Abstract
The endocannabinoid system (ECS) is a major lipid signalling network that plays important pro‐homeostatic (allostatic) roles not only in the nervous system but also in peripheral organs. There is increasing evidence that there is a dietary component in the modulation of the ECS. Cannabinoid receptors in hominids co‐evolved with diet, and the ECS constitutes a feedback loop for food selection and energy metabolism. Here, it is postulated that the mismatch of ancient lipid genes of hunter‐gatherers and pastoralists with the high‐carbohydrate diet introduced by agriculture could be compensated for via dietary modulation of the ECS. In addition to the fatty acid precursors of endocannabinoids, the potential role of dietary cannabimimetic phytochemicals in agriculturist nutrition is discussed. Dietary secondary metabolites from vegetables and spices able to enhance the activity of cannabinoid‐type 2 (CB2) receptors may provide adaptive metabolic advantages and counteract inflammation. In contrast, chronic CB1 receptor activation in hedonic obese individuals may enhance pathophysiological processes related to hyperlipidaemia, diabetes, hepatorenal inflammation and cardiometabolic risk. Food able to modulate the CB1/CB2 receptor activation ratio may thus play a role in the nutrition transition of Western high‐calorie diets. In this review, the interplay between diet and the ECS is highlighted from an evolutionary perspective. The emerging potential of cannabimimetic food as a nutraceutical strategy is critically discussed.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- 2‐AG
2‐arachidonoyl glycerol
- AA
arachidonic acid
- AEA
N‐arachidonoylethanolamine, anandamide
- apoE
apolipoprotein E
- BAT
brown adipose tissue
- BCP
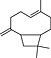
β‐caryophyllene
- CNR1
gene encoding CB1 receptors
- DHA
docosahexaenoic acid
- DIM
3,3′‐diindolylmethane
- EC
endocannabinoid
- ECS
endocannabinoid system
- EPA
eicosapentanoic acid
- FAAH
fatty acid amide hydrolase
- NAE
N‐acylethanolamine
- PEA
palmitoylethanolamide
- PUFA
polyunsaturated fatty acid
- SNP
single nucleotide polymorphism
- T2DM
diabetes mellitus type 2
- THC
Δ9‐tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid 1
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes e |
| AMY1 receptor | ALDH2 |
| CB1 receptor | COX‐2 |
| CB2 receptor | FAAH (FAAH1) |
| GPR55 | MAGL (MGL) |
| Ligand‐gated ion channels b | PLA2 |
| GABAA receptor | |
| NMDA receptor | |
| Voltage‐gated ion channels c | |
| TRPV1 | |
| Nuclear hormone receptors d | |
| PPARγ |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
The endocannabinoid system (ECS) is an ancient panorgan eicosanoid signalling network in which arachidonic acid (AA) derived lipids act in concert with particular receptors and enzymes resulting in the complex modulation of numerous central and peripheral physiological and pathophysiological processes (Pertwee, 2005, 2009; Di Marzo, 2008a; Pacher and Mechoulam, 2011; DiPatrizio and Piomelli, 2015). The ECS comprises classical GPCR cannabinoid receptors (CB1 and CB2) and potentially also the orphan receptor GPR55 (Pertwee, 2007; Ryberg et al., 2007), which are differentially activated by the endocannabinoids (ECs) 2‐arachidonoyl glycerol (2‐AG) and N‐arachidonoylethanolamine (anandamide, AEA) (Devane et al., 1992; Mechoulam et al., 1995; Sugiura et al., 1995; Hanus, 2009). AEA and 2‐AG, which are generated from discrete phospholipid precursors at the inner cellular membrane leaflet, also modulate different ion channels and nuclear receptors, like, for example, transient receptor potential vanilloid 1 (TRPV1), GABAA receptors and PPAR‐γ (O'Sullivan, 2007; Ross, 2009; De Petrocellis and Di Marzo, 2010; Pertwee, 2010; Sigel et al., 2011). Importantly, the enzymes degrading the ECs AEA and 2‐AG, namely, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), but also α‐β hydrolases‐6 (ABHD6) have been shown to regulate local and paracrine EC concentrations (Di Marzo, 2008a; Marrs et al., 2010). In inflamed tissue, COX‐2 catalyses the oxygenation of both AA and ECs, leading to an additional control of tissue EC concentration during inflammation (Hermanson et al., 2014). Finally, there is an as yet unidentified facilitated EC cellular reuptake mechanism in certain neuronal cell types and immune cells that can be selectively inhibited and may thus present another level of biological regulation (Nicolussi and Gertsch, 2015). CB1 receptors are involved in the control of behaviour (e.g. motivation, reward, memory processing and habituation to stress) and are thus expressed widely in the CNS where they act as major neuronal circuit breakers, generating a negative retrograde feedback at both glutamatergic and GABAergic synapses in the CNS (Freund et al., 2003; Kano, 2014). CB1 receptors are not only among the most frequent GPCR species in the brain, but functional CB1 receptors are also expressed peripherally and overall probably evolved under the selection pressure of fundamental physiological stress stimuli (Bowles et al., 2015; Morena et al., 2016). These include physical activity, famine, the fight or flight response, traumata and microbial infections. The peripheral signal transduction pathways of CB1 receptors are still poorly understood. Activation of the ECS is associated with the major stress response (i.e. via the hypothalamic pituitary adrenal axis) or just physical activity (Tantimonaco et al., 2014). While the glucocorticoid receptor regulates expression of the CNR1 gene encoding CB1 receptors (Hillard, 2014), ECs modulate stress factors in the brain (McEwen et al., 2015). This regulation appears to be dynamic and not static. For instance, studies in rodents indicate that acute glucocorticoid administration enhances the activity of ECs whereas chronic exposure to glucocorticoids down‐regulates the ECS (McPartland et al., 2014). In healthy mammals, CB1 receptor signalling may facilitate their survival after excessive physical activity, stress and trauma by restoring homeostasis, suppressing negative memories and reducing anxiety at the level of the CNS (Ruehle et al., 2012), as well as reactivating appetite and catabolic processes at the peripheral level (Watkins and Kim, 2015). The CB1 receptor‐mediated neuronal responses are linked to central reward and motivation (Hernandez and Cheer, 2015), and hedonically positive sensory properties of food lead to activation of CB1 receptor‐mediated reward circuits, which control food selection in rodents (DiPatrizio and Simansky, 2008; Deshmukh and Sharma, 2012; Hernandez and Cheer, 2012; Thompson et al., 2016). The selection of palatable food (i.e. lipid and sugar craving) is already present in newborns. It has been shown that milk suckling in newborns is partly mediated via CB1 receptor activation (Mechoulam et al., 2006). Accordingly, CB1 receptors are present in taste buds, and their activation enhances neural responses to sweet foods (DiPatrizio and Piomelli, 2012). Milk is also a significant source of AA, which can trigger EC biosynthesis in the brain (Berger et al., 2001), thus having potentially broad physiological effects. ECs are not only involved in the initiation of suckling and, therefore, in the feeding and growth of the offspring but also impact behaviour (Manduca et al., 2012). The amount of 2‐AG in human milk is in the range of 7–20 nM, that is, close to receptor‐active concentrations and about 100 times higher than AEA (Di Marzo et al., 1998). Distinct dairy fat compositions have been shown to modulate the levels of ECs in plasma in a yet poorly understood manner (Pintus et al., 2013; Dunn et al., 2014). It remains unclear whether fat intake in humans is directly linked to ECS‐mediated pathophysiological effects (i.e. via chronic CB1 receptor activation). For instance, carnivores and pastoralists ingest ECs and significant amounts of the EC precursor AA from raw meat or dairy products without adverse effects. While the ingestion of ECs may not lead to systemic physiological effects beyond the gastrointestinal (GI) tract because they are locally metabolized (Di Marzo et al., 1998), the modulation of the EC concentrations by bioavailable polyunsaturated fatty acids (PUFAs) like AA is well described and will be discussed below.
Experiments with genetically modified mice have shown that central CB1 receptors can exert paradoxical effects on food intake, depending on whether they are localized to presynaptic terminals of excitatory or inhibitory neurons (Bellocchio et al., 2010). In order to better understand this apparent hormetic complexity, the functioning of the ECS needs be put into context with evolution, that is, the environmental selection pressures and dietary habits, which are very different among different mammals, but also between distinct human populations (e.g. hunter‐gatherers versus Western societies or upon the introduction of agriculture). Comparisons with great apes and the fossil and archaeological records suggest that among the most important changes in diet was an increase in plant carbohydrates during human evolution (Aiello and Wells, 2002). Generally, in the context of high‐calorie diets as found in plant starch farming societies, chronic CB1 receptor activation is associated with increased obesity, an unfavourable lipid profile, insulin resistance, exacerbation of inflammation in the liver and kidney (Di Marzo, 2008b; Gruden et al., 2016) and cardiometabolic risk (Janero, 2012).In contrast, CB2 receptors, which show 68% homology to CB1 receptors in the transmembrane region, appear to be primarily expressed in the periphery in immune cells like monocytes/macrophages where they negatively modulate inflammatory stress, for example, via attenuation of Toll‐like receptor‐induced signal transduction pathways (Tomar et al., 2015). In the liver and kidney, CB2 receptor activation is clearly protective (Pacher and Mechoulam, 2011). Many other cell types seem to express low amounts of CB2 receptors, although they may not be present functionally at the cell surface (Kleyer et al., 2012). CB2 receptors have been shown to protect tissues from fibrotic processes, possibly also by modulating macrophage polarization (Pacher and Mechoulam, 2011; Gertsch, 2016). Intriguingly, CB2 receptors have more recently been shown to stimulate a number of positive metabolic processes leading to antidiabetic effects and cardiometabolic protection (vide infra). As proposed here, one role of CB2 receptors in diet‐driven metabolic processes could be to antagonize the pathophysiological metabolic effects mediated by CB1 receptors, at least in a high‐calorie and pro‐inflammatory dietary context. Although CB2 receptors in the brain are clearly expressed in microglia cells (Maresz et al., 2005), the presence or physiological relevance of functional CB2 receptors in subsets of neurons is still debated. Importantly, both CB receptors play pro‐homeostatic physiological roles, which may however differ in distinct animal species. The metabolic effects of the overall EC concentrations in tissues are clearly complex as these lipids are promiscuous in their action (Di Marzo and De Petrocellis, 2012) and in addition to CB receptors also target different channels and nuclear receptors (vide supra). For instance, AEA also activates the PPARs (O'Sullivan, 2007), a family of transcription factors that regulate energy balance by promoting either energy deposition or energy dissipation (Medina‐Gomez et al., 2007). Under normal physiological conditions, PPARγ is mainly expressed in adipose tissue together with CB1 receptors where it regulates diverse functions such as the development of fat cells and their capacity to store lipids. Since there are numerous PPAR modulators (e.g. PPARγ activators) in vegetable diets (Wang et al., 2014; Li et al., 2015), PPAR‐active phytochemicals in diet may play a co‐regulatory role in modulating the ECS. From an evolutionary perspective, depending on dietary habits, the role of the ECS in energy metabolism could be distinctly different between herbivores, carnivores and omnivores. The ECS is also a regulator of intestinal function and the brain‐gut axis. It generally inhibits neural activity in pathways involved in the physiological regulation of the GI tract, including visceral sensation, pain, motility but also different forms of inflammation (Izzo, 2007; Izzo et al., 2015; Sharkey and Wiley, 2016). The dysregulation of the ECS has been implicated in numerous human diseases, and its pharmacological modulation is a very promising strategy to prevent or treat inflammatory, neurodegenerative, cardiovascular, metabolic disorders, ischaemia damage, as well as pain and maybe certain types of cancer (Di Marzo, 2008a; Pacher, 2009; Maccarrone et al., 2015).
Although several comprehensive reviews on the connection between diet and the ECS have been published (Matias et al., 2006; Osei‐Hyiaman et al., 2006; Carr et al., 2008; Di Marzo et al., 2009; DiPatrizio and Piomelli, 2012; Bisogno and Maccarrone, 2014; Kleberg et al., 2014), little emphasis has been put on the evolutionary context and the differential roles CB1 and CB2 receptors might play in food selection and metabolic stress. One exception is the excellent review by DiPatrizio and Piomelli (2012) providing open questions on the role of the CB1 receptors in energy needs and maintaining metabolic balance in mammals. Here, an evolutionary perspective on the link between diet and the ECS is provided, with emphasis on the changes introduced by agriculture and potential health implications of a ‘cannabimimetic diet’.
Cannabimimetics in the plant kingdom and vegetable food
Secondary metabolism in plants is an enormously rich source of chemically diverse molecules (Firn and Jones, 2003; Koch et al., 2005), and it is therefore not surprising to find numerous biologically active natural products in plants, including food plants (Nilius and Appendino, 2013; Atanasov et al., 2015, Russo, 2016). From a phylogenetic perspective, secondary metabolite ligands of mammalian receptors do already occur in the plant kingdom. Apart from peptides, virtually all neurotransmitters and hormones have already been ‘invented’ by plants and fungi (Murch, 2006), pointing towards receptor evolution driven by chemical environmental selection pressures (i.e. ligand‐based selection of mutations based on function). Nevertheless, chemical diversity in plants is shaped by environmental conditions and predation pressure, leading to vitamin‐like (i.e. essential), nonspecific (Gertsch, 2016) or even xenohormetic secondary metabolites (Lamming et al., 2004; Howitz and Sinclair, 2008) in the Animalia food pyramid. Neurotransmitters, modulators and hormones naturally have very short half‐lives and are not generally orally bioavailable due to the metabolic enzymes present in most tissues, including the GI tract. Noteworthy, ECs are present widely in lower plants, including mosses and ferns but not in flowering plants that serve as food for mammals (Gachet et al., 2017). However, based on the chemotype diversity and substrate plasticity of secondary metabolism, numerous ‘similars’ are being produced from related chemical scaffolds, mimicking or modulating the action of mammalian receptor ligands or enzyme substrates (Appendino et al., 2014). If such phytochemicals by chance have a better metabolic stability than an endogenous ligand, they are likely to exert targeted pharmacological effects. Through diet, such effects can be chronic. In the context of this short review and perspective, Δ9‐tetrahydrocannabinol (THC) from Cannabis sativa L. mimics the effects of 2‐AG and AEA by activating cannabinoid receptors, a coincidence that has led to the medicinal and recreational use and cultivation of this plant species and ultimately the discovery of the ECS (Mechoulam, 2002). Nutritious cannabis seeds lacking phytocannabinoids have played a role as food and have been cultivated since millennia (Chen et al., 2012). Although the ECS has been elucidated thanks to research on phytocannabinoids from cannabis, there are food‐derived natural products that are able to indirectly modulate this system, at least in the periphery. Given the apparent prominent modulatory role of the ECS in energy metabolism, the elucidation of ECS active dietary factors beyond common nutrients could serve as basis to search for a covariance between genes and diet.
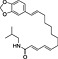
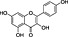
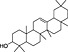
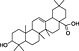
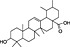
In order to identify secondary metabolite signatures in plants that potentially modulate ECS proteins, we are currently screening global plant extract libraries within the frameworks of MedPlant.EU and the Swiss NCCR TransCure. Different widespread (canonical) triterpenoids have already been shown to inhibit metabolic enzymes of the major EC 2‐AG (King et al., 2009; Bento et al., 2011b; Chicca et al., 2012; Parkkari et al., 2014), ubiquitous plant flavonoids were found to inhibit FAAH, the metabolic enzyme degrading AEA and other N‐acylethanolamines (NAEs) (Thors et al., 2007; 2008; 2010) and to weakly modulate CB receptors (Khedr et al., 2016) (Table 1). Intriguingly, the ubiquitous plant sesquiterpene β‐caryophyllene (BCP) has been shown to exert potent CB2 receptor‐mediated cannabimimetic effects in mice (Gertsch, 2008; Gertsch et al., 2008; Bento et al., 2011a; Horváth et al., 2012; Cheng et al., 2014; Klauke et al., 2014) and N‐alkylamides from maca (Lepidium meyenii Walp.) and black pepper (Piper nigrum L.) show cannabimimetic effects in vitro and in vivo respectively (Hajdu et al., 2014; Nicolussi et al., 2014). The polyacetylene falcarinol (carotatoxin) present in carrots and other vegetables was shown to inhibit CB1 receptor activation by AEA in vitro (Leonti et al., 2010). Several isoprenylated analogues of the naturally occurring plant stilbenoid trans‐resveratrol bind to both CB1 and CB2 receptors with low affinity (Brents et al., 2012). The widespread dietary triterpnoids oleanolic acid (present in olive oil) and ursolic acid (Table 1) have been shown to inhibit ABDH12 (Parkkari et al., 2014), an enzyme that controls 2‐AG levels in immune cells. Thus, different dietary phytochemicals have already been shown to directly or indirectly modulate the ECS (here referred to as cannabimimetics) (Gertsch et al., 2010; Russo, 2016). The emerging question is whether such phytochemicals in spices and food are bioavailable and exert physiological effects in vivo and how diet modulates the ECS and vice versa? Currently, the best evidence of a dietary link to ECS modulation stems from studies on PUFAs, that is, the indirect effects of Ω6 and Ω3 fatty acids on EC production and/or ECS proteins (vide infra). Yet the more recent discovery of CB2 receptor‐selective cannabimimetics in spices may play a widely unrecognized physiological role, coinciding with the emerging evidence that certain spices and vegetables in the diet can reduce the risk of metabolic syndrome and diabetes mellitus type 2 (T2DM) and associated risk factors (Sikand et al., 2015).
Table 1.
Phytochemicals in diet that may modulate the ECS
| Plant secondary metabolite | Chemical structure | Dietary origin | Target/effect | Potency (IC50, K i)/efficacy | In vivo evidence | Selected references |
|---|---|---|---|---|---|---|
| BCP |
(1).
|
Most widespread, numerous food plants, spices | CB2 receptor agonist | ~200 nM/partial (in vitro), full (in vivo) | strong | Gertsch et al., 2008; Horváth et al., 2012; Klauke et al., 2014 |
| DIM |
(2).
|
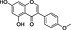
Brassicaceae vegetables | CB2 receptor agonist | ~1 μM/partial | missing | Yin et al., 2009 |
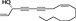
| Falcarinol |
(3).
|
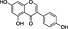
Apiaceae, carrots (Daucus carrota), ginseng | CB1 receptor inverse agonist | ~200 nM/full | missing (indirect) | Leonti et al., 2010 |
| Macamide |
(4).
|
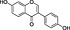
Macca (Lepidium meyenii) | AEA reuptake inhibitor (CB1 binding) | ~200 nM/partial | missing | Hajdu et al., 2014 |
| Guineensine |
(5).
|
Piper spp., black pepper | AEA reuptake inhibitor | ~200 nM/full | good | Nicolussi et al., 2014 |
| Biochanin A |
(6).
|
Soybeans (Glycine max), chickpeas (Cicer arietinum) | FAAH1 inhibitor | 0.5–2 μM/full | some | Thors et al., 2010 |
| Genistein |
(7).
|
Fava beans (Vicia faba), lupin (Lupinus spp.), soy | FAAH inhibitor/AEA uptake inhibitor | 1–3 μM/full | missing | Thors et al., 2007; Thors et al., 2010 |
| Daidzein |
(8).
|
Fava beans (Vicia faba), lupin (Lupinus spp.), soy | FAAH inhibitor/AEA uptake inhibitor | 2–4 μM/full | missing | Thors et al., 2007; Thors et al., 2010 |
| Kaempferol |
(9).
|
Widespread in food plants | FAAH inhibitor | 2–4 μM | missing | Thors et al., 2008 |
| β‐amyrin |
(10).
|
Widespread in vegetables | MAGL/ABHD6/ABHD12 inhibitor | ~1 μM/partial | some | Bento et al., 2011b; Chicca et al., 2012 |
| Oleanolic acid |
(11).
|
Relatively widespread in food plants (olive oil) | ABHD12 inhibitor | ~1.5 μM/full | some | Parkkari et al., 2014 |
| Ursolic acid |
(12).
|
Widespread in food plants | ABHD12 inhibitor | ~2 μM/partial | missing | Parkkari et al., 2014 |
| Pristimerin |
(13).
|
Scarce in food, Celastraceae | MAGL inhibitor | 100 nM/full reversible | missing | King et al., 2009 |
| Euphol |
(14).
|
Scarce in food | MAGL inhibitor | 300 nM/full reversible | missing | King et al., 2009 |
| NAEs |
(15).
|
Present in higher plants, widespread in fresh food plants | FAAH and NAAA inhibitors | nM metabolized in vivo | some | Gachet et al., 2017; Petrosino and Di Marzo, 2017 |
The endocannabinoid food‐medicine continuum in the context of life‐style
Bioactive phytochemicals form the molecular basis of the food‐medicine continuum, which has its origin in the co‐evolution of diet and biochemical processes underlying animal physiology (Etkin and Ross, 1982; Johns, 1990; Moerman, 1996; Heinrich and Prieto, 2008). Just like essential fatty acids and vitamins have evolved from the constant animal–plant interactions in time and space, there are probably numerous yet poorly understood dietary biochemical modulations that shape our fitness. Almost certainly, many of these plant–animal interactions remain to be discovered. Based on the concept of phytochemical network pharmacology (Hopkins, 2008; Gertsch, 2011), weak yet constant modulatory effects on different nodes of the ECS (even below detection in vitro) may suffice to exert significant physiological effects over time. Such effects are of particular relevance for dietary interventions. As illustrated by recent genetic association studies with CB receptors (vide infra), dietary selection pressures might also explain some of the pronounced species differences observed with ligands targeting cannabinoid receptors, in particular CB2 receptors (McPartland et al., 2007). The development of the ECS reflects convergent, divergent and parallel evolution involving duplications and mutations of EC receptors, resulting in gene extinctions or new structures/functions (McPartland et al., 2006). Given the importance of the constant flux of phytochemicals from vegetable food, some of these later adaptive events may have been associated with dietary changes, such as the introduction of agriculture and the differential use of spices rich in cannabimimetics (vide infra). For instance, a recent study has shown that the continuous consumption of Ω3 PUFAs by the Inuit in Greenland causes dietary genetic and physiological adaptations reflected by mutations in desaturase genes (Fumagalli et al., 2015). Therefore, dietary habits can change physiology within a relatively short time span (~10 000 years) via both genetic and epigenetic mechanisms. Despite the fact that some ECS genes show evidence of adaptive evolution, the system is under strong purifying selection (McPartland et al., 2007), suggesting that diet could indeed be a major factor in this process.
As reviewed by Leonard et al. (2010), research in human evolutionary biology has shown that many of the key features that distinguish humans from other primates (e.g. our large brain size) also have implications for our distinctive nutritional needs (Aiello and Wheeler, 1995; Leonard and Robertson, 1997; Leonard, 2002). To accommodate the metabolic demands of our large brains, humans consume diets that are very dense in energy and nutrients. For instance, there are intriguing differences in lipid consumption between humans and monkeys (vide infra) revealing that humans strictly prefer lipid‐rich foods (Montmayeur and le Coutre, 2010). CB1 receptor activation is associated with increased energy intake and decrease energy expenditure by controlling the activity of neural pathways involved in the sensing and hedonic processing of fatty foods (DiPatrizio and Piomelli, 2012). Consequently, the ECS is a lipid signalling network that has implications for lipid intake. In the gut, ECs may promote fat intake by activating CB1 receptors on vagal fibres and enteric neurons (Izzo and Sharkey, 2010). Kirkham (2009) further emphasized the central role of the ECS to process lipid food stimuli that exert an influence over consumption via innate and learned appetites, generating the complex psychological experiences of hunger, lipid craving and delight independently of energy status. As pointed out by Leonard et al. (2010), in contrast to the levels seen in human populations, monkeys obtain only a small share of calories from dietary fat. Popovich et al. (1997) estimated that lowland gorillas derive only approximately 3% of their energy from dietary fats. The need for an energy‐rich diet in Paleolithic and Neolithic times has shaped our ability to detect and metabolize high‐fat foods. Food preferences are based on lipid sensory inputs (Sclafani, 2001; Gaillard et al., 2008; Le Coutre and Schmitt, 2008) and that our brains have the ability to assess the energy content of foods with remarkable accuracy (Toepel et al., 2009). Additionally, compared with monkeys, humans have an enhanced capacity to digest and metabolize higher fat diets. Our GI tract has an expanded small intestine and reduced colon, consistent with the consumption of a high‐quality diet consisting of large amounts of animal food (Milton, 1987). Since natural fat intake differs widely between animal species, the translation from animal studies to humans needs to be interpreted with care. In contrast to most non‐carnivorous animals, humans ingest significant amounts of AA through animal products like meat, dairy products and eggs (an estimated 0.1–0.6 g·day−1). In contrast to the fish and algae‐derived Ω3 PUFAs docosahexaenoic acid (DHA) and eicosapentanoic acid (EPA), which tend to lower EC levels, the intake of AA has been shown by different studies to be associated with increased EC levels in different tissues (reviewed in McPartland et al., 2014). The consumption of Ω3 and Ω6 essential fatty acids in Western diets (USA) has changed markedly with industrialization, with an increase in γ‐linoleic acid (LA) availability from an estimated 3% to about 8% of energy supply (Blasbalg et al., 2011). Higher food plants (Angiosperms), which constitute most of mammalian vegetable diet, do not generally synthesize AA but the precursor LA that is essential for AA biosynthesis in animals. Although high AA intake has been associated with pro‐oxidative and inflammatory systemic responses (Ferretti et al., 1997; Ling et al., 2012) and some evidence suggests that the consumption of a diet high in AA is associated with the development of leptin resistance and obesity (Cheng et al., 2015), the role of this Ω6 PUFA is pleiotropic and complex (playing distinct positive and negative roles in different circumstances). Recent data suggest that there is even a possibility of chronic AA administration having a reducing anti‐inflammatory effect on the kidney (Katakura et al., 2015). Moreover, no evidence is available from randomized, controlled intervention studies among healthy, noninfant humans to show that addition of the AA precursor LA to the diet increases the concentration of inflammatory markers (Johnson and Fritsche, 2012). Biochemically, dietary AA is incorporated into membrane phospholipids from where it is released by a PLA2‐mediated enzymatic hydrolysis or exists as triacylglycerol. Free AA (arachidonate) can either be tissue and/or inflammation‐dependently metabolized into prostaglandins, leukotrienes, thromboxanes, prostacyclin or ECs. 2‐AG is a significant precursor (store) of AA in brain that is regulated via 2‐AG hydrolysis (Nomura et al., 2011). Overall, AA intake is of fundamental importance for human brain development where AA and DHA constitute the major PUFAs in the CNS. The vital importance of AA was shown by a FADS1 KO study in which AA supplementation prevented the lethal phenotype (Fan et al., 2012). To assure there are sufficient amounts of AA in the brain, rather than generating AA de novo from LA, our organs seem to preferentially take it from diet. Dietary AA seems to be efficiently taken up and transported into different organ tissues, including the liver and brain by poorly understood mechanisms. It has been shown that orally ingested labelled arachidonate is directly incorporated into phospholipids of the brain and other organs (Likhodii and Cunnane, 1999). A recent LC–MS/MS study revealed that plasma concentrations of AA in human, young healthy individuals are in the range of 2.5–8 μM and inversely correlate with cortisol levels (Gachet et al., 2015; Gachet and Gertsch, 2016). In streptozotocin‐induced diabetes animal models, AA was found to be depleted and Δ5‐desaturation inhibition described as a fundamental feature of diabetes (Holman et al., 1983). A more recent study showed that a lower AA/dihomo‐γ‐linolenic acid ratio is associated with metabolic abnormalities in obese individuals (Zhao et al., 2016). The current literature on human studies with Ω3 PUFA enriched diets (e.g. krill, Euphausia superba Dana oil) on the effects of the ECS is somewhat unclear and may reflect differences between human populations. In obese individuals, the Ω6/Ω3 PUFA ratio in plasma generally correlates with a decrease in EC levels (Banni et al., 2011). It has been suggested that early dietary interventions based on Ω3 PUFAs may represent an alternative strategy to drugs for reducing endocannabinoid tone and improving metabolic parameters in the metabolic syndrome (Demizieux et al., 2016). For example, krill diet let to a concomitant reduction of triglyceridaemia and EC levels and was associated with a decreased waist/hip and visceral fat/skeletal muscle mass ratio (Berge et al., 2013). In this study, it was suggested that treatments with krill formulations may produce different effects on plasma EC levels depending on different cohorts of subjects, duration of treatments (4 vs. 24 weeks) and dosage (2 vs. 4 g·day−1). Most likely, the effects of PUFAs on the human ECS strictly depend on the lifestyle. Accordingly, hunter‐gatherers and pastoralist societies such as the ones in the Sub‐Saharan region do not show increased obesity or T2DM, despite their constant high intake of Ω6 PUFA (AA). Generally, a lower ratio of Ω6/Ω3 PUFAs is desirable in reducing the risk of many of the chronic diseases of high prevalence in industrialized societies or societies with high‐carbohydrate intake (Simopoulos, 2002; Wang and Chan, 2015).
Finch and Stanford (2004) have shown that the evolution of key ‘meat‐adaptive’ genes in hominid evolution, such as apolipoprotein E (apoE), are critical for the promotion of the enhanced lipid metabolism necessary for subsisting on diets with greater levels of animal material. A beneficial interplay between apoE and CB1 receptor activation has been proposed (Zhao et al., 2010; Bartelt et al., 2011). In agreement with the concept of genetic adaptation to diet, the CB1 (CNR1) single nucleotide polymorphism (SNP) 1359 G/A (p.Thr453Thr; rs1049353), a common polymorphism in Caucasians, has been reported to be associated with less fat intake (fatty acids and cholesterol) but more carbohydrate intake in obese females (de Luis et al., 2016). The latter association is interesting as it could suggest that CB1 receptors originally served to motivate lipid intake from meat are under dietary pressure in Western societies. Moreover, carriers of this SNP have a better lipid profile and a lower body mass index (Storr et al., 2010; de Luis et al., 2015). In contrast, in a small group of 60 diabetic individuals a lack of association of G1359A polymorphism with obesity, cardiovascular risk factors was reported (de Luis et al., 2010). However, subjects with C385C genotype of FAAH1 showed an improvement in insulin and homeostatic model assessment‐R levels with a high PUFA hypocaloric diet after losing weight for 3 months (de Luis et al., 2013). In women with obesity, an overall association of the mutant‐type group G1359A and A1359A with a better cardiovascular profile (triglyceride, high‐density lipoprotein cholesterol, insulin and homeostasis model assessment levels) than the SNP lacking group was reported (de Luis et al., 2011). These emerging human genetic data may suggest a possible purifying selection of genes in the ECS with respect to dietary habits.
If chronic CB1 receptor activation in humans would cause consistent hyperphagia independently of lifestyle, beyond the well‐documented acute appetite‐stimulating effects, and/or foster insulin resistance or T2DM, then this should be clearly observed in the human populations that regularly smoke high THC cannabis for recreational purposes. THC is a potent partial human CB1 receptor agonist but only a very inefficient human CB2 receptor agonist, at least in vitro. Indeed, acute cannabis use is classically associated with snacking behaviour (munchies). Studies generally suggest that acute cannabis use stimulates appetite, also in the therapeutic context of hypohagia in AIDS and cancer patients (Whiting et al., 2015). Nevertheless, as for large epidemiological studies in the general population, findings consistently indicate that cannabis users tend to have rather lower body mass indices than nonusers (Hayatbakhsh et al., 2010). The reason for this discrepancy between animal studies and humans and the overall unclear picture could be that cannabis consumers have a differential stress response from the rest of the population, and above all, they are not generally obese. Perceived stress, emotional eating, anhedonia, depression, dietary restraint and disinhibition are risk factors for obesity. Consequently, neuropsychological effects counteracting stress through cannabis consumption may mask the molecular mechanisms studied in mice. Although in one human study chronic cannabis smoking was associated with visceral adiposity and adipose tissue insulin resistance (Muniyappa et al., 2013), there is no explicit evidence that cannabis smoking causes insulin resistance or T2DM (Alshaarawy and Anthony, 2015). Nevertheless, a recent study has shown that increased years of cannabis or cigarette smoking are important factors in metabolic health (Yankey et al., 2016), concluding that each year increase in marijuana use was significantly associated (maybe not causative) with increased odds of metabolic syndrome and hypertension. From a mechanistic point of view, the best evidence for the negative metabolic effects mediated via CB1 receptors stems from animal experiments. Chronic CB1 receptor activation in mice clearly causes obesity‐related insulin resistance; this is probably mediated by hepatic CB1 receptor‐induced inhibition of insulin signalling and clearance (Liu et al., 2012; Picone and Kendall, 2015). Moreover, peripherally restricted CB1 receptor antagonists retain efficacy in reducing weight and improving metabolic abnormalities in mouse models of obesity (Kunos and Tam, 2011).
The function of CB1 receptors may make sense in the context of hunter‐gatherer nutrition where fat is the primary nutrient and physical activity is high, but might have led to a conflict with high‐carbohydrate intake in professional agriculturist nutrition. An emerging question is whether different lifestyles determine the role of the ECS in allostasis. Diets of hunter‐gatherers show substantial variation in their carbohydrate content. However, the range of energy intake from carbohydrates in the diets of most hunter‐gatherer societies is markedly lower from the amounts currently recommended for healthy Western humans (Ströhle and Hahn, 2011). In line with the extreme carbohydrate craving in humans, who have more copies of the salivary amylase genes than primates and thus more efficiently digest starch (Perry et al., 2007), the onset of agriculture was probably one of the most dramatic and important developments in human history (Diamond, 2002). Carbohydrate farming incited the most important dietary transition, which is still ongoing to the present day of post‐agriculturist nutrition (i.e. based on refined sugars). The generation and excess use of sugars could be seen in analogy to the detrimental impact of the first distilled alcohol on humans. The sudden availability of excess sugars in combination with fats in diet may have led to a collision of genes that evolved to cope with high energy demands due to constant physical activity (Neel, 1962). Excessive consumption of high‐energy, palatable food without physical activity contributes to obesity, which results in the metabolic syndrome, heart disease and T2DM (Mazier et al., 2015). In obese individuals, increased EC levels are also found in the liver, adipose tissue, pancreas and skeletal muscle, where they contribute to hepatic steatosis and insulin resistance, adipocyte hypertrophy and inflammation, reduced glucose uptake and oxygen consumption in the muscle and reduced beta cell function (Silvestri et al., 2011; Cristino et al., 2014a,b). Thus, CB1 receptors may have evolved as pro‐homeostatic (i.e. allostatic) receptors in the context of survival challenges (food restriction, fight or flight response, hunting, physical and psychological traumata) not entirely compatible with the lifestyles of contemporary post‐agriculturists.
The introduction of cannabimimetic spices during agriculture
In 1997, Eaton et al. revisited their seminal paper on the dietary origin of chronic metabolic disorders as the result of a mismatch between ancient genes and high‐calorie diets (Eaton and Konner, 1985; Eaton et al., 1997). The multimillion year evolutionary process during nearly all of which genetic change reflected the life circumstances of our ancestors was suddenly disturbed by the introduction of agriculture about 12 000 years ago. Dietary carbohydrates once essential for the cognitive and social development of Paleolithic humans gradually turned into a metabolic stress factor as a function of their glycaemic indices. Epidemiological evidence points towards a pandemic diet‐induced glucotoxicity due to excess sugar intake (Hite et al., 2011). Likewise, excessive intake of fat can lead to lipotoxic pathophysiological effects, yet there is a more direct strong link between glucotoxicity and the metabolic syndrome and T2DM in humans (Guldbrand et al., 2014). The independent Swedish Council on Health Technology Assessment has concluded that dietary fat is not associated with obesity (Hansen, 2013) and, consequently, T2DM and cardiometabolic risk. The committee reviewed 16 000 studies published through until 2013 and recommended that a low‐carbohydrate, high‐fat diet should be the most effective measure against obesity. Yet the reality of post‐agriculturist societies is a concomitant high‐carbohydrate and high‐fat intake. Most of the rodent studies on the pathophysiological role of the ECS in energy homeostasis stem from high‐fat diets and not from high‐carbohydrate diets, with few exceptions. Interestingly, CB1 receptor inverse agonist/antagonist‐treated rats fed with either high fat or high‐carbohydrate diet showed differential responses (Rivera et al., 2013), indicating that the dietary context for the role of the ECS is important.
With the onset of agriculture, the cultivation and consumption of green leafy vegetables and spices was also initiated. The regular use of green leafy vegetables and spices can be seen as an innovation of agriculturists as both an adaptive process to environment and taste (Heinrich et al., 2006; Krebs, 2009; Leonti, 2012). Spices are typically rich in essential oils and terpenes, thus providing a source of potential lipid modulators of the endogenous lipid systems, including the ECS, TRP channels (TRPV1 and TRPA1), the PPARs and the overall eicosanoid system (see also Russo, 2016). Just like salt increases the palatability of food, certain hot or flavoured spices can do the same. However, there are many spices in agriculturist diets that exert pharmacological effects. For instance, phenylpropanoids from ginger (Zingiber officinale L.) have been shown to pleiotropically interfere with the arachidonate signalling system by targeting COX‐2 (van Breemen et al., 2011) and PLA2 (Nievergelt et al., 2011), leading to potent anti‐inflammatory effects by disrupting IL‐1β expression (Nievergelt et al., 2011). Numerous plant volatiles among spices modulate ion channels (Maffei et al., 2011), such as TRPV1 that signals to the ECS. NAEs like palmitoylethanolamide (PEA) are very abundant in flowering plants (Gachet et al., 2017), which constitute a major source of food. Given the emerging pharmacology of PEA (Petrosino and Di Marzo, 2017), it will be interesting to assess the biological contribution and significance of NAEs from diet. Overall, this dietary adaptation to eating green leafy vegetables and spices rich in essential oils and NAEs may not be a coincidence but a biological function to counteract metabolic stress induced by the excessive carbohydrate intake. However, the epidemiological evidence does not portray a clear picture. As shown by a recent big epidemiological study from China, ‘spicy food’ was, quite unexpectedly, positively associated with body weight (Sun et al., 2014). In Chinese cuisine, spicy food is more meat‐based rather than vegetable‐based with heavy salt and/or oil use for flavour and palatability, the primary spice being hot pepper (Capsicum spp.). This contrasts with the findings of other herbal spices that have been shown to reduce body weight and improve glucose tolerance (Grant et al., 2009; Bower et al., 2016; Sikand et al., 2015). While the roles of vitamins, minerals and Ω3 PUFAs for human health have been studied in detail, the role of secondary plant metabolites that directly or indirectly interact with our physiology beyond flavonoids remains largely unknown. One reason for this is the enormous difficulties in studying mixtures of poorly bioavailable natural products in vivo (Gertsch, 2011). Nevertheless, the introduction of numerous spices during agriculture is intriguing and might reflect an adaptive process to high‐calorie diets.
Diet‐induced shifting of the CB1/CB2 receptor activation ratio?
Research from animal models but also humans (i.e. RIO studies with rimonabant) impressively shows that in high‐calorie diets, CB1 receptor activation is causally associated with obesity and the metabolic syndrome and thus directly modulates energy balance (Mazier et al., 2015; Gatta‐Cherifi and Cota, 2016). In metabolically healthy obese individuals, overactive CB1 receptors in adipocytes, pancreas and liver may foster the onset of a metabolic syndrome, but probably not in non‐obese individuals (Cable et al., 2014). Therefore, a dietary CB1 receptor antagonist in combination with high‐calorie diets could potentially reduce the risk of CB1 receptor‐mediated metabolic pathologies in the context of high‐calorie diets. The only dietary antagonist/inverse agonist of CB1 receptors reported so far is the acetylenic oxylipin falcarinol, which predominantly occurs in carrots (Daucus carota L.), but also in many other Apiaceae vegetables such as parsley (Petroselinum crispum L.), celery (Apium graveolens L.), parsnips (Pastinaca sativa L.), fennel (Foeniculum vulgare Mill.) and in ginseng (Panax ginseng C.A. Meyer). This natural product was introduced into the human diet upon the transition from hunter‐gatherers to agriculturists. In addition to apparently irreversibly inhibiting CB1 receptors in vitro (Leonti et al., 2010), falcarinol also covalently blocks the aldehyde dehydrogenase 2 family by alkylation of the active site (ALDH2; Heydenreuter et al., 2015), activates nuclear factor erythroid‐2 related factor 2 (Nrf2; Qu et al., 2015) and weakly interacts with PPARγ (El‐Houri et al., 2015) and GABAA receptor subtypes (Czyzewska et al., 2014). It was recently shown that falcarinol inhibits adipocyte differentiation and andipogenesis and improves glucose uptake (El‐Houri et al., 2015), but shows opposite effects on lipolysis to the CB1 inverse agonist/antagonist rimonabant. Overall, the effect of falcarinol on adipogenesis would be in agreement with its inhibitory effects on CB1 receptors. Purple carrot juice and β‐carotene have been compared for their effects in a rat model of metabolic syndrome based on a high‐carbohydrate, high‐fat diet in which carrot juice improved glucose tolerance, as well as cardiovascular and hepatic structure and function independent of β‐carotene (Poudyal et al., 2010). Interestingly, significantly lower glucose, insulin and C‐peptide responses and higher satiety scores were elicited with raw carrots than with microwaved ones in humans (Gustafsson et al., 1995), which is in agreement with the loss of falcarinol content upon cooking. In a study addressing the effect of dosage on the metabolic response to vegetables added to a mixed lunch meal, it was found that the larger the carrot portion, the lower the glucose and insulin/C‐peptide responses and the higher the satiety scores (Gustafsson et al., 1994), which may suggest that the large carrot meals could provide sufficient falcarinol to exert this effect. A benefit of ginseng supplementation in improving glucose control and insulin sensitivity in patients with T2DM or impaired glucose intolerance has been concluded from a recent meta‐analysis (Gui et al., 2016). Although these data are promising, there is not yet any mechanistic in vivo evidence that this negative dietary mechanism on CB1 receptor signalling exists.
More recent evidence points towards a protective action of CB2 receptors in energy metabolism and diabetes (vide infra). Since many of the beneficial (i.e. therapeutic) effects mediated via CB1 receptors can also be obtained with CB2 receptor‐selective agonists, which do not show any central side effects (Buckley, 2008; Pacher and Mechoulam, 2011), the emerging role of this cannabinoid receptor in the context of diet is remarkable. Although CB2 receptors can enhance obesity and insulin resistance in high‐fat diets in certain mouse strains (Deveaux et al., 2009; Agudo et al., 2010), CB2 receptor activation seems, generally, to cause the opposite effects to those of CB1 receptors in rodents (Rossi et al., 2016; Verty et al., 2015; Onaivi et al., 2008). Unlike with the studies using CB1 knockout mice, global CB2 knockout mice, due to developmental adaptive processes, may not be suitable models to study the role of this receptor in energy balance and metabolism. Another problem could be the pronounced species differences in CB2 receptors and the lack of knowledge of CB2 receptors in humans, in particular in the context of high‐fat diet. In light of the evolutionary discussion related to hunter‐gatherer and pastoralist diet (vide supra), the possibility that in humans, high‐fat diet more strongly activates CB2 receptors than in mice cannot be excluded, thus compensating for CB1 receptor activation. Therefore, data from rodents could be misleading. It would certainly be interesting to assess CB2 receptor density and signalling in pastoralists. Nevertheless, CB2 receptors play a role in inhibiting food intake in the satiated state in rats, whereas the CB1 receptor promoted food intake in the fasted condition (Ting et al., 2015). A possible role for central cannabinoid CB2 receptors in body weight control and glucose homeostasis was deduced from a study artificially triggering CB2 expression in mouse brain (Romero‐Zerbo et al., 2012). In humans, the minor allele of rs3123554 in the CB2 receptor was associated cross‐sectionally with lower body weight, whereas during intervention, the same allele led to a smaller reduction in body weight (Ketterer et al., 2014). In this study, it was proposed that reduced cerebral insulin sensitivity in carriers of this allele might contribute to these disadvantageous effects during lifestyle intervention. Moreover, an association between the CB2 receptor Q63R functional variant and the age at menarche in a cohort of Italian obese girls was reported (Bellini et al., 2015). These studies clearly provide a rationale to consider a possible protective role for CB2 receptors in diet‐induced metabolic malignancies (Figure 1). CB2 receptor activation could for example be protective in high‐carbohydrate diets (Bermudez‐Silva et al., 2007) in obese individuals. Obesity is associated with a low‐grade inflammatory state and adipocyte hyperplasia/hypertrophy. This suggests that CB2 receptor activity, possibly via modulation of immune cells like macrophages, could potentially modulate food intake and could have significant effects on energy metabolism and pro‐inflammatory obesity (Schmitz et al., 2016). Moreover, CB2 receptors could be protective in atherosclerosis, restenosis, stroke, myocardial infarction and heart failure (Steffens and Pacher, 2012). At present, few dietary phytochemicals have been shown to activate CB2 receptors in vivo. The best studied phytochemical is β‐caryophyllene (BCP), which has been independently shown to exert numerous CB2 receptor‐mediated cannabimimetic effects in rodents (e.g. Gertsch et al., 2008; Horváth et al., 2012; Bahi et al., 2014; Klauke et al., 2014). As outlined in a previous commentary, BCP is a common phytochemical widely present in vegetables and spices (Gertsch, 2008). It is one of the most widespread plant volatiles and can be synthesized by virtually all plants. Although the exact molecular mechanism of action of this virtually water insoluble sesquiterpene remains unclear and CB2 receptor interaction/activation data in vitro can vary (unpublished observations), in vivo data from rodent experiments are convincing and point towards broad CB2 receptor‐mediated protective effects in various animal models (Gertsch et al., 2008; Bento et al., 2011a; Horváth et al., 2012; Cheng et al., 2014; Klauke et al., 2014). Oral administration of high doses of BCP has beneficial effects on glucose homeostasis in diabetic rats similar to glibenclamide, a standard antidiabetic drug (Basha and Sankaranarayanan, 2014 and 2016). Glucose‐stimulated insulin secretion is essential for the control of metabolic fuel homeostasis, and its impairment is a key element in the failure of beta cells in T2DM. BCP has been shown to dose‐dependently stimulate insulin secretion in MIN6 cells in a CB2 receptor‐dependent manner (Suijun et al., 2014). BCP potently inhibits solid tumour growth and lymph node metastasis of B16F10 melanoma cells in high‐fat diet‐induced obese C57BL/6N mice (Jung et al., 2015). BCP can be found in cows milk where it accumulates with a BCP rich diet (Borge et al., 2016). Attempts to establish structure–activity relationships with BCP at the CB2 receptor have failed (Chicca et al., 2014) as this simple bicyclic hydrocarbon scaffold offers limited possibilities. Another interesting natural CB2 receptor agonist is 3,3′‐diindolylmethane (DIM), which is an anticarcinogenic metabolite generated upon ingestion and hydrolysis of glucobrassicin commonly found in vegetables of the Brassicaceae family. It has been shown to be a partial CB2 agonist (Yin et al., 2009). The intake of BCP and potentially DIM could, at least in theory, directly shift the CB1/CB2 receptor activation ratio away from CB1 receptor activation.
Figure 1.
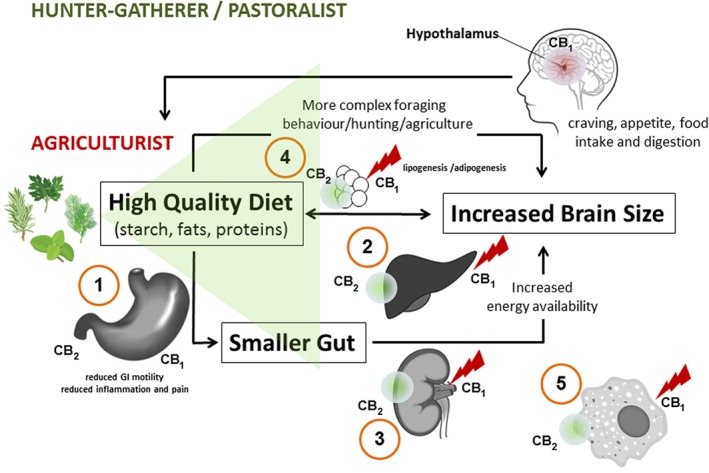
Hypothetical evolutionary model of the differential roles of CB1 and CB2 receptors in human (patho)physiology. The ECS integrates dietary stimuli from different lifestyles leading to a potential mismatch in agriculturist societies where high‐calorie food (sugars and fats) predominates. To compensate for the detrimental effects of chronic CB1 receptor activation in peripheral organs, CB2 receptors may have evolved as a protective mechanism. While both CB1 and CB2 receptors are protective in the GI tract (1), in the liver (2), kidney (3) and adipocytes (4), CB2 receptor activation could counteract the pro‐obesity and pro‐fibrotic action of CB1 receptor activation. In addition, CB2 receptor activation may ameliorate chronic inflammation [e.g. via macrophage polarization (5)] and metabolic disease. Some phytochemicals introduced during agriculture (spices, leafy vegetables, etc.) may modulate the CB1/CB2 receptor activation ratio, thus linking diet with physiology.
Hunting, pastoralism and agriculture – possible crossroads for the endocannabinoid system
Many metabolic human genes that evolved in the context of carnivory and a hunter‐gatherer lifestyle may not have necessarily been associated with famine (Pijl, 2011; Berbesque et al., 2014). However, carnivory demands efficient lipid metabolism. Dietary fat stimulates the intestinal release of the incretin hormones glucagon‐like peptide 1 (GLP‐1; Edfalk et al., 2008; Mandøe et al., 2015) and glucose‐dependent insulinotropic polypeptide (GIP) (Thomsen et al., 1999), metabolically connecting animal and plant foods. A hunter‐gatherer diet rich in animal food (about 65% of total energy intake) does not lead to metabolic problems or cardiometabolic risk (Cordain et al., 2002). Indeed, as already pointed out, pastoralist societies of the sub‐Saharan region, which have a history more ancient than agriculture, provide strong evidence that the consumption of milk and meat (proteins and fat) in a high physical activity context do not correlate with increased cardiovascular disease. For instance, despite a diet high in saturated fat, Fulani adults in Nigeria have a lipid profile indicative of a low risk of cardiovascular disease (Glew et al., 2001). This is in agreement with findings that high‐fat diets may not cause obesity and cardiometabolic pathologies in the context of sufficient physical activity (Hansen, 2013). While hunter–hunter gatherers have limited carbohydrate intake (Ströhle and Hahn, 2011), their energy demands are covered through fat‐based diets. This would fit the hypothesis that the ECS evolved in the context of sympathetic stimuli (Szabo et al., 2001), linking the CNS to metabolism and potentially also the control of brown adipose tissue (BAT) thermogenesis (Labbé et al., 2015). Interestingly, endogenous CB1 receptor negative allosteric modulatory peptides (pepcans; RVD‐hemopressin) have been discovered in noradrenergic neurons and chromaffin cells of adrenal glands in mice (Bauer et al., 2012, Hofer et al., 2015), thus potentially revealing an endogenous modulatory mechanism that has negative effects on CB1 receptors. Further research is needed to understand the physiological role of these peptides in the context of diet. Hypothalamic CB1 receptor signalling is a key determinant of energy expenditure under basal conditions and plays a role in conveying the effects of leptin on food intake (Cardinal et al., 2012). Since CB1 receptors are strongly expressed in adipocytes, in addition to the hypothalamic regulation of thermogenesis, these receptors may have additional roles, for example, in the differentiation of white adipose tissue (WAT) and BAT. Apart from diet, weight control, exercise and the use of recreational substances like alcohol, tobacco and coffee also modulate the ECS (McPartland et al., 2014). In contemporary post‐agricultural societies, sugars and fats constitute a major source of energy that can be obtained at basically no physical cost. It would be interesting to compare the functioning of the ECS between hunter‐gatherers, pastoralists and agriculturists in different energetic circumstances, taking into account possible genetic adaptations. Experimentally more accessible, the role of the ECS in fast‐growing meat‐producing animal strains versus normal growing animals of the same species should be studied to better link genetics with food intake. Along this line, cursorial animals produce AEA upon exercise, whereas non‐cursorial animals do not (Raichlen et al., 2012), suggesting a physical activity reward (runners high) potentially interlinked to energy metabolism (Fuss et al., 2015). Upon moving to urban centres or as income rises, developing nations typically replace plant‐based diets with more refined carbohydrates, isolated animal fats, vegetable oils and caloric sweeteners, a phenomenon known as the ‘nutrition transition’ (Popkin et al., 2012), which goes along with less physical activity.
Prospects for nutraceutical research?
With the nutrition transition ongoing in industrialized societies and the interrelated phenomena of glucotoxicity and lipotoxicity, CB2 receptor‐selective cannabimimetic dietary lipids should be considered as potential novel food supplements. They are generally anti‐inflammatory and anti‐fibrogenic and may potentially counteract CB1 receptor signalling. An interesting candidate is the FDA‐approved nontoxic food additive CB2 receptor agonist BCP (CAS 87–44‐5) (Schmitt et al., 2016), which also targets PPARs (Sharma et al., 2016) and is already an active ingredient of certain nutraceuticals. However, a clinical assessment of the controlled intake of higher doses of this phytochemical in diseases related to the metabolic syndrome and T2DM, such as, for example, hepatorenal inflammation, would be necessary. It was recently suggested that BCP could have potential in preventing or ameliorating non‐alcoholic fatty liver disease via stimulation of the CB2 receptor‐mediated calcium‐triggered activation of AMP‐activated protein kinase (AMPK; Kamikubo et al., 2016). BCP is orally bioavailable and accumulates in adipose tissue (unpublished data) with yet unclear clearance mechanisms. It is estimated that the daily intake of BCP from spices and vegetables is less than 10 mg but may vary with diet. The spices and vegetables of the Mediterranean and Indian cuisines may already contain sufficient amounts of cannabimimetics like BCP, DIM and EC modulating PUFAs. Dietary black pepper, which is a major source of BCP, also contains the potent AEA reuptake inhibitor guineensine (Nicolussi et al., 2014), an interesting dietary natural product, which could exert weak indirect agonistic effects on CB receptors if orally bioavailable. Clearly, phytochemicals able to inhibit peripheral CB1 receptors could represent novel therapeutic agents in diet. Since the intake of falcarinol is limited to few vegetables and spices, such as carrots and parsley, and the pharmacokinetics of this plant lipid remains unknown; further research is necessary. There is compelling evidence that numerous vegetables and spices exert beneficial effects in the context of obesity, the metabolic syndrome and diabetes (Leiherer et al., 2013). There could even be a diet‐mediated metabolic plant feedback beyond nutrients and vitamins (Gertsch, 2016). Diet is important for the development of the immune system, stress axis and neurobiological fitness in infants, and the ECS appears to play a critical role in this process (Harrison and Baune, 2014; Moretti et al., 2014). It will thus be interesting to assess the possibility of ‘reprogramming’ energy metabolism in infants of obese parents, for example, by tuning the Ω3/6 PUFAs in early development. Intervention studies have demonstrated an improvement in immune function in infants fed diets supplemented with AA and DHA compared with normal diets (Richard et al., 2016). Thus, increased EC levels in infants may be beneficial and result in positive health outcomes, including a reduction in the risk of developing allergic and atopic disease early in life. However, in adults, in addition to absolute amounts of Ω6 and Ω3 fatty acid intake, the Ω6/3 ratio plays an important role in increasing the development of obesity via AA‐derived eicosanoid metabolites, including ECs. This can be reversed by increasing the intake of EPA and DHA (Simopoulos, 2016). In dietary obese mice, DHA/EPA administered as phospholipids prevented glucose intolerance and obesity more effectively than the corresponding tracylgylcerols, and only the phospholipid form reduced plasma insulin and adipocyte hypertrophy, being also more effective in modulating 2‐AG levels and reducing hepatic steatosis and low‐grade inflammation of WAT (Rossmeisl et al., 2012). Overall, an age‐ and food‐dependent balanced Ω6/3 ratio seems to be important for health and in the prevention and management of obesity in adults; the link between Ω3 fatty acid intake and ECS function is of great interest in nutrition. Noteworthy, ethanolamide metabolites of EPA and DHA (i.e. EPEA and DHEA) have been shown to exist, and interact and activate CB1 and CB2 receptors, although less potently than classical ECs (Brown et al., 2010). In vivo, EPA and DHA‐derived ECs could nonetheless act as CB receptor ligands or bona fide ECs, although further research is necessary to determine their physiological role and signalling effects via CB receptors. Intriguingly, EPEA and DHEA become detectable in vivo after consumption of diets rich in EPA and DHA (Wood et al., 2010). Quite surprisingly, the fact that low levels of Ω3 PUFAs in humans are linked to neuropsychiatric diseases (Hashimoto et al., 2014) might also be due to their fundamental, yet poorly understood interaction, with CB1 receptors and modulation of synaptic plasticity (Lafourcade et al., 2011). Inhibitory long‐term depression of inhibitory inputs and long‐term potentiation via NMDA glutamate receptors have both been shown to be impaired in Ω3 PUFA‐deficient mice (Thomazeu et al., 2016). A translation of the current state of knowledge into a potential nutraceutical strategy may involve the formulation of CB2 receptor active dietary cannabimimetics together with Ω3 PUFAs.
Discussion
‘Little strokes fell the big oaks’
The pro‐homeostatic (allostatic) role of the ECS needs to be seen in the light of the evolutionary pressures, including dietary habits, but also development and ageing. Recent human genetic studies show that the ECS system is under purification pressure, most likely also driven by diet. Without an evolutionary perspective, it is difficult to draw general conclusions on the functioning of the ECS in energy metabolism. It is even likely that there is opposite roles of ECs and CB receptors, depending on diet and age. Thus, we need to also better understand the role of CB receptors in energy metabolism in elderly people. Furthermore, species differences, in particular for CB2 receptors, are likely to limit the conclusions from animal studies. The potential hormetic effects of ECs on their receptors (CB, GPR55, PPARs, ion channels, etc.) should be studied in more detail as frequently inverse dose–response effects are observed in vitro and in animal experiments (mostly inbred strains). Independent of fatty acid intake, in high‐calorie diets and conditions of obesity, CB2 receptors may mediate protective effects, thus enabling metabolic stress adaptation to high‐carbohydrate diets in agriculturist societies. Noteworthy, CB2 receptor activation can potentially prevent or ameliorate diabetes‐associated nephropathy (Barutta et al., 2011; Barutta et al., 2014), suggesting that CB2 receptor‐selective cannabimimetics in the diet may have a broad anti‐inflammatory and protective role (Figure 1). However, the differential activation, by ECs, of CB1 versus CB2 receptors that are found to be co‐expressed in peripheral cells is far from being understood. By elucidating beneficial cannabimimetics in the diet (e.g. those that shift the CB1/CB2 receptor activation ratio), we will be able to change dietary patterns and take into account the ECS in nutrition. Never in the history of human diets have we consumed more carbohydrates and less phytochemicals than today. It is highly likely that numerous small modulatory effects of phytochemicals (the little strokes), such as PUFAs, BCP, DIM, guineensine, falcarinol, β‐amyrin, oleanolic acid and flavonoids. on the different proteins of the ECS may have significant physiological effects. The dietary input into the ECS drives the complex physiological path of appetite stimulation, energy intake and metabolism to craving, modulation of obesity, metabolic stress and cardiometabolic problems. As with Ω3 fatty acids, nutrition that favours CB2 receptor activation should be beneficial and could open up new prospects for nutraceuticals. Ultimately, unsatisfactory diets augment drift and diminish gene flow, which reduces genetic variation in local populations and prevents the spread of genes involved in homeostasis, thereby disrupting adaptive processes and contributing to the onset of lifestyle diseases. Clearly, nutrition is fundamental in the context of population dynamics and healthy ageing. In the end, we are what we eat and eat what we are (our biochemical blueprint).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The EU MedPlant, a Marie Curie Initial Training Network (ITN), is acknowledged for financial support.
Gertsch, J. (2017) Cannabimimetic phytochemicals in the diet – an evolutionary link to food selection and metabolic stress adaptation?. British Journal of Pharmacology, 174: 1464–1483. doi: 10.1111/bph.13676.
Paper dedicated to Prof. em. Dr. Dr. hc Otto Sticher on the occasion of his 80th birthday.
References
- Agudo J, Martin M, Roca C, Molas M, Bura AS, Zimmer A et al. (2010). Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 53: 2629–2640. [DOI] [PubMed] [Google Scholar]
- Aiello LC, Wheeler P (1995). The expensive‐tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol 36: 199–221. [Google Scholar]
- Aiello LC, Wells JCK (2002). Energetics and the evolution of the genus Homo. Ann Rev Anthropol 31: 323–338. [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaarawy O, Anthony JC (2015). Cannabis smoking and diabetes mellitus: results from meta‐analysis with eight independent replication samples. Epidemiology 26: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G, Minassi A, Taglialatela‐Scafati O (2014). Recreational drug discovery: natural products as lead structures for the synthesis of smart drugs. Nat Prod Rep 31: 880–904. [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Waltenberger B, Pferschy‐Wenzig EM, Linder T, Wawrosch C, Uhrin P et al. (2015). Discovery and resupply of pharmacologically active plant‐derived natural products: a review. Biotechnol Adv 33: 1582–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S (2014). β‐Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav 135: 119–124. [DOI] [PubMed] [Google Scholar]
- Banni S, Carta G, Murru E, Cordeddu L, Giordano E, Sirigu AR et al. (2011). Krill oil significantly decreases 2‐arachidonoylglycerol plasma levels in obese subjects. Nutr Metab (Lond) 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Orlando P, Mele C, Ligresti A, Toedter K, Scheja L et al. (2011). Altered endocannabinoid signalling after a high‐fat diet in Apoe(−/−) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia 54: 2900–2910. [DOI] [PubMed] [Google Scholar]
- Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP et al. (2011). Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 60: 2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta F, Grimaldi S, Franco I, Bellini S, Gambino R, Pinach S et al. (2014). Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin‐induced diabetic mice. Kidney Int 86: 979–990. [DOI] [PubMed] [Google Scholar]
- Basha RH, Sankaranarayanan C (2014). β‐Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin‐induced diabetic rats. Acta Histochem 116: 1469–1479. [DOI] [PubMed] [Google Scholar]
- Basha RH, Sankaranarayanan C (2016). β‐Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem Biol Interact 245: 50–58. [DOI] [PubMed] [Google Scholar]
- Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B et al. (2012). Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J Biol Chem 287: 36944–36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini G, Grandone A, Torella M, Miraglia del Giudice E, Nobili B, Perrone L et al. (2015). The cannabinoid receptor 2 Q63R variant modulates the relationship between childhood obesity and age at menarche. PLoS One 10: e0140142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P et al. (2010). Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13: 281–283. [DOI] [PubMed] [Google Scholar]
- Bento AF, Marcon R, Dutra RC, Claudino RF, Cola M, Leite DF et al. (2011a). β‐Caryophyllene inhibits dextran sulfate sodium‐induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am J Pathol 178: 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbesque JC, Marlowe FW, Shaw P, Thompson P (2014). Hunter‐gatherers have less famine than agriculturalists. Biol Lett 10 : 20130853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge K, Piscitelli F, Hoem N, Silvestri C, Meyer I, Banni S et al. (2013). Chronic treatment with krill powder reduces plasma triglyceride and anandamide levels in mildly obese men. Lipids Health Dis 12: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V (2001). Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N‐acylethanolamines in piglets. Proc Natl Acad Sci U S A 98: 6402–6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez‐Silva FJ, Sanchez‐Vera I, Suárez J, Serrano A, Fuentes E, Juan‐Pico P et al. (2007). Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol 565: 207–211. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Maccarrone M (2014). Endocannabinoid signaling and its regulation by nutrients. Biofactors 40: 373–380. [DOI] [PubMed] [Google Scholar]
- Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR (2011). Changes in consumption of omega‐3 and omega‐6 fatty acids in the United States during the 20th century. Am J Clin Nutr 93: 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borge GI, Sandberg E, Øyaas J, Abrahamsen RK (2016). Variation of terpenes in milk and cultured cream from Norwegian alpine rangeland‐fed and in‐door fed cows. Food Chem 199: 195–202. [DOI] [PubMed] [Google Scholar]
- Bower A, Marquez S, De Mejia EG (2016). The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr 56: 2728–2746. [DOI] [PubMed] [Google Scholar]
- Bowles NP, Karatsoreos IN, Li X, Vemuri VK, Wood JA, Li Z et al. (2015). A peripheral endocannabinoid mechanism contributes to glucocorticoid‐mediated metabolic syndrome. Proc Natl Acad Sci U S A 112: 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Medina‐Bolivar F, Seely KA, Nair V, Bratton SM, Nopo‐Olazabal L et al. (2012). Natural prenylated resveratrol analogs arachidin‐1 and ‐3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica 42: 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA et al. (2010). Cannabinoid receptor‐dependent and ‐independent anti‐proliferative effects of omega‐3 ethanolamides in androgen receptor‐positive and ‐negative prostate cancer cell lines. Carcinogenesis 31: 1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE (2008). The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol 153: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable JC, Tan GD, Alexander SP, O'Sullivan SE (2014). The effects of obesity, diabetes and metabolic syndrome on the hydrolytic enzymes of the endocannabinoid system in animal and human adipocytes. Lipids Health Dis 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal P, Bellocchio L, Clark S, Cannich A, Klugmann M, Lutz B et al. (2012). Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology 153: 4136–4143. [DOI] [PubMed] [Google Scholar]
- Carr TP, Jesch ED, Brown AW (2008). Endocannabinoids, metabolic regulation, and the role of diet. Nutr Res 28: 641–650. [DOI] [PubMed] [Google Scholar]
- Chen T, Wu Y, Zhang Y, Wang B, Hu Y, Wang C et al. (2012). Archaeobotanical study of ancient food and cereal remains at the Astana Cemeteries, Xinjiang, China. PLoS One 7: e45137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Yu Y, Zhang Q, Szabo A, Wang H, Huang XF (2015). Arachidonic acid impairs hypothalamic leptin signaling and hepatic energy homeostasis in mice. Mol Cell Endocrinol 412: 12–18. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Dong Z, Liu S (2014). β‐Caryophyllene ameliorates the Alzheimer‐like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology 94: 1–12. [DOI] [PubMed] [Google Scholar]
- Chicca A, Marazzi J, Gertsch J (2012). The antinociceptive triterpene β‐amyrin inhibits 2‐arachidonoylglycerol (2‐AG) hydrolysis without directly targeting cannabinoid receptors. Br J Pharmacol 167: 1596–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicca A, Caprioglio D, Minassi A, Petrucci V, Appendino G, Taglialatela‐Scafati O et al. (2014). Functionalization of β‐caryophyllene generates novel polypharmacology in the endocannabinoid system. ACS Chem Biol 9: 1499–1507. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Miller JB, Mann N, Hill K (2002). The paradoxical nature of hunter‐gatherer diets: meat‐based, yet non‐atherogenic. Eur J Clin Nutr 56 (Suppl 1): S42–S52. [DOI] [PubMed] [Google Scholar]
- Cristino L, Palomba L, Di Marzo V (2014b). New horizons on the role of cannabinoid CB1 receptors in palatable food intake, obesity and related dysmetabolism. Int J Obes Suppl 4 (Suppl 1): S26–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, Becker T, Di Marzo V (2014a). Endocannabinoids and energy homeostasis: an update. Biofactors 40: 389–397. [DOI] [PubMed] [Google Scholar]
- Czyzewska MM, Chrobok L, Kania A, Jatczak M, Pollastro F, Appendino G et al. (2014). Dietary acetylenic oxylipin falcarinol differentially modulates GABAA receptors. J Nat Prod 77: 2671–2677. [DOI] [PubMed] [Google Scholar]
- de Luis DA, Izaola O, Aller R, Lopez JJ, Torres B, Diaz G et al. (2016). Association of G1359A polymorphism of the cannabinoid receptor gene (CNR1) with macronutrient intakes in obese females. J Hum Nutr Diet 29: 118–123. [DOI] [PubMed] [Google Scholar]
- de Luis DA, González Sagrado M, Aller R, Izaola O, Conde R, Romero E et al. (2010). G1359A polymorphism of the cannabinoid receptor gene (CNR1) and insulin resistance in patients with diabetes mellitus type 2. Nutr Hosp 25: 34–38. [PubMed] [Google Scholar]
- de Luis DA, González Sagrado M, Aller R, Izaola O, Conde R (2011). Influence of G1359A polymorphism of the cannabinoid receptor gene on anthropometric parameters and insulin resistance in women with obesity. Metabolism 60: 272–276. [DOI] [PubMed] [Google Scholar]
- de Luis DA, Izaola O, Aller R, de La Fuente B, Pacheco D (2013). Effects of C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) on weight loss, adipocytokines levels, and insulin resistance after a high polyunsaturated fat diet in obese patients. J Endocrinol Invest 36: 965–969. [DOI] [PubMed] [Google Scholar]
- de Luis DA, Aller R, Izaola O, Díaz Soto G, López Gómez JJ, Gomez Hoyos E et al. (2015). Effects of a high‐protein/low‐carbohydrate versus a standard hypocaloric diet on weight and cardiovascular risk factors during 9 months: role of a genetic variation in the cannabinoid receptor gene (CNR1) (G1359A polymorphism). Ann Nutr Metab 66: 125–131. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V (2010). Non‐CB1, non‐CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G‐protein‐coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol 5: 103–121. [DOI] [PubMed] [Google Scholar]
- Demizieux L, Piscitelli F, Troy‐Fioramonti S, Iannotti FA, Borrino S, Gresti J et al. (2016). Early low‐fat diet enriched with linolenic acid reduces liver endocannabinoid tone and improves late glycemic control after a high‐fat diet challenge in mice. Diabetes 65: 1824–1837. [DOI] [PubMed] [Google Scholar]
- Deshmukh RR, Sharma PL (2012). Stimulation of accumbens shell cannabinoid CB(1) receptors by noladin ether, a putative endocannabinoid, modulates food intake and dietary selection in rats. Pharmacol Res 66: 276–282. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G et al. (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946–1949. [DOI] [PubMed] [Google Scholar]
- Deveaux V, Cadoudal T, Ichigotani Y, Teixeira‐Clerc F, Louvet A, Manin S et al. (2009). Cannabinoid CB2 receptor potentiates obesity‐associated inflammation, insulin resistance and hepatic steatosis. PLoS One 4: e5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J (2002). Evolution, consequences and future of plant and animal domestication. Nature 418: 700–707. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Sepe N, De Petrocellis L, Berger A, Crozier G, Fride E et al. (1998). Trick or treat from food endocannabinoids? Nature 396: 636–637. [DOI] [PubMed] [Google Scholar]
- Di Marzo V (2008a). Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 7: 438–455. [DOI] [PubMed] [Google Scholar]
- Di Marzo V (2008b). The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 51: 1356–1367. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Ligresti A, Cristino L (2009). The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond) 33(Suppl 2): S18–S24. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L (2012). Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci 367: 3216–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ (2008). Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci 28: 9702–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Piomelli D (2012). The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci 35: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Piomelli D (2015). Intestinal lipid‐derived signals that sense dietary fat. J Clin Invest 125: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TN, Keenan AH, Thomas AP, Newman JW, Adams SH (2014). A diet containing a nonfat dry milk matrix significantly alters systemic oxylipins and the endocannabinoid 2‐arachidonoylglycerol (2‐AG) in diet‐induced obese mice. Nutr Metab (Lond) 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SB, Eaton SB 3rd, Konner MJ (1997). Paleolithic nutrition revisited: a twelve‐year retrospective on its nature and implications. Eur J Clin Nutr 51: 207–216. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Konner M (1985). Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 312: 283–289. [DOI] [PubMed] [Google Scholar]
- Edfalk S, Steneberg P, Edlund H (2008). Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Houri RB, Kotowska D, Christensen KB, Bhattacharya S, Oksbjerg N, Wolber G et al. (2015). Polyacetylenes from carrots (Daucus carota) improve glucose uptake in vitro in adipocytes and myotubes. Food Funct 6: 2135–2144. [DOI] [PubMed] [Google Scholar]
- Etkin NL, Ross PJ (1982). Food as medicine and medicine as food: an adaptive framework for the interpretation of plant utilization among the Hausa of northern Nigeria. Soc Sci Med 16: 1559–1573. [DOI] [PubMed] [Google Scholar]
- Fan YY, Monk JM, Hou TY, Callway E, Vincent L, Weeks B et al. (2012). Characterization of an arachidonic acid‐deficient (Fads1 knockout) mouse model. J Lipid Res 53: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A, Nelson GJ, Schmidt PC, Kelley DS, Bartolini G, Flanagan VP (1997). Increased dietary arachidonic acid enhances the synthesis of vasoactive eicosanoids in humans. Lipids 32: 435–439. [DOI] [PubMed] [Google Scholar]
- Finch CE, Stanford CB (2004). Meat‐adaptive genes and the evolution of slower aging in humans. Q Rev Biol 79: 3–50. [DOI] [PubMed] [Google Scholar]
- Firn RD, Jones CG (2003). Natural products – a simple model to explain chemical diversity. Nat Prod Rep 20: 382–391. [DOI] [PubMed] [Google Scholar]
- Bento AF, Marcon R, Dutra RC, Claudino RF, Cola M, Leite DFP et al. (2011b). β‐caryophyllene inhibits dextran sulfate sodium‐induced colitis in mice through cb2 receptor activation and PPARγ pathway. Am J Pathol 178: 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83: 1017–1066. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Moltke I, Grarup N, Racimo F, Bjerregaard P, Jørgensen ME et al. (2015). Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 349: 1343–1347. [DOI] [PubMed] [Google Scholar]
- Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B et al. (2015). A runner's high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A 112: 13105–13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet MS, Schubert A, Calarco S, Boccard J, Gertsch J (2017). Targeted metabolomics shows plasticity in the evolution of signaling lipids and uncovers old and new endocannabinoids in the plant kingdom. Sci Rep 7: 41177. DOI: 10.1038/srep41177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet MS, Gertsch J (2016). Quantitative analysis of arachidonic acid, endocannabinoids, N‐acylethanolamines and steroids in biological samples by LCMS/MS: fit to purpose. J Chromatogr B Analyt Technol Biomed Life Sci 1012‐1013: 215–221. [DOI] [PubMed] [Google Scholar]
- Gachet MS, Rhyn P, Bosch OG, Quednow BB, Gertsch J (2015). A quantitiative LC–MS/MS method for the measurement of arachidonic acid, prostanoids, endocannabinoids, N‐acylethanolamines and steroids in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 976‐977: 6–18. [DOI] [PubMed] [Google Scholar]
- Gaillard D, Passilly‐Degrace P, Besnard P (2008). Molecular mechanisms of fat preference and overeating. Ann N Y Acad Sci 1141: 163–175. [DOI] [PubMed] [Google Scholar]
- Gatta‐Cherifi B, Cota D (2016). New insights on the role of the endocannabinoid system in the regulation of energy balance. Int J Obes (Lond) 40: 210–219. [DOI] [PubMed] [Google Scholar]
- Gertsch J (2016). The metabolic plant feedback hypothesis: how plant secondary metabolites nonspecifically impact human health. Planta Med 82: 920–929. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ et al. (2008). Beta‐caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A 105: 9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J (2008). Anti‐inflammatory cannabinoids in diet: towards a better understanding of CB(2) receptor action? Commun Integr Biol 1: 26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J, Pertwee RG, Di Marzo V (2010). Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol 160: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J (2011). Botanical drugs, synergy, and network pharmacology: forth and back to intelligent mixtures. Planta Med 77: 1086–1098. [DOI] [PubMed] [Google Scholar]
- Glew RH, Williams M, Conn CA, Cadena SM, Crossey M, Okolo SN et al. (2001). Cardiovascular disease risk factors and diet of Fulani pastoralists of northern Nigeria. Am J Clin Nutr 74: 730–736. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Bensoussan A, Chang D, Kiat H, Klupp NL et al. (2009). Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev (4): CD006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G, Barutta F, Kunos G, Pacher P (2016). Role of the endocannabinoid system in diabetes and diabetic complications. Br J Pharmacol 173: 1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui QF, Xu ZR, Xu KY, Yang YM (2016). The efficacy of ginseng‐related therapies in type 2 diabetes mellitus: an updated systematic review and meta‐analysis. Medicine (Baltimore) 95: e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldbrand H, Lindström T, Dizdar B, Bunjaku B, Östgren CJ, Nystrom FH et al. (2014). Randomization to a low‐carbohydrate diet advice improves health related quality of life compared with a low‐fat diet at similar weight‐loss in type 2 diabetes mellitus. Diabetes Res Clin Pract 106: 221–227. [DOI] [PubMed] [Google Scholar]
- Gustafsson K, Asp NG, Hagander B, Nyman M (1994). Dose–response effects of boiled carrots and effects of carrots in lactic acid in mixed meals on glycaemic response and satiety. Eur J Clin Nutr 48: 386–396. [PubMed] [Google Scholar]
- Gustafsson K, Asp NG, Hagander B, Nyman M, Schweizer T (1995). Influence of processing and cooking of carrots in mixed meals on satiety, glucose and hormonal response. Int J Food Sci Nutr 46: 3–12. [DOI] [PubMed] [Google Scholar]
- Hajdu Z, Nicolussi S, Rau M, Lorántfy L, Forgo P, Hohmann J et al. (2014). Identification of endocannabinoid system‐modulating N‐alkylamides from Heliopsis helianthoides var. scabra and Lepidium meyenii . J Nat Prod 77: 1663–1669. [DOI] [PubMed] [Google Scholar]
- Hansen A (2013). Swedish health advisory body says too much carbohydrate, not fat, leads to obesity. BMJ 347: f6873. [DOI] [PubMed] [Google Scholar]
- Hanus LO (2009). Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med Res Rev 29: 213–271. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Baune BT (2014). Modulation of early stress‐induced neurobiological changes: a review of behavioural and pharmacological interventions in animal models. Transl Psychiatry 4: e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Maekawa M, Katakura M, Hamazaki K, Matsuoka Y (2014). Possibility of polyunsaturated fatty acids for the prevention and treatment of neuropsychiatric illnesses. J Pharmacol Sci 124: 294–300. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, O'Callaghan MJ, Mamun AA, Williams GM, Clavarino A, Najman JM (2010). Cannabis use and obesity and young adults. Am J Drug Alcohol Abuse 36: 350–356. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Prieto JM (2008). Diet and healthy ageing 2100: will we globalise local knowledge systems? Ageing Res Rev 7: 249–274. [DOI] [PubMed] [Google Scholar]
- Hermanson DJ, Gamble‐George JC, Marnett LJ, Patel S (2014). Substrate‐selective COX‐2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci 35: 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Cheer JF (2012). Effect of CB1 receptor blockade on food‐reinforced responding and associated nucleus accumbens neuronal activity in rats. J Neurosci 32: 11467–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Cheer JF (2015). To act or not to act: endocannabinoid/dopamine interactions in decision‐making. Front Behav Neurosci 9: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydenreuter W, Kunold E, Sieber SA (2015). Alkynol natural products target alDH2 in cancer cells by irreversible binding to the active site. Chem Commun (Camb) 51: 15784–15787. [DOI] [PubMed] [Google Scholar]
- Hillard CJ (2014). Stress regulates endocannabinoid‐CB1 receptor signaling. Semin Immunol 26: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hite AH, Berkowitz VG, Berkowitz K (2011). Low‐carbohydrate diet review: shifting the paradigm. Nutr Clin Pract 26: 300–308. [DOI] [PubMed] [Google Scholar]
- Hofer SC, Ralvenius WT, Gachet MS, Fritschy JM, Zeilhofer HU, Gertsch J (2015). Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology 98: 78–89. [DOI] [PubMed] [Google Scholar]
- Holman RT, Johnson SB, Gerrard JM, Mauer SM, Kupcho‐Sandberg S, Brown DM (1983). Arachidonic acid deficiency in streptozotocin‐induced diabetes. Proc Natl Acad Sci U S A 80: 2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth B, Mukhopadhyay P, Kechrid M, Patel V, Tanchian G, Wink DA et al. (2012). β‐Caryophyllene ameliorates cisplatin‐induced nephrotoxicity in a cannabinoid 2 receptor‐dependent manner. Free Radic Biol Med 52: 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL (2008). Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4: 682–690. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Sinclair DA (2008). Xenohormesis: sensing the chemical cues of other species. Cell 133: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Sharkey KA (2010). Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther 126: 21–38. [DOI] [PubMed] [Google Scholar]
- Izzo AA (2007). The cannabinoid CB(2) receptor: a good friend in the gut. Neurogastroenterol Motil 19: 704–708. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Muccioli GG, Ruggieri MR, Schicho R (2015). Endocannabinoids and the digestive tract and bladder in health and disease. Handb Exp Pharmacol 231: 423–447. [DOI] [PubMed] [Google Scholar]
- Janero DR (2012). Cannabinoid‐1 receptor (CB1R) blockers as medicines: beyond obesity and cardiometabolic disorders to substance abuse/drug addiction with CB1R neutral antagonists. Expert Opin Emerg Drugs 17: 17–29. [DOI] [PubMed] [Google Scholar]
- Johns T (1990). With Bitter Herbs They Shall Eat: Chemical Ecology and the Origins of Human Diet and Medicine (Arizona Studies in Human Ecology). University of Arizona Press: Tucson, AZ, p. 356 , Hardcover, 1990. [Google Scholar]
- Johnson GH, Fritsche K (2012). Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet 112: 1029–1041, 1041.e1‐15. [DOI] [PubMed] [Google Scholar]
- Jung JI, Kim EJ, Kwon GT, Jung YJ, Park T, Kim Y et al. (2015). β‐Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high‐fat diet‐induced obese C57BL/6N mice. Carcinogenesis 36: 1028–1039. [DOI] [PubMed] [Google Scholar]
- Kamikubo R, Kai K, Tsuji‐Naito K, Akagawa M (2016). β‐Caryophyllene attenuates palmitate‐induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol Nutr Food Res 60: 2228–2242. [DOI] [PubMed] [Google Scholar]
- Kano M (2014). Control of synaptic function by endocannabinoid‐mediated retrograde signaling. Proc Jpn Acad Ser B Phys Biol Sci 90: 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura M, Hashimoto M, Inoue T, Mamun AA, Tanabe Y, Arita M et al. (2015). Chronic arachidonic acid administration decreases docosahexaenoic acid‐ and eicosapentaenoic acid‐derived metabolites in kidneys of aged rats. PLoS One 10: e0140884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer C, Heni M, Stingl K, Tschritter O, Linder K, Wagner R et al. (2014). Polymorphism rs3123554 in CNR2 reveals gender‐specific effects on body weight and affects loss of body weight and cerebral insulin action. Obesity (Silver Spring) 22: 925–931. [DOI] [PubMed] [Google Scholar]
- Khedr AI, Ibrahim SR, Mohamed GA, Ahmed HE, Ahmad AS, Ramadan MA et al. (2016). New ursane triterpenoids from Ficus pandurata and their binding affinity for human cannabinoid and opioid receptors. Arch Pharm Res 39: 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AR, Dotsey EY, Lodola A, Jung KM, Ghomian A, Qiu Y et al. (2009). Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem Biol 16: 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC (2009). Endocannabinoids and the non‐homeostatic control of appetite. Curr Top Behav Neurosci 1: 231–253. [DOI] [PubMed] [Google Scholar]
- Klauke AL, Racz I, Pradier B, Markert A, Zimmer AM, Gertsch J et al. (2014). The cannabinoid CB₂ receptor‐selective phytocannabinoid beta‐caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropsychopharmacol 24: 608–620. [DOI] [PubMed] [Google Scholar]
- Kleberg K, Hassing HA, Hansen HS (2014). Classical endocannabinoid‐like compounds and their regulation by nutrients. Biofactors 40: 363–372. [DOI] [PubMed] [Google Scholar]
- Kleyer J, Nicolussi S, Taylor P, Simonelli D, Furger E, Anderle P et al. (2012). Cannabinoid receptor trafficking in peripheral cells is dynamically regulated by a binary biochemical switch. Biochem Pharmacol 83: 1393–1412. [DOI] [PubMed] [Google Scholar]
- Koch MA, Schuffenhauer A, Scheck M, Wetzel S, Casaulta M, Odermatt A et al. (2005). Charting biologically relevant chemical space: a structural classification of natural products (SCONP). Proc Natl Acad Sci U S A 102: 17272–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G, Tam J (2011). The case for peripheral CB₁ receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol 163: 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs JR (2009). The gourmet ape: evolution and human food preferences. Am J Clin Nutr 90: 707S–711S. [DOI] [PubMed] [Google Scholar]
- Labbé SM, Caron A, Lanfray D, Monge‐Rofarello B, Bartness TJ, Richard D (2015). Hypothalamic control of brown adipose tissue thermogenesis. Front Syst Neurosci 9: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I et al. (2011). Nutritional omega‐3 deficiency abolishes endocannabinoid‐mediated neuronal functions. Nat Neurosci 14: 345–350. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA (2004). Small molecules that regulate lifespan: Evidence for xenohormesis. Mol Microbiol 53: 1003–1009. [DOI] [PubMed] [Google Scholar]
- le Coutre J, Schmitt JAJ (2008). Food ingredients and cognitive performance. Curr Opin Clin Nutr Metab Care 11: 706–710. [DOI] [PubMed] [Google Scholar]
- Leiherer A, Mündlein A, Drexel H (2013). Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul Pharmacol 58: 3–20. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Robertson ML (1997). Comparative primate energetics and hominid evolution. Am J Phys Anthropol 102: 265–281. [DOI] [PubMed] [Google Scholar]
- Leonard WR (2002). Food for thought: dietary change was a driving force in human evolution. Sci Am 287: 106–115. [PubMed] [Google Scholar]
- Leonard WR, Snodgrass JJ, Robertson ML (2010). Evolutionary perspectives on fat ingestion and metabolism in humans In: Montmayeur JP, le Coutre J. (eds). Fat Detection: Taste, Texture, and Post Ingestive Effects. CRC Press/Taylor & Francis: Boca Raton (FL); Chapter 1. [Google Scholar]
- Heinrich M, Nebel S, Leonti M, Rivera D, Obón CM (2006). ‘Local Food‐Nutraceuticals’: bridging the gap between local knowledge and global needs. Forum Nutr 59: 1–17. [DOI] [PubMed] [Google Scholar]
- Leonti M, Casu L, Raduner S, Cottiglia F, Floris C, Altmann KH et al. (2010). Falcarinol is a covalent cannabinoid CB1 receptor antagonist and induces pro‐allergic effects in skin. Biochem Pharmacol 79: 1815–1826. [DOI] [PubMed] [Google Scholar]
- Leonti M (2012). The co‐evolutionary perspective of the food‐medicine continuum and wild gathered and cultivated vegetables. Genet Resour Crop Evol 59: 1295–1302. [Google Scholar]
- Li L, Bonneton F, Chen XY, Laudet V (2015). Botanical compounds and their regulation of nuclear receptor action: the case of traditional Chinese medicine. Mol Cell Endocrinol 401: 221–237. [DOI] [PubMed] [Google Scholar]
- Ling PR, Malkan A, Le HD, Puder M, Bistrian BR (2012). Arachidonic acid and docosahexaenoic acid supplemented to an essential fatty acid‐deficient diet alters the response to endotoxin in rats. Metabolism 61: 395–406. [DOI] [PubMed] [Google Scholar]
- Likhodii SS, Cunnane SC (1999). Uptake of 13C‐tracer arachidonate and gamma‐linolenate by the brain and liver of the suckling rat observed using 13C‐NMR. J Neurochem 72: 2548–2555. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J et al. (2012). Hepatic cannabinoid receptor‐1 mediates diet‐induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 142: 1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Bab I, Bíró T, Cabral GA, Dey SK, Di Marzo V et al. (2015). Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 36: 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Gertsch J, Appendino G (2011). Plant volatiles: production, function and pharmacology. Nat Prod Rep 28: 1359–1380. [DOI] [PubMed] [Google Scholar]
- Matias I, Bisogno T, Di Marzo V (2006). Endogenous cannabinoids in the brain and peripheral tissues: regulation of their levels and control of food intake. Int J Obes (Lond) 30(Suppl 1): S7–S12. [DOI] [PubMed] [Google Scholar]
- Mandøe MJ, Hansen KB, Hartmann B, Rehfeld JF, Holst JJ, Hansen HS (2015). The 2‐monoacylglycerol moiety of dietary fat appears to be responsible for the fat‐induced release of GLP‐1 in humans. Am J Clin Nutr 102: 548–555. [DOI] [PubMed] [Google Scholar]
- Manduca A, Campolongo P, Trezza V (2012). Cannabinoid modulation of mother‐infant interaction: is it just about milk? Rev Neurosci 23: 707–722. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN (2005). Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95: 437–445. [DOI] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J et al. (2010). The serine hydrolase ABHD6 controls the accumulation and efficacy of 2‐AG at cannabinoid receptors. Nat Neurosci 13: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazier W, Saucisse N, Gatta‐Cherifi B, Cota D (2015). The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metab 26: 524–537. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN et al. (2015). Mechanisms of stress in the brain. Nat Neurosci 18: 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JM, Matias I, Di Marzo V, Glass M (2006). Evolutionary origins of the endocannabinoid system. Gene 370: 64–74. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG (2007). Meta‐analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol 152: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JM, Guy GW, Di Marzo V (2014). Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One 9: e89566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben‐Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR et al. (1995). Identification of an endogenous 2‐monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50: 83–90. [DOI] [PubMed] [Google Scholar]
- Mechoulam R (2002). Discovery of endocannabinoids and some random thoughts on their possible roles in neuroprotection and aggression. Prostaglandins Leukot Essent Fatty Acids 66: 93–99. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Berry EM, Avraham Y, Di Marzo V, Fride E (2006). Endocannabinoids, feeding and suckling – from our perspective. Int J Obes (Lond) 30 (Suppl 1): S24–S28. [DOI] [PubMed] [Google Scholar]
- Medina‐Gomez G, Gray S, Vidal‐Puig A (2007). Adipogenesis and lipotoxicity: role of peroxisome proliferator‐activated receptor gamma (PPARgamma) and PPARgammacoactivator‐1 (PGC1). Public Health Nutr 10 (10A): 1132–1137. [DOI] [PubMed] [Google Scholar]
- Milton K (1987). Primate diets and gut morphology: implications for human evolution In: Harris M, Ross EB. (eds). Food and Evolution: Toward a Theory of Human Food Habits. Temple University Press: Philadelphia, PA, pp. 93–116. [Google Scholar]
- Moerman DE (1996). An analysis of the food plants and drug plants of native North America. J Ethnopharmacol 52: 1–22. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, le Coutre J. (eds) (2010). Fat Detection: Taste, Texture, and Post Ingestive Effects. CRC Press, Taylor & Francis: Boca Raton (FL). [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN (2016). Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 41: 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Δ9‐Tetrahydrocannabinol‐induced anti‐inflammatory responses in adolescent mice switch to proinflammatory conditions in adulthood. [DOI] [PubMed]
- Moretti S, Castelli M, Franchi S, Raggi MA, Mercolini L, Protti M et al. (2014). Δ9‐Tetrahydrocannabinol‐induced anti‐inflammatory responses in adolescent mice switch to proinflammatory in adulthood. J Leukoc Biol 96: 523–534. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Sable S, Ouwerkerk R, Mari A, Gharib AM, Walter M et al. (2013). Metabolic effects of chronic cannabis smoking. Diabetes Care 36: 2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murch S (2006). In: Baluska F, Mancuso S, Volkmann D. (eds). Neurotransmitters, neuroregulators and neurotoxins in plants, neuonal aspects of plant. Springer: Berlin, pp. 137–151. 10.1007/978-3-540-28516-8_10. [Google Scholar]
- Neel JV (1962). Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 14: 353–362. [PMC free article] [PubMed] [Google Scholar]
- Nicolussi S, Gertsch J (2015). Endocannabinoid transport revisited. Vitam Horm 98: 441–485. [DOI] [PubMed] [Google Scholar]
- Nicolussi S, Viveros‐Paredes JM, Gachet MS, Rau M, Flores‐Soto ME, Blunder M et al. (2014). Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice. Pharmacol Res 80: 52–65. [DOI] [PubMed] [Google Scholar]
- Nievergelt A, Marazzi J, Schoop R, Altmann KH, Gertsch J (2011). Ginger phenylpropanoids inhibit IL‐1beta and prostanoid secretion and disrupt arachidonate‐phospholipid remodeling by targeting phospholipases A2. J Immunol 187: 4140–4150. [DOI] [PubMed] [Google Scholar]
- Nilius B, Appendino G (2013). Spices: the savory and beneficial science of pungency. Rev Physiol Biochem Pharmacol 164: 1–76. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC et al. (2011). Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R (2008). Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci 1139: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei‐Hyiaman D, Harvey‐White J, Bátkai S, Kunos G (2006). The role of the endocannabinoid system in the control of energy homeostasis. Int J Obes (Lond) 30 (Suppl 1): S33–S38. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE (2007). Cannabinoids go nuclear: evidence for activation of peroxisome proliferator‐activated receptors. Br J Pharmacol 152: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P (2009). Cannabinoid CB1 receptor antagonists for atherosclerosis and cardiometabolic disorders: new hopes, old concerns? Arterioscler Thromb Vasc Biol 29: 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mechoulam R (2011). Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res 50: 193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkari T, Haavikko R, Laitinen T, Navia‐Paldanius D, Rytilahti R, Vaara M et al. (2014). Discovery of triterpenoids as reversible inhibitors of α/β‐hydrolase domain containing 12 (ABHD12). PLoS One 9: e98286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry GH, Dominy NJ, Claw KG, Lee AS, Fiegler H, Redon R et al. (2007). Diet and the evolution of human amylase gene copy number variation. Nat Genet 39: 1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2007). GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol 152: 984–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2005). Pharmacological actions of cannabinoids. Handb Exp Pharmacol 168: 1–51. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2009). Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 156: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2010). Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 17: 1360–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V (2017). The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol 174: 1349–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone RP, Kendall DA (2015). Minireview: from the bench, toward the clinic: therapeutic opportunities for cannabinoid receptor modulation. Mol Endocrinol 29: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijl H (2011). Obesity: evolution of a symptom of affluence. Neth J Med 69: 159–166. [PubMed] [Google Scholar]
- Pintus S, Murru E, Carta G, Cordeddu L, Batetta B, Accossu S et al. (2013). Sheep cheese naturally enriched in α‐linolenic, conjugated linoleic and vaccenic acids improves the lipid profile and reduces anandamide in the plasma of hypercholesterolaemic subjects. Br J Nutr 109: 1453–1462. [DOI] [PubMed] [Google Scholar]
- Poudyal H, Panchal S, Brown L (2010). Comparison of purple carrot juice and β‐carotene in a high‐carbohydrate, high‐fat diet‐fed rat model of the metabolic syndrome. Br J Nutr 104: 1322–1332. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Adair LS, Ng SW (2012). Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich DG, Jenkins DJA, Kendall CWC, Dierenfeld ES, Carroll RW, Tariq N et al. (1997). The western lowland gorilla diet has implications for the health of humans and other hominoids. J Nutr 127: 2000–2005. [DOI] [PubMed] [Google Scholar]
- Qu C, Li B, Lai Y, Li H, Windust A, Hofseth LJ et al. (2015). Identifying panaxynol, a natural activator of nuclear factor erythroid‐2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage‐induced cardiomyocyte hypertrophy. J Ethnopharmacol 168: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A (2012). Wired to run: exercise‐induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner's high’. J Exp Biol 215: 1331–1336. [DOI] [PubMed] [Google Scholar]
- Richard C, Lewis ED, Field CJ (2016). Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant's immune system early in life. Appl Physiol Nutr Metab 41: 461–475. [DOI] [PubMed] [Google Scholar]
- Ruehle S, Rey AA, Remmers F, Lutz B (2012). The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol 26: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera P, Luque‐Rojas MJ, Pastor A, Blanco E, Pavón FJ, Serrano A et al. (2013). Diet‐dependent modulation of hippocampal expression of endocannabinoid signaling‐related proteins in cannabinoid antagonist‐treated obese rats. Eur J Neurosci 37: 105–117. [DOI] [PubMed] [Google Scholar]
- Romero‐Zerbo SY, Garcia‐Gutierrez MS, Suárez J, Rivera P, Ruz‐Maldonado I, Vida M et al. (2012). Overexpression of cannabinoid CB2 receptor in the brain induces hyperglycaemia and a lean phenotype in adult mice. J Neuroendocrinol 24: 1106–1119. [DOI] [PubMed] [Google Scholar]
- Ross RA (2009). The enigmatic pharmacology of GPR55. Trends Pharmacol Sci 30: 156–163. [DOI] [PubMed] [Google Scholar]
- Rossi F, Bellini G, Luongo L, Manzo I, Tolone S, Tortora C et al. (2016). Cannabinoid receptor 2 as anti‐obesity target: inflammation, fat storage and browning modulation. J Clin Endocrinol Metab 101: 3469–3478. [DOI] [PubMed] [Google Scholar]
- Rossmeisl M, Jilkova ZM, Kuda O, Jelenik T, Medrikova D, Stankova B et al. (2012). Metabolic effects of n‐3 PUFA as phospholipids are superior to triglycerides in mice fed a high‐fat diet: possible role of endocannabinoids. PLoS One 7: e38834. doi:10.1371/journal.pone.0038834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB (2016). Beyond cannabis: plants and the endocannabinoid system. Trends Pharmacol Sci 37: 594–605. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J et al. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152: 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt D, Levy R, Carroll B (2016). Toxicological evaluation of β‐caryophyllene oil: subchronic toxicity in rats. Int J Toxicol 35: 558–567. [DOI] [PubMed] [Google Scholar]
- Schmitz K, Mangels N, Häussler A, Ferreirós N, Fleming I, Tegeder I (2016). Pro‐inflammatory obesity in aged cannabinoid‐2 receptor‐deficient mice. Int J Obes (Lond) 40: 366–379. [DOI] [PubMed] [Google Scholar]
- Sclafani A (2001). Psychobiology of food preferences. Int J Obes Relat Metab Disord 5: S13–S16. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Wiley JW (2016). The role of the endocannabinoid system in the brain‐gut axis. Gastroenterology 151: 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Al Kaabi JM, Nurulain SM, Goyal SN, Kamal MA, Ojha S (2016). Polypharmacological properties and therapeutic potential of β‐caryophyllene: a dietary phytocannabinoid of pharmaceutical promise. Curr Pharm Des 22: 3237–3264. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Rácz I, Marazzi J, Smart TG, Zimmer A et al. (2011). The major central endocannabinoid directly acts at GABA(A) receptors. Proc Natl Acad Sci U S A 108: 18150–18155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand G, Kris‐Etherton P, Boulos NM (2015). Impact of functional foods on prevention of cardiovascular disease and diabetes. Curr Cardiol Rep 17: 39. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Ligresti A, Di Marzo V (2011). Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocr Metab Disord 12: 153–162. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP (2016). An increase in the omega‐6/omega‐3 fatty acid ratio increases the risk for obesity. Nutrients 8: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP (2002). The importance of the ratio of omega‐6/omega‐3 essential fatty acids. Biomed Pharmacother 56: 365–379. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr M, Emmerdinger D, Diegelmann J, Pfennig S, Ochsenkühn T, Göke B et al. (2010). The cannabinoid 1 receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn's disease. PLoS One 5: e9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens S, Pacher P (2012). Targeting cannabinoid receptor CB(2) in cardiovascular disorders: promises and controversies. Br J Pharmacol 167: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A, Hahn A (2011). Diets of modern hunter‐gatherers vary substantially in their carbohydrate content depending on ecoenvironments: results from an ethnographic analysis. Nutr Res 31: 429–435. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K et al. (1995). 2‐Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215: 89–97. [DOI] [PubMed] [Google Scholar]
- Suijun W, Zhen Y, Ying G, Yanfang W (2014). A role for trans‐caryophyllene in the moderation of insulin secretion. Biochem Biophys Res Commun 444: 451–454. [DOI] [PubMed] [Google Scholar]
- Sun D, Lv J, Chen W, Li S, Guo Y, Bian Z et al. (2014). Spicy food consumption is associated with adiposity measures among half a million Chinese people: the China Kadoorie Biobank study. BMC Public Health 14: 1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Nordheim U, Niederhoffer N (2001). Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J Pharmacol Exp Ther 297: 819–826. [PubMed] [Google Scholar]
- Tantimonaco M, Ceci R, Sabatini S, Catani MV, Rossi A, Gasperi V et al. (2014). Physical activity and the endocannabinoid system: an overview. Cell Mol Life Sci 71: 2681–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EE, Jagielo‐Miller JE, Vemuri VK, Makriyannis A, McLaughlin PJ (2016). CB1 antagonism produces behaviors more consistent with satiety than reduced reward value in food‐maintained responding in rats. J Psychopharmacol 30: 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J et al. (1999). Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr 69: 1135–1143. [DOI] [PubMed] [Google Scholar]
- Thors L, Eriksson J, Fowler CJ (2007). Inhibition of the cellular uptake of anandamide by genistein and its analogue daidzein in cells with different levels of fatty acid amide hydrolase‐driven uptake. Br J Pharmacol 152: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thors L, Belghiti M, Fowler CJ (2008). Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. Br J Pharmacol 155: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thors L, Burston JJ, Alter BJ, McKinney MK, Cravatt BF, Ross RA et al. (2010). Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br J Pharmacol 160: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomazeau A, Bosch‐Bouju C, Manzoni O, Layé S (2016). Nutritional n‐3 PUFA Deficiency Abolishes Endocannabinoid Gating of Hippocampal Long‐Term Potentiation. Cereb Cortex. pii: bhw052. [DOI] [PubMed]
- Ting CH, Chi CW, Li CP, Chen CY (2015). Differential modulation of endogenous cannabinoid CB1 and CB2 receptors in spontaneous and splice variants of ghrelin‐induced food intake in conscious rats. Nutrition 31: 230–235. [DOI] [PubMed] [Google Scholar]
- Toepel U, Knebel J‐F, Hudry J, le Coutre J, Murray MM (2009). The brain tracks the energetic value in food images. Neuroimage 44: 967–974. [DOI] [PubMed] [Google Scholar]
- Tomar S, Zumbrun EE, Nagarkatti M, Nagarkatti PS (2015). Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide‐induced acute liver failure through regulation of macrophage polarization and microRNAs. J Pharmacol Exp Ther 353: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankey BN, Strasser S, Okosun IS (2016). A cross‐sectional analysis of the association between marijuana and cigarette smoking with metabolic syndrome among adults in the United States. Diabetes Metab Syndr 10(2 Suppl 1): S89–S95. [DOI] [PubMed] [Google Scholar]
- van Breemen RB, Tao Y, Li W (2011). Cyclooxygenase‐2 inhibitors in ginger (Zingiber officinale). Fitoterapia 82: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty AN, Stefanidis A, McAinch AJ, Hryciw DH, Oldfield B (2015). Anti‐obesity effect of the CB2 receptor agonist JWH‐015 in diet‐induced obese mice. PLoS One 10: e0140592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Waltenberger B, Pferschy‐Wenzig EM, Blunder M, Liu X, Malainer C et al. (2014). Natural product agonists of peroxisome proliferator‐activated receptor gamma (PPARγ): a review. Biochem Pharmacol 92: 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chan CB (2015). n‐3 polyunsaturated fatty acids and insulin secretion. J Endocrinol 224: R97–R106. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Kim J (2015). The endocannabinoid system: directing eating behavior and macronutrient metabolism. Front Psychol 5: 1506. doi:10.3389/fpsyg.2014.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV et al. (2015). Cannabinoids for medical use: a systematic review and meta‐analysis. JAMA 313: 2456–2473. [DOI] [PubMed] [Google Scholar]
- Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi‐Keefe CJ, Makriyannis A (2010). Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid‐system metabolites in brain and plasma. J Lipid Res 51: 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F et al. (2009). Lipid G protein‐coupled receptor ligand identification using beta‐arrestin PathHunter assay. J Biol Chem 284: S12328–S12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ni Y, Ma X, Zhao A, Bao Y, Liu J et al. (2016). A panel of free fatty acid ratios to predict the development of metabolic abnormalities in healthy obese individuals. Sci Rep 6: 28418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu Y, Zhang W, Xue J, Wu YZ, Xu W et al. (2010). WIN55212‐2 ameliorates atherosclerosis associated with suppression of pro‐inflammatory responses in ApoE‐knockout mice. Eur J Pharmacol 649: 285–292. [DOI] [PubMed] [Google Scholar]