Abstract
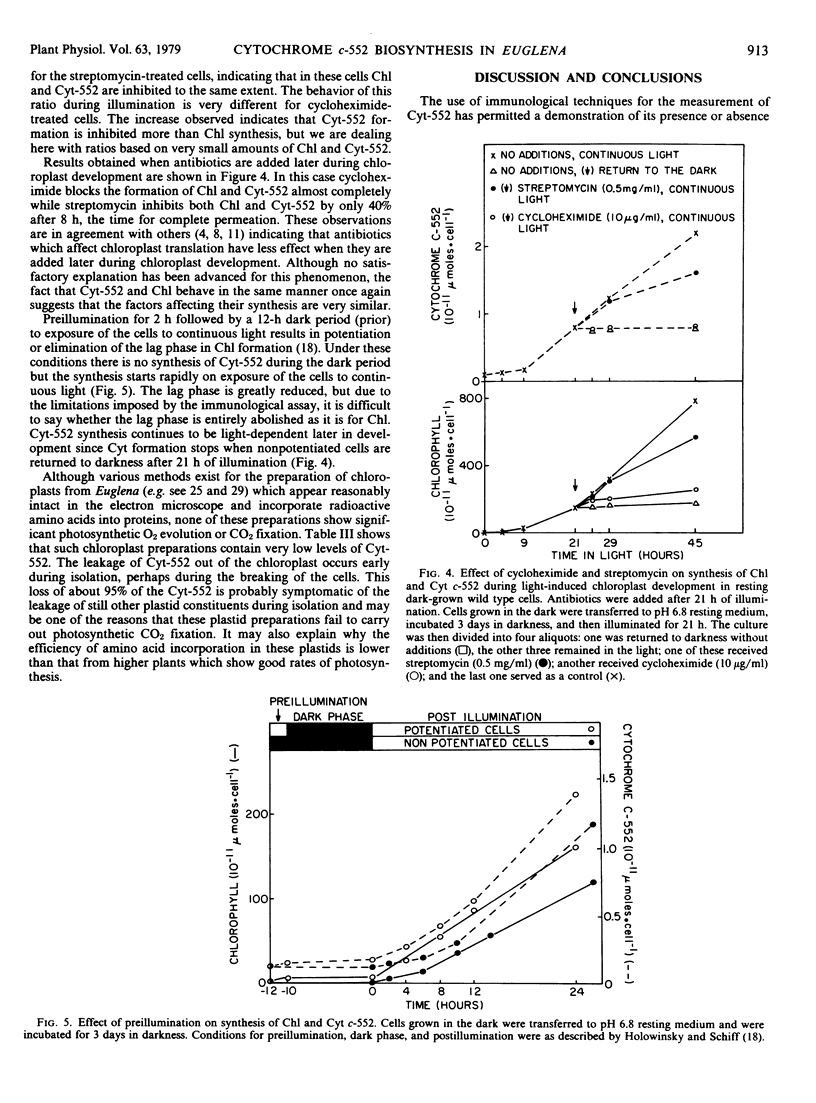
Lack of a suitable assay has thwarted attempts to measure cytochrome c-552 in dark-grown wild type cells of Euglena gracilis var. bacillaris in mutants and in other situations where the concentrations are low. Purification methods are described based on electrofocusing which provide a cytochrome c-552 preparation homogeneous enough to elicit a single reactive antibody in rabbits; this antibody is then used as a specific and sensitive assay for cytochrome c-552. Dark-grown cells of wild type and of mutants O1BS, O2BX, G1BU and P1BXL (which make normal sized chloroplasts with abnormal internal structure in the light) have 0.02 to 0.1 × 10−11 micromoles of cytochrome c-552 per cell, 10 to 150 times less than light-grown cells. Light-grown cells of these mutants and of wild type show a ratio of chlorophyll to cytochrome of about 300 (mole to mole). Cytochrome c-552 is undetectable in dark-grown Y1BXD, Y3BUD, and W34ZUD which cannot carry plastid development beyond the proplastid in light; the light-grown cells of these mutants have levels of cytochrome similar to or lower than dark-grown wild type cells. Cytochrome c-552 is undetectable in light- and dark-grown mutants in which plastid DNA is undetectable (such as Y2BUL, W3BUL, W8BHL, and W10BSmL) consistent with the view, but not proving, that this molecule may be coded, at least in part, in plastid DNA. During light-induced chloroplast development in resting cells, cytochrome c-552 formation behaves in all respects like chlorophyll except that the dark-grown cells contain low amounts of the cytochrome c-552 but lack chlorophyll. Thus, both cytochrome c-552 and chlorophyll show the same lag period even when the length is changed by nutritional manipulation; preillumination largely eliminates the lag in the formation of both molecules, cycloheximide and streptomycin both inhibit the biosynthesis of chlorophyll and cytochrome c-552 in the same manner, and the formation of both during chloroplast development is strictly light-dependent. It is shown that chloroplasts isolated from Euglena by methods thought to give intact organelles, lack 95% of the cytochrome c-552; this and the loss of similar molecules may explain why these isolated chloroplasts are not photosynthetically active.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avadhani N. G., Buetow D. E. Isolation of active polyribosomes from the cytoplasm, mitochondria and chloroplasts of Euglena gracilis. Biochem J. 1972 Jun;128(2):353–365. doi: 10.1042/bj1280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Bovarnick J. G., Chang S. W., Schiff J. A., Schwartzbach S. D. Events surrounding the early development of Euglena chloroplasts: experiments with streptomycin in non-dividing cells. J Gen Microbiol. 1974 Jul;83(0):51–62. doi: 10.1099/00221287-83-1-51. [DOI] [PubMed] [Google Scholar]
- Bovarnick J. G., Schiff J. A., Freedman Z., Egan J. M. Events surrounding the early development of Euglena chloroplasts: cellular origins of chloroplast enzymes in euglena. J Gen Microbiol. 1974 Jul;83(0):63–71. doi: 10.1099/00221287-83-1-63. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich M. A., Meyer T., Tedro S. M., Kamen M. D. The question of histidine content in c-type cytochromes. Proc Natl Acad Sci U S A. 1971 Mar;68(3):629–631. doi: 10.1073/pnas.68.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- Freyssinet G., Schwob C. Relation between Paramylum Content and the Length of the Lag Period of Chlorophyll Synthesis during Greening of Dark-grown Euglena gracilis. Plant Physiol. 1976 May;57(5):824–830. doi: 10.1104/pp.57.5.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid A., Levine L. Multiple molecular forms of prostaglandin 15-hydroxydehydrogenase and 9-ketoreductase in chicken kidney. Prostaglandins. 1977 Mar;13(3):503–516. doi: 10.1016/0090-6980(77)90028-4. [DOI] [PubMed] [Google Scholar]
- Heizmann P. H., Salvador G. F., Nigon V. Occurrence of plastidial rRNAs and plastidial structures in bleached mutants of Euglena gracilis. Exp Cell Res. 1976 May;99(2):253–260. doi: 10.1016/0014-4827(76)90581-4. [DOI] [PubMed] [Google Scholar]
- Holowinsky A. W., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. I. Induction by preillumination. Plant Physiol. 1970 Mar;45(3):339–347. doi: 10.1104/pp.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine L. Levels of 13,14-dihydro-15-keto-PGE2 in some biological fluids as measured by radioimmunoassay. Prostaglandins. 1977;14(6):1125–1139. doi: 10.1016/0090-6980(77)90290-8. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Lyman H., Siegelman H. W. Large-scale autotrophic culture of Euglena gracilis. J Protozool. 1967 May;14(2):297–299. doi: 10.1111/j.1550-7408.1967.tb02000.x. [DOI] [PubMed] [Google Scholar]
- PERINI F., SCHIFF J. A., KAMEN M. D. IRON-CONTAINING PROTEINS IN EUGLENA. II. FUNCTIONAL LOCALIZATION. Biochim Biophys Acta. 1964 Jul 29;88:91–98. doi: 10.1016/0926-6577(64)90156-1. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The purification and amino acid sequence of cytochrome C-552 from Euglena gracilis. Biochem J. 1974 May;139(2):449–459. doi: 10.1042/bj1390449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMILLIE R. M., EVANS W. R., LYMAN H. METABOLIC EVENTS DURING THE FORMATION OF A PHOTOSYNTHETIC FROM A NONPHOTOSYNTHETIC CELL. Brookhaven Symp Biol. 1964 Mar;16:89–108. [PubMed] [Google Scholar]
- Salisbury J. L., Vasconcelos A. C., Floyd G. L. Isolation of Intact Chloroplasts of Euglena gracilis by Isopycnic Sedimentation in Gradients of Silica. Plant Physiol. 1975 Sep;56(3):399–403. doi: 10.1104/pp.56.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Freyssinet G., Schiff J. A. The chloroplast and cytoplasmic ribosomes of euglena: I. Stability of chloroplast ribosomes prepared by an improved procedure. Plant Physiol. 1974 Apr;53(4):533–542. doi: 10.1104/pp.53.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Schiff J. A. Chloroplast and cytoplasmic ribosomes of Euglena: selective binding of dihydrostreptomycin to chloroplast ribosomes. J Bacteriol. 1974 Oct;120(1):334–341. doi: 10.1128/jb.120.1.334-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg A. J., Schiff J. A. Events Surrounding the Early Development of Euglena Chloroplasts: 7. Inhibition of Carotenoid Biosynthesis by the Herbicide SAN 9789 (4-Chloro-5-(methylamino)-2-(alpha,alpha,alpha,-trifluoro-m-tolyl)-3-(2H)pyridazinone) and Its Developmental Consequences. Plant Physiol. 1976 Feb;57(2):260–269. doi: 10.1104/pp.57.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner G. F., Hauska G. Localization of the reaction site of cytochrome 552 in chloroplasts from Euglena gracilis. Effects of a specific antibody on endogenous, external and reincorporated cytochrome 552 in chloroplast membranes. Arch Biochem Biophys. 1974 Sep;164(1):136–144. doi: 10.1016/0003-9861(74)90015-0. [DOI] [PubMed] [Google Scholar]
- Zeldin M. H., Schiff J. A. RNA metabolism during light-induced chloroplast development in euglena. Plant Physiol. 1967 Jul;42(7):922–932. doi: 10.1104/pp.42.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]