Abstract
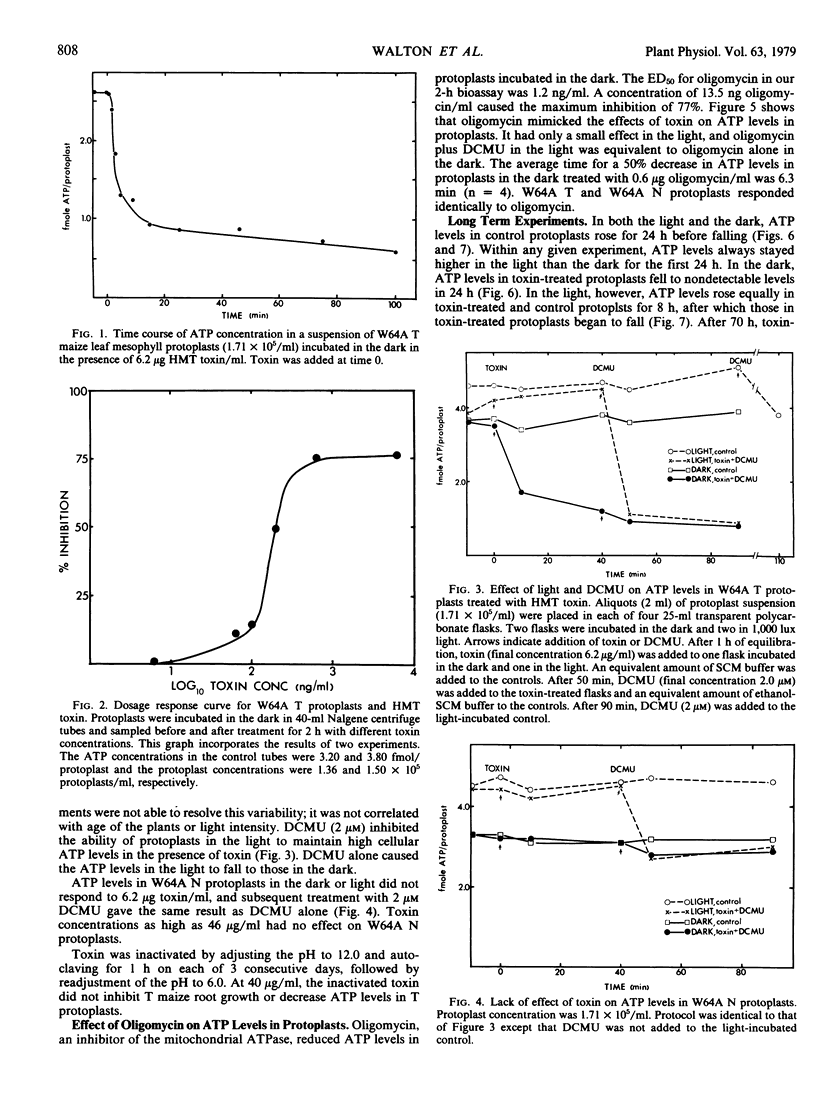
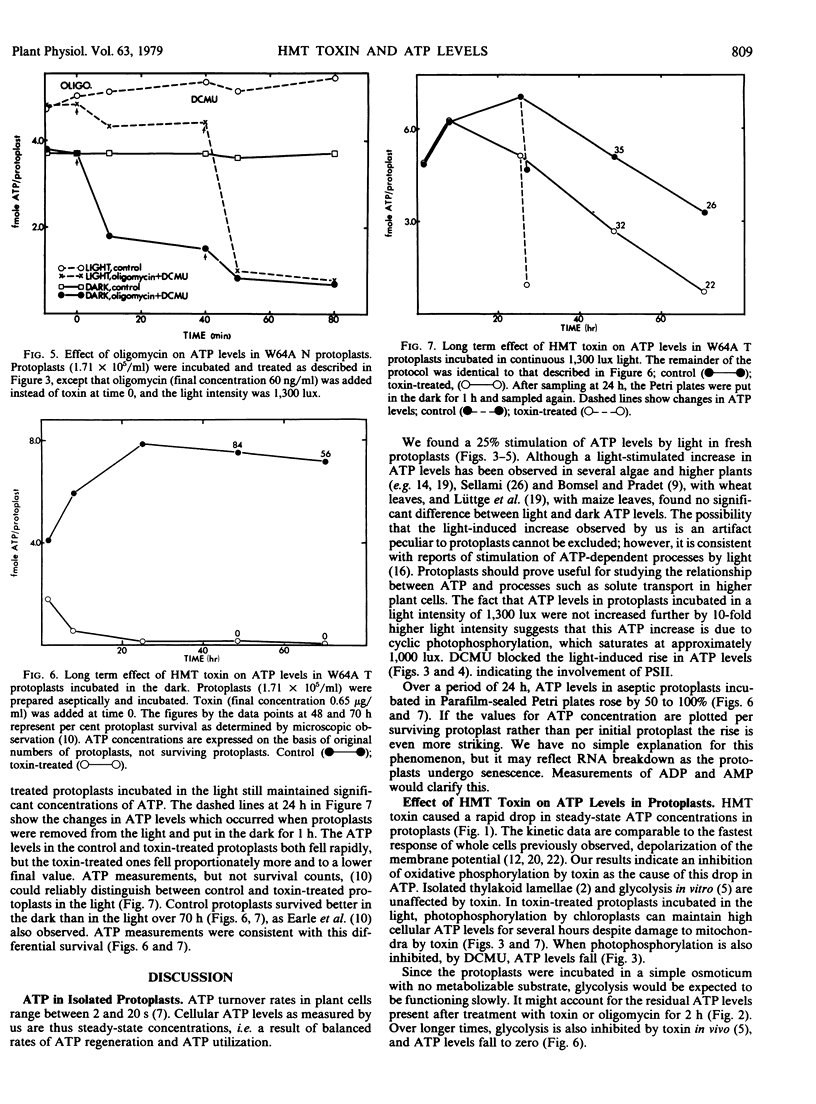
Helminthosporium maydis race T (HMT) toxin caused a reduction in the steady-state ATP levels when leaf mesophyll protoplasts isolated from maize containing Texas male-sterile (T) but not male-fertile (N) cytoplasm were incubated in the dark. At a toxin concentration 10 times the mean effectived dose for inhibition of root growth, the ATP levels began to fall in 30 to 90 seconds, fell by 50% in about 4 minutes, and reached 23% of the original levels in 100 minutes. This is faster than any previously observed response of whole cells or tissues to HMT toxin. In protoplasts incubated in the light, ATP levels were 25% higher than in the dark and were either unaffected or only slightly diminished by toxin. 3-(3,4-Dichlorophenyl)-1, 1-dimethylurea (DCMU), an inhibitor of photosynthetic electron transport, overcame the effect of light on both toxin-treated and control protoplasts. Oligomycin, an inhibitor of mitochondrial ATP synthesis, mimicked the effects of toxin in the dark, in the light, and in the light plus DCMU, but it was not specific for T cytoplasm. During the first 24 hours of culture, ATP levels in control protoplasts increased in both the light and dark. In the dark, ATP was not detectable after 24-hour incubation in the presence of toxin, whereas in the light a substantial amount of ATP remained. Our results are compatible with the hypothesis that mitochondria in vivo are inhibited by HMT toxin. Other responses of cells and tissues to toxin can be explained in terms of reduced ATP levels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich H. C., Gracen V. E., York D., Earle E. D., Yoder O. C. Ultrastructural effects of Helminthosporium maydis race T toxin on mitochondria of corn roots and protoplasts. Tissue Cell. 1977;9(1):167–177. doi: 10.1016/0040-8166(77)90057-x. [DOI] [PubMed] [Google Scholar]
- Arntzen C. J., Haugh M. F., Bobick S. Induction of Stomatal Closure by Helminthosporium maydis Pathotoxin. Plant Physiol. 1973 Dec;52(6):569–574. doi: 10.1104/pp.52.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarski M. A., Izawa S., Scheffer R. P. Reversible Effects of Toxin from Helminthosporium maydis Race T on Oxidative Phosphorylation by Mitochondria from Maize. Plant Physiol. 1977 Apr;59(4):540–545. doi: 10.1104/pp.59.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar B. S., Daly J. M., Rehfeld D. W. Inhibition of Dark CO(2) Fixation and Photosynthesis in Leaf Discs of Corn Susceptible to the Host-specific Toxin Produced by Helminthosporium maydis, Race T. Plant Physiol. 1975 Jul;56(1):1–7. doi: 10.1104/pp.56.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe C., Cole C. V., Ross C. Oligomycin inhibition of phosphate uptake and ATP labeling in excised maize roots. Plant Physiol. 1969 Jul;44(7):1040–1044. doi: 10.1104/pp.44.7.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Earle E. D., Gracen V. E., Yoder O. C., Gemmill K. P. Cytoplasm-specific Effects of Helminthosporium maydis Race T Toxin on Survival of Corn Mesophyll Protoplasts. Plant Physiol. 1978 Mar;61(3):420–424. doi: 10.1104/pp.61.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick H., Bauman L. F., Nicholson R. L., Hodges T. K. Influence of Helminthosporium maydis, Race T, Toxin on Potassium Uptake in Maize Roots: II. Sensitivity of Development of the Augmented Uptake Potential to Toxin and Inhibitors of Protein Synthesis. Plant Physiol. 1977 Jan;59(1):103–106. doi: 10.1104/pp.59.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Phosphate absorption rates and adenosine 5'-triphosphate concentrations in corn root tissue. Plant Physiol. 1974 Sep;54(3):250–256. doi: 10.1104/pp.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Arntzen C. J. Depolarization of the Electrogenic Transmembrane Electropotential of Zea mays L. by Bipolaris (Helminthosporium) maydis Race T Toxin, Azide, Cyanide, Dodecyl Succinic Acid, or Cold Temperature. Plant Physiol. 1978 Nov;62(5):781–783. doi: 10.1104/pp.62.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Arntzen C. J. Selective Inhibition of K, Na, Cl, and PO(4) Uptake in Zea mays L. by Bipolaris (Helminthosporium) maydis Race T Pathotoxin: Evidence for a Plasmalemma Target Site? Plant Physiol. 1977 Sep;60(3):363–369. doi: 10.1104/pp.60.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J., Koeppe D. E. Southern corn leaf blight: susceptible and resistant mitochondria. Science. 1971 Jul 2;173(3991):67–69. doi: 10.1126/science.173.3991.67. [DOI] [PubMed] [Google Scholar]
- Sellami A. Evolution des adenosine phosphates et de la charge energetique dans les compartiments chloroplastique et nonchloroplastique des feuilles de ble. Biochim Biophys Acta. 1976 Mar 12;423(3):524–539. doi: 10.1016/0005-2728(76)90205-x. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Adenine nucleotide levels in Neurospora, as influenced by conditions of growth and by metabolic inhibitors. J Bacteriol. 1973 May;114(2):752–766. doi: 10.1128/jb.114.2.752-766.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L., Lu C. Y., Shane L. Correlated changes in membrane potential and ATP concentrations in Neurospora. Nature. 1970 Apr 18;226(5242):274–276. doi: 10.1038/226274a0. [DOI] [PubMed] [Google Scholar]


