [PubMed] [Google Scholar]
- Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Immunother Vaccines. 2013;4:354. doi: 10.3389/fimmu.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin L, Ahlen G, Alheim M, Weiland O, Barnfield C, Liljestrom P, Sallberg M. Codon optimization and mRNA amplification effectively enhances the immunogenicity of the hepatitis C virus nonstructural 3/4A gene. Gene Ther. 2004;11:522–533. doi: 10.1038/sj.gt.3302184. [DOI] [PubMed] [Google Scholar]
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol. 1997a;71:2715–2721. doi: 10.1128/jvi.71.4.2715-2721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TM, Ulmer JB, Caulfield MJ, Deck RR, Friedman A, Wang S, Liu X, Donnelly JJ, Liu MA. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997b;3:362–371. [PMC free article] [PubMed] [Google Scholar]
- Gableh F, Saeidi M, Hemati S, Hamdi K, Soleimanjahi H, Gorji A, Ghaemi A. Combination of the toll like receptor agonist and α-Galactosylceramide as an efficient adjuvant for cancer vaccine. J Biomed Sci. 2016;23:16. doi: 10.1186/s12929-016-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez M de la L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68:861–869. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- Garmory HS, Brown KA, Titball RW. DNA vaccines: improving expression of antigens. Genet Vaccines Ther. 2003;1:2. doi: 10.1186/1479-0556-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of nonsmall-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Garu A, Moku G, Gulla SK, Chaudhuri A. Genetic Immunization with In Vivo Dendritic Cell Targeting Liposomal DNA Vaccine Carrier Induces Long-lasting Anti-tumor Immune Response. Mol Ther. 2015 doi: 10.1038/mt.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloni M, Rizzi M, Castiglioni P, Zanetti M. T cell immunity using transgenic B lymphocytes. Proc Natl Acad Sci U S A. 2004;101:3892–3897. doi: 10.1073/pnas.0400138101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjatic S, Altorki NK, Tang DN, Tu SM, Kundra V, Ritter G, Old LJ, Logothetis CJ, Sharma P. NY-ESO-1 DNA vaccine induces T-cell responses that are suppressed by regulatory T cells. Clin Cancer Res. 2009;15:2130–2139. doi: 10.1158/1078-0432.CCR-08-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patiño JA, Perales MA, Mortazavi F, Bacich D, Heston W, Latouche JB, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, Hess PR, Jankowski MK, Jones PD, Leibman NF, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72:1631–1638. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- Hawkins WG, Gold JS, Dyall R, Wolchok JD, Hoos A, Bowne WB, Srinivasan R, Houghton AN, Lewis JJ. Immunization with DNA coding for gp100 results in CD4 T-cell independent antitumor immunity. Surgery. 2000;128:273–280. doi: 10.1067/msy.2000.107421. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PDl efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon H, Oran A, Brocker T, Jacob J. B Lymphocytes Participate in Cross-Presentation of Antigen following Gene Gun Vaccination. J Immunol. 2005;174:5233–5242. doi: 10.4049/jimmunol.174.9.5233. [DOI] [PubMed] [Google Scholar]
- Hoppes R, Oostvogels R, Luimstra JJ, Wals K, Toebes M, Bies L, Ekkebus R, Rijal P, Celie PHN, Huang JH, et al. Altered Peptide Ligands Revisited: Vaccine Design through Chemically Modified HLA-A2-Restricted T Cell Epitopes. J Immunol. 2014;193:4803–4813. doi: 10.4049/jimmunol.1400800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- Igoucheva O, Grazzini M, Pidich A, Kemp DM, Larijani M, Farber M, Lorton J, Rodeck U, Alexeev V. Immunotargeting and eradication of orthotopic melanoma using a chemokine-enhanced DNA vaccine. Gene Ther. 2013;20:939–948. doi: 10.1038/gt.2013.17. [DOI] [PubMed] [Google Scholar]
- Isaacs JT, Antony L, Dalrymple SL, Brennen WN, Gerber S, Hammers H, Wissing M, Kachhap S, Luo J, Xing L, et al. Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013;73:1386–1399. doi: 10.1158/0008-5472.CAN-12-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune System. Science (80-) 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob JB, Kong YM, Nalbantoglu I, Snower DP, Wei WZ. Tumor regression following DNA vaccination and regulatory T cell depletion in neu transgenic mice leads to an increased risk for autoimmunity. J Immunol. 2009;182:5873–5881. doi: 10.4049/jimmunol.0804074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Johnson LE, Frye TP, McNeel DG. Immunization with a prostate cancer xenoantigen elicits a xenoantigen epitope-specific T-cell response. Oncoimmunology. 2012;1:1546–1556. doi: 10.4161/onci.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Kapadia D, Sadikovic A, Vanloubbeeck Y, Brockstedt D, Fong L. Interplay between CD8α+ Dendritic Cells and Monocytes in Response to Listeria monocytogenes Infection Attenuates T Cell Responses. PLoS One. 2011;6:e19376. doi: 10.1371/journal.pone.0019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, He CY, Chen ZY. A robust system for production of minicircle DNA vectors. Nat Biotechnol. 2010;28:1287–1289. doi: 10.1038/nbt.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, Zhang M, Cooper ZA, Frederick DT, Li Y, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18:5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyushnenkova EN, Riabov VB, Kouiavskaia DV, Wietsma A, Zhan M, Alexander RB. Breaking immune tolerance by targeting CD25+ regulatory T cells is essential for the anti-tumor effect of the CTLA-4 blockade in an HLA-DR transgenic mouse model of prostate cancer. Prostate. 2014;74:1423–1432. doi: 10.1002/pros.22858. [DOI] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- Kobiyama K, Jounai N, Aoshi T, Tozuka M, Takeshita F, Coban C, Ishii KJ. Innate Immune Signaling by, and Genetic Adjuvants for DNA Vaccination. Vaccines. 2013;1:278–292. doi: 10.3390/vaccines1030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa M, Canamero M, Gomez CE, Najera JL, Gil J, Esteban M. Immunization with recombinant DNA and modified vaccinia virus Ankara (MVA) vectors delivering PSCA and STEAP1 antigens inhibits prostate cancer progression. Vaccine. 2011;29:1504–1513. doi: 10.1016/j.vaccine.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Kursar M, Bonhagen K, Fensterle J, Köhler A, Hurwitz R, Kamradt T, Kaufmann SHE, Mittrücker HW. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J Exp Med. 2002;196:1585–1592. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambracht-Washington D, Qu B, Fu M, Anderson LD, Eagar TN, Stüve O, Rosenberg RN. A peptide prime-DNA boost immunization protocol provides significant benefits as a new generation Aβ42 DNA vaccine for Alzheimer disease. J Neuroimmunol. 2013;254:63–68. doi: 10.1016/j.jneuroim.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layek B, Lipp L, Singh J. APC targeted micelle for enhanced intradermal delivery of hepatitis B DNA vaccine. J Control Release. 2015;207:143–153. doi: 10.1016/j.jconrel.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Lazoura E, Lodding J, Farrugia W, Ramsland Pa, Stevens J, Wilson Ia, Pietersz Ga, Apostolopoulos V. Enhanced major histocompatibility complex class I binding and immune responses through anchor modification of the non-canonical tumour-associated mucin 18 peptide. Immunology. 2006;119:306–316. doi: 10.1111/j.1365-2567.2006.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Park JB, Cheong M, Choi YS, Park D, Sin JI. Antitumor Therapeutic and Antimetastatic Activity of Electroporation-Delivered Human Papillomavirus 16 E7 DNA Vaccines: A Possible Mechanism for Enhanced Tumor Control. DNA Cell Biol. 2011;30:975–985. doi: 10.1089/dna.2011.1266. [DOI] [PubMed] [Google Scholar]
- Lee J, Li L, Gretz N, Gebert J, Dihlmann S. Absent in Melanoma 2 (AIM2) is an important mediator of interferon-dependent and -independent HLA-DRA and HLA-DRB gene expression in colorectal cancers. Oncogene. 2012;31:1242–1253. doi: 10.1038/onc.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Shi JL, Wu XY, Fu F, Yu J, Yuan XY, Peng Z, Cong XY, Xu SJ, Sun WB, et al. Improvement of the Immunogenicity of Porcine Circovirus Type 2 DNA Vaccine by Recombinant ORF2 Gene and CpG Motifs. Viral Immunol. 2015;28:290–296. doi: 10.1089/vim.2014.0121. [DOI] [PubMed] [Google Scholar]
- Lin CT, Tsai YC, He L, Calizo R, Chou HH, Chang TC, Soong YK, Hung CF, Lai CH. A DNA vaccine encoding a codon-optimized human papillomavirus type 16 E6 gene enhances CTL response and anti-tumor activity. J Biomed Sci. 2006;13:481–488. doi: 10.1007/s11373-006-9086-6. [DOI] [PubMed] [Google Scholar]
- Ling Yong CL, Siak-Wei Ow D, Tandiono T, Mei Heng LL, Kwok-Keung Chan K, Ohl CD, Klaseboer E, Ohl SW, Boon-Hwa Choo A. Microbubble-mediated sonoporation for highly efficient transfection of recalcitrant human B-cell lines. Biotechnol J. 2014;9:1081–1087. doi: 10.1002/biot.201300507. [DOI] [PubMed] [Google Scholar]
- Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Yue R, Yang Y, Cui Y, Yang L, Zhao D, Zhou X. AIM2 inhibits autophagy and IFN-β production during M. bovis infection. Oncotarget. 2016;7:46972–46987. doi: 10.18632/oncotarget.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz FKM, Wilde S, Voigt K, Kieback E, Mosetter B, Schendel DJ, Uckert W. Codon optimization of the human papillomavirus E7 oncogene induces a CD8+ T cell response to a cryptic epitope not harbored by wild-type E7. PLoS One. 2015;10:e0121633. doi: 10.1371/journal.pone.0121633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, Stevenson F, Ottensmeier CH. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20:1269–1278. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhang F, Xu S, Fire AZ, Kay MA. The Extragenic Spacer Length Between the 5|[prime]| and 3|[prime]| Ends of the Transgene Expression Cassette Affects Transgene Silencing From Plasmid-based Vectors. Mol Ther. 2012;20:2111–2119. doi: 10.1038/mt.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang F, Kay MA. A Mini-intronic Plasmid (MIP): A Novel Robust Transgene Expression Vector In Vivo and In Vitro. Mol Ther. 2013;21:954–963. doi: 10.1038/mt.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Kapp Ja. Peptide affinity for MHC influences the phenotype of CD8(+) T cells primed in vivo. Cell Immunol. 2001;214:89–96. doi: 10.1006/cimm.2001.1884. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, Chattergoon MA, Baine Y, Higgins TJ, Ciccarelli RB, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar LEG, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. Minicircle DNA Vectors Achieve Sustained Expression Reflected by Active Chromatin and Transcriptional Level. Mol Ther. 2012;21:131–138. doi: 10.1038/mt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan RH, McWilliams JA, Jordan KR, Dow SW, Wilson DB, Slansky JE. Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J Clin Invest. 2006;116:2543–2551. doi: 10.1172/JCI26936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeel DG. Prostate cancer immunotherapy. Curr Opin Urol. 2007;17:175–181. doi: 10.1097/MOU.0b013e3280eb10eb. [DOI] [PubMed] [Google Scholar]
- McNeel DG, Disis ML. Tumor vaccines for the management of prostate cancer. Arch Immunol Ther Exp (Warsz) 2000;48:85–93. [PubMed] [Google Scholar]
- McNeel DG, Malkovsky M. Immune-based therapies for prostate cancer. Immunol Lett. 2005;96:3–9. doi: 10.1016/j.imlet.2004.06.009. [DOI] [PubMed] [Google Scholar]
- McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeel DG, Becker JT, Johnson LE, Olson BM. DNA Vaccines for Prostate Cancer. Curr Cancer Ther Rev. 2012;8:254–263. doi: 10.2174/157339412804143113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeel DG, Becker JT, Eickhoff JC, Johnson LE, Bradley E, Pohlkamp I, Staab MJ, Liu G, Wilding G, Olson BM. Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res. 2014;20:3692–3704. doi: 10.1158/1078-0432.CCR-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeel DG, Eickhoff J, Jeraj R, Staab MJ, Straus J, Rekoske B, Liu G. DNA vaccine with pembrolizumab elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). 2016 national meeting of the Society for Immunotherapy of Cancer; Washington DC. 2016. abstract 369. [Google Scholar]
- Mehta AR, Armstrong AJ. Tasquinimod in the treatment of castrate-resistant prostate cancer - current status and future prospects. Ther Adv Urol. 2016;8:9–18. doi: 10.1177/1756287215603558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher R, Grosch HW, Hasilik A. Plasmid vectors with a 5′-hybrid intron facilitate high-level glycoprotein expression in CHO-cells. Biochim Biophys Acta - Gene Struct Expr. 2002;1575:49–53. doi: 10.1016/s0167-4781(02)00242-7. [DOI] [PubMed] [Google Scholar]
- Mennuni C, Calvaruso F, Facciabene A, Aurisicchio L, Storto M, Scarselli E, Ciliberto G, La Monica N. Efficient induction of T-cell responses to carcinoembryonic antigen by a heterologous prime-boost regimen using DNA and adenovirus vectors carrying a codon usage optimized cDNA. Int J Cancer. 2005;117:444–455. doi: 10.1002/ijc.21188. [DOI] [PubMed] [Google Scholar]
- Miller AM, Ozenci V, Kiessling R, Pisa P. Immune monitoring in a phase 1 trial of a PSA DNA vaccine in patients with hormone-refractory prostate cancer. J Immunother. 1997;28:389–395. doi: 10.1097/01.cji.0000165353.19171.41. [DOI] [PubMed] [Google Scholar]
- Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T Cells Are Enriched in the Tumor and Peripheral Blood of Prostate Cancer Patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- Mincheff M, Tchakarov S, Zoubak S, Loukinov D, Botev C, Altankova I, Georgiev G, Petrov S, Meryman HT. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol. 2000;38:208–217. doi: 10.1159/000020281. [DOI] [PubMed] [Google Scholar]
- Mitsui M, Nishikawa M, Zang L, Ando M, Hattori K, Takahashi Y, Watanabe Y, Takakura Y. Effect of the content of unmethylated CpG dinucleotides in plasmid DNA on the sustainability of transgene expression. J Gene Med. 2009;11:435–443. doi: 10.1002/jgm.1317. [DOI] [PubMed] [Google Scholar]
- Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey D, A S, Rajenderan S, Casey G, C G, Tangney M. Plasmid Transgene Expression in vivo: Promoter and Tissue Variables. In Gene Therapy - Tools and Potential Applications (InTech) 2013 [Google Scholar]
- Mossadegh N, Gissmann L, Müller M, Zentgraf H, Alonso A, Tomakidi P. Codon optimization of the human papillomavirus 11 (HPV 11) L1 gene leads to increased gene expression and formation of virus-like particles in mammalian epithelial cells. Virology. 2004;326:57–66. doi: 10.1016/j.virol.2004.04.050. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin V, Morgan ME, Eleveld-Trancikova D, Haanen JBaG, Wielders E, Looman MWG, Janssen RaJ, Figdor CG, Jansen BJH, Adema GJ. Targeting dendritic cells with antigen via dendritic cell-associated promoters. Cancer Gene Ther. 2012;19:303–311. doi: 10.1038/cgt.2012.2. [DOI] [PubMed] [Google Scholar]
- Mousavi Niri N, Memarnejadian A, Pilehvar-Soltanahmadi Y, Agha Sadeghi M, Mahdavi M, Kheshtchin N, Arab S, Namdar A, Jadidi F, Zarghami N, et al. Improved Anti-Treg Vaccination Targeting Foxp3 Efficiently Decreases Regulatory T Cells in Mice. J Immunother. 2016;39:269–275. doi: 10.1097/CJI.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Muthana M, Scott SD, Farrow N, Morrow F, Murdoch C, Grubb S, Brown N, Dobson J, Lewis CE. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 2008;15:902–910. doi: 10.1038/gt.2008.57. [DOI] [PubMed] [Google Scholar]
- Naftzger C, Takechi Y, Kohda H, Hara I, Vijayasaradhi S, Houghton AN. Immune response to a differentiation antigen induced by altered antigen: a study of tumor rejection and autoimmunity. Proc Natl Acad Sci U S A. 1996;93:14809–14814. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Hoai T, Baldenhofer G, Ahmed MS, Pham-Duc M, Gries M, Lipp M, Dorken B, Pezzutto A, Westermann J. CCL19 (ELC) improves TH1-polarized immune responses and protective immunity in a murine Her2/neu DNA vaccination model. J Gene Med. 2012a;14:128–137. doi: 10.1002/jgm.1651. [DOI] [PubMed] [Google Scholar]
- Nguyen-Hoai T, Hohn O, Vu MD, Baldenhofer G, Sayed Ahmed MS, Dörken B, Norley S, Lipp M, Pezzutto A, Westermann J. CCL19 as an adjuvant for intradermal gene gun immunization in a Her2/neu mouse tumor model: improved vaccine efficacy and a role for B cells as APC. Cancer Gene Ther. 2012b;19:880–887. doi: 10.1038/cgt.2012.78. [DOI] [PubMed] [Google Scholar]
- Ni J, Nolte B, Arnold A, Fournier P, Schirrmacher V. Targeting anti-tumor DNA vaccines to dendritic cells via a short CD11c promoter sequence. Vaccine. 2009;27:5480–5487. doi: 10.1016/j.vaccine.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Nie Y, Wang YY. Innate immune responses to DNA viruses. Protein Cell. 2013;4:1–7. doi: 10.1007/s13238-012-2122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Björk A, Vallon-Christersson J, Isaacs JT, Leanderson T. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer. 2010;9:107. doi: 10.1186/1476-4598-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn MJ, McElmurry RT, Lees CJ, DeFeo AP, Chen ZY, Kay MA, Naldini L, Freeman G, Tolar J, Blazar BR. Minicircle DNA-based Gene Therapy Coupled With Immune Modulation Permits Long-term Expression of α-L-Iduronidase in Mice With Mucopolysaccharidosis Type I. Mol Ther. 2011;19:450–460. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal SK, Kortylewski M. Breaking bad habits: Targeting MDSCs to alleviate immunosuppression in prostate cancer. Oncoimmunology. 2015;5:e1078060. doi: 10.1080/2162402X.2015.1078060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis E, Nicklin S, Baker A, White S. Promoters and Control Elements: Designing Expression Cassettes for Gene Therapy. Curr Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, Egevad L, Hellstrom M, Kiessling R, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91:688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlenko M, Leder C, Moreno S, Levitsky V, Pisa P. Priming of CD8+ T-cell responses after DNA immunization is impaired in TLR9-and MyD88-deficient mice. Vaccine. 2007;25:6341–6347. doi: 10.1016/j.vaccine.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Peled A, Abraham M, Avivi I, Rowe JM, Beider K, Wald H, Tiomkin L, Ribakovsky L, Riback Y, Ramati Y, et al. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin Cancer Res. 2014;20:469–479. doi: 10.1158/1078-0432.CCR-13-1302. [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Tangen C, Hussain M, Lara PN, Jones J, Talpin ME, Burch P, Greene G, Small E, Crawford ED. SWOG 99-16: Randomized phase III trial of docetaxel (D)/estramustine (E) versus mitoxantrone(M)/prednisone(p) in men with androgen-independent prostate cancer (AIPCA) ASCO Meet Abstr. 2004;22:3. [Google Scholar]
- Plank C, Zelphati O, Mykhaylyk O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection—Progress and prospects. Adv Drug Deliv Rev. 2011;63:1300–1331. doi: 10.1016/j.addr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant Role for Directly Transfected Dendritic Cells in Antigen Presentation to CD8+ T Cells after Gene Gun Immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- Qin L, Jiang G, Han J, Letvin NL. Regulatory T Cells Modulate DNA Vaccine Immunogenicity at Early Time via Functional CD4(+) T Cells and Antigen Duration. Front Immunol. 2015;6:510. doi: 10.3389/fimmu.2015.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsimandresy RA, Dorfleutner A, Stehlik C. An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Mol Innate Immun. 2013;4:440. doi: 10.3389/fimmu.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer Immunol Res. 2015;3:946–955. doi: 10.1158/2326-6066.CIR-14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekoske BT, Olson BM, McNeel DG. Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology. 2016;5:e1165377. doi: 10.1080/2162402X.2016.1165377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu E, Chen ZY, Xu H, He CY, Kay MA. Histone modifications are associated with the persistence or silencing of vector-mediated transgene expression in vivo. Mol Ther J Am Soc Gene Ther. 2007;15:1348–1355. doi: 10.1038/sj.mt.6300177. [DOI] [PubMed] [Google Scholar]
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob J-J, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015a;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015b;572:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- Rolla S, Ria F, Occhipinti S, Di Sante G, Iezzi M, Spadaro M, Nicolo C, Ambrosino E, Merighi IF, Musiani P, et al. Erbb2 DNA Vaccine Combined with Regulatory T Cell Deletion Enhances Antibody Response and Reveals Latent Low-Avidity T Cells: Potential and Limits of Its Therapeutic Efficacy. J Immunol. 2010;184:6124–6132. doi: 10.4049/jimmunol.0901215. [DOI] [PubMed] [Google Scholar]
- Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Brave A, Wahren B, Pisa P. Skin Electroporation: Effects on Transgene Expression, DNA Persistence and Local Tissue Environment. PLoS One. 2009;4:e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P, Bronte V, Apolloni E, Cabrelle A, Ronca R, et al. Aptamer-mediated blockade of IL4Ra triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373–1383. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012;11:189–209. doi: 10.1586/erv.11.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadian A, Tabarraei A, Soleimanjahi H, Fotouhi F, Gorji A, Ghaemi A. Comparing the effect of Toll-like receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch Virol. 2014;159:1951–1960. doi: 10.1007/s00705-014-2024-4. [DOI] [PubMed] [Google Scholar]
- Sakamaki I, Kwak LW, Cha S, Yi Q, Lerman B, Chen J, Surapaneni S, Bateman S, Qin H. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia. 2014;28:329–337. doi: 10.1038/leu.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A, Fichou N, Allard M, Dubreil L, De Beaurepaire L, Viel A, Jégou D, Bösch S, Bach JM. MicroRNA-29b Modulates Innate and Antigen-Specific Immune Responses in Mouse Models of Autoimmunity. PLoS One. 2014;9:e106153. doi: 10.1371/journal.pone.0106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santegoets SJ, Stam AG, Lougheed SM, Gall H, Jooss K, Sacks N, Hege K, Lowy I, Scheper RJ, Gerritsen WR, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer. 2014;2:31. doi: 10.1186/s40425-014-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-Like Receptor 9 Signaling by Adaptor Protein 3. Science (80-) 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Amara RR, Yeow WS, Pitha PM, Robinson HL. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J Virol. 2002;76:6652–6659. doi: 10.1128/JVI.76.13.6652-6659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, Gänsbacher B, Plank C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Görgens A, Giebel B, Schadendorf D, Paschen A. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133:1653–1663. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, Wagner C, Zemann A, Lührs P, Kutil R, Goos M, Stingl G, Wagner SN. CpG Motifs Are Efficient Adjuvants for DNA Cancer Vaccines. J Invest Dermatol. 2004;123:371–379. doi: 10.1111/j.0022-202X.2004.23208.x. [DOI] [PubMed] [Google Scholar]
- Shah MAA, Ali Z, Ahmad R, Qadri I, Fatima K, He N. DNA Mediated Vaccines Delivery Through Nanoparticles. J Nanosci Nanotechnol. 2015;15:41–53. doi: 10.1166/jnn.2015.9603. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fitzgerald KA. Innate Immune Sensing of DNA. PLoS Pathog. 2011;7:e1001310. doi: 10.1371/journal.ppat.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Sundstedt A, Ciesielski M, Miles KM, Celander M, Adelaiye R, Orillion A, Ciamporcero E, Ramakrishnan S, Ellis L, et al. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol Res. 2015;3:136–148. doi: 10.1158/2326-6066.CIR-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota H, Petrenko L, Hattori T, Klinman DM. Contribution of IRF-3 mediated IFNbeta production to DNA vaccine dependent cellular immune responses. Vaccine. 2009;27:2144–2149. doi: 10.1016/j.vaccine.2009.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Simon S, Vignard V, Florenceau L, Dreno B, Khammari A, Lang F, Labarriere N. PD-1 expression conditions T cell avidity within an antigen-specific repertoire. Oncoimmunology. 2015;5:e1104448. doi: 10.1080/2162402X.2015.1104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovin S, Gregor P, Wolchok J, Pedraza A, Orlandi F, Jefferson M, Jefferson M, Fernandez C, Rudolph J, Houghton A, et al. A xenogeneic PSMA DNA vaccine for patients (pts) with non-castrate metastatic (NCMPC) and castrate metastatic prostate cancer (CMPC)–A phase I trial of proof of principle. ASCO Meet Abstr. 2007;25:3073. [Google Scholar]
- Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci U S A. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HA, McNeel DG. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J Immunother. 2011;34:569–580. doi: 10.1097/CJI.0b013e31822b5b1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Ha, Rekoske BT, McNeel DG. DNA vaccines encoding altered peptide ligands for SSX2 enhance epitope-specific CD8+ T-cell immune responses. Vaccine. 2014;32:1707–1715. doi: 10.1016/j.vaccine.2014.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava MK, Zhu L, Harris-White M, Kar UK, Kar U, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7:e40677. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AC, Casares S, Brumeanu TD, Klinman DM, Bona CA. CpG motifs of DNA vaccines induce the expression of chemokines and MHC class II molecules on myocytes. Eur J Immunol. 2001;31:301–310. doi: 10.1002/1521-4141(200101)31:1<301::AID-IMMU301>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science. 2012;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschak JJ, Wang S, Fitzgerald KA, Lu S. Identification of Aim2 as a Sensor for DNA Vaccines. J Immunol. 2014;194:630–636. doi: 10.4049/jimmunol.1402530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschak JJ, Wang S, Fitzgerald KA, Lu S. A cGAS-Independent STING/IRF7 Pathway Mediates the Immunogenicity of DNA Vaccines. J Immunol (Baltimore, Md 1950) 2016;196:310–316. doi: 10.4049/jimmunol.1501836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Moore ML, Grayson JM, Dubey P. Increased CD8+ T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012;72:1975–1985. doi: 10.1158/0008-5472.CAN-11-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer JB, Otten GR. Priming of CTL responses by DNA vaccines: direct transfection of antigen presenting cells versus cross-priming. Dev Biol (Basel) 2000;104:9–14. [PubMed] [Google Scholar]
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier. Mol Pharm. 2011;8:543–554. doi: 10.1021/mp100369n. [DOI] [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vandermeulen G, Richiardi H, Escriou V, Ni J, Fournier P, Schirrmacher V, Scherman D, Préat V. Skin-specific promoters for genetic immunisation by DNA electroporation. Vaccine. 2009;27:4272–4277. doi: 10.1016/j.vaccine.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–386. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Wang Q, Jiang W, Chen Y, Liu P, Sheng C, Chen S, Zhang H, Pan C, Gao S, Huang W. In Vivo Electroporation of Minicircle DNA as a Novel Method of Vaccine Delivery to Enhance HIV-1-Specific Immune Responses. J Virol. 2013;88:1924–1934. doi: 10.1128/JVI.02757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JE, McNeel DG. GVAX: an allogeneic, whole-cell, GM-CSF-secreting cellular immunotherapy for the treatment of prostate cancer. Expert Opin Biol Ther. 2007;7:1893–1902. doi: 10.1517/14712598.7.12.1893. [DOI] [PubMed] [Google Scholar]
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- Weitzenfeld P, Ben-Baruch A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett. 2014;352:36–53. doi: 10.1016/j.canlet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Wen FT, Thisted RA, Rowley DA, Schreiber H. A systematic analysis of experimental immunotherapies on tumors differing in size and duration of growth. Oncoimmunology. 2012;1:172–178. doi: 10.4161/onci.1.2.18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA. Vector Design for Improved DNA Vaccine Efficacy, Safety and Production. Vaccines. 2013;1:225–249. doi: 10.3390/vaccines1030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science. 2012;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Bin W, Huali J, Wei J, Jiesheng T, Feng G, Ying L. Bacterial magnetic particles (BMPs)-PEI as a novel and efficient non-viral gene delivery system. J Gene Med. 2007;9:679–690. doi: 10.1002/jgm.1068. [DOI] [PubMed] [Google Scholar]
- Xiang ZQ, Spitalnik S, Tran M, Wunner WH, Cheng J, Ertl HC. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- Yamano T, Kaneda Y, Huang S, Hiramatsu SH, Hoon DSB. Enhancement of immunity by a DNA melanoma vaccine against TRP2 with CCL21 as an adjuvant. Mol Ther J Am Soc Gene Ther. 2006;13:194–202. doi: 10.1016/j.ymthe.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Yamano T, Kaneda Y, Hiramatsu SH, Huang S, Tran AN, Giuliano AE, Hoon DSB. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther. 2007;14:451–459. doi: 10.1038/sj.cgt.7701035. [DOI] [PubMed] [Google Scholar]
- Yan J, Tingey C, Lyde R, Gorham TC, Choo DK, Muthumani A, Myles D, Weiner LP, Kraynyak KA, Reuschel EL, et al. Novel and enhanced anti-melanoma DNA vaccine targeting the tyrosinase protein inhibits myeloid-derived suppressor cells and tumor growth in a syngeneic prophylactic and therapeutic murine model. Cancer Gene Ther. 2014;21:507–517. doi: 10.1038/cgt.2014.56. [DOI] [PubMed] [Google Scholar]
- Yan M, Jene N, Byrne D, Millar EKA, O’Toole SA, McNeil CM, Bates GJ, Harris AL, Banham AH, Sutherland RL, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13:R47. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C, Choi JG, Abraham S, Shankar P, Manjunath N. Targeting DNA vaccines to myeloid cells using a small peptide. Eur J Immunol. 2014;45:82–88. doi: 10.1002/eji.201445010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Tan Z, Lee BK, Tang J, Wu X, Cheung KW, Lo NTL, Man K, Liu L, Chen Z. Antigen spreading-induced CD8+T cells confer protection against the lethal challenge of wild-type malignant mesothelioma by eliminating myeloid-derived suppressor cells. Oncotarget. 2015;6:32426–32438. doi: 10.18632/oncotarget.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, Luo S, Liang X, Ji T, Gu Z, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res. 2014;3:196–205. doi: 10.1158/2326-6066.CIR-14-0177. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]


