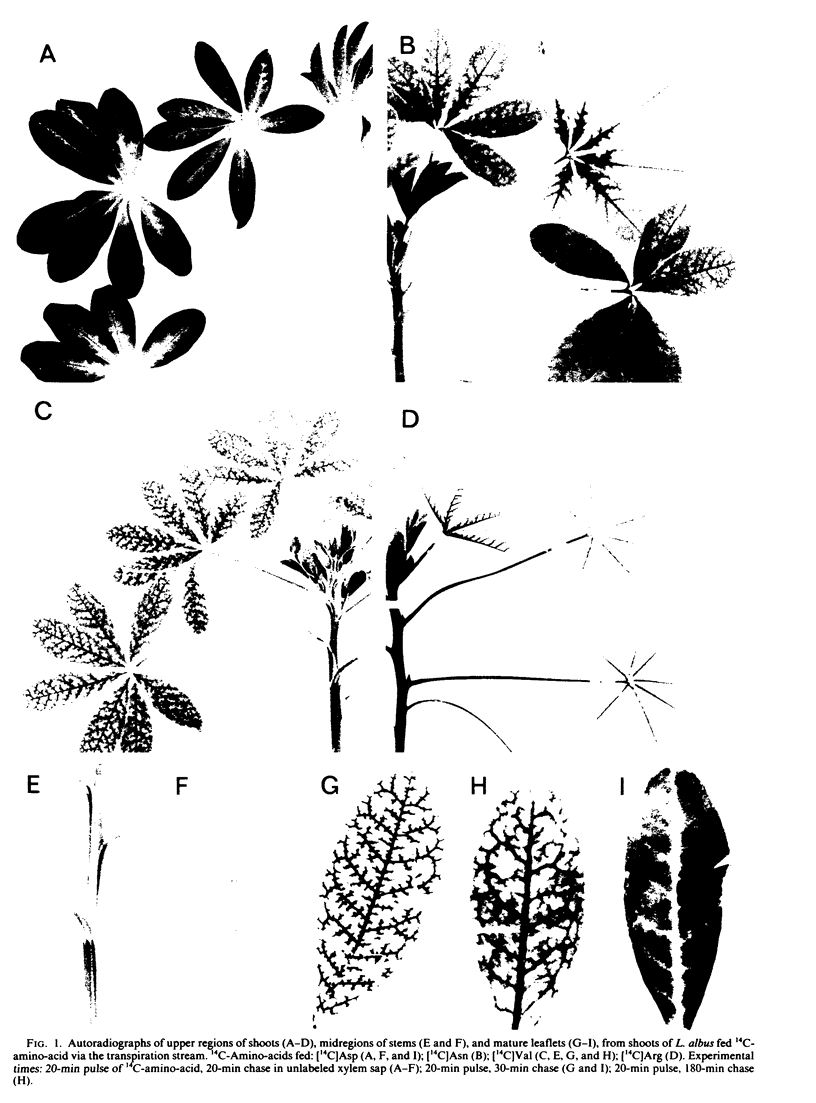
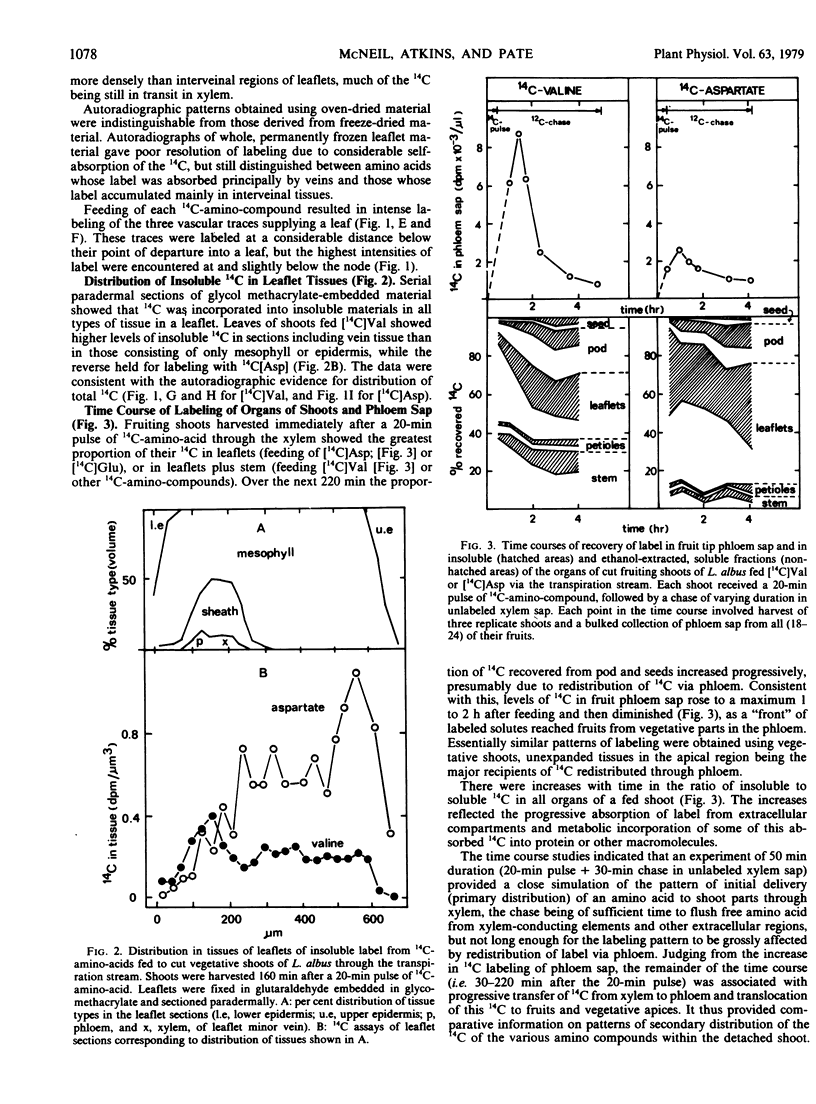
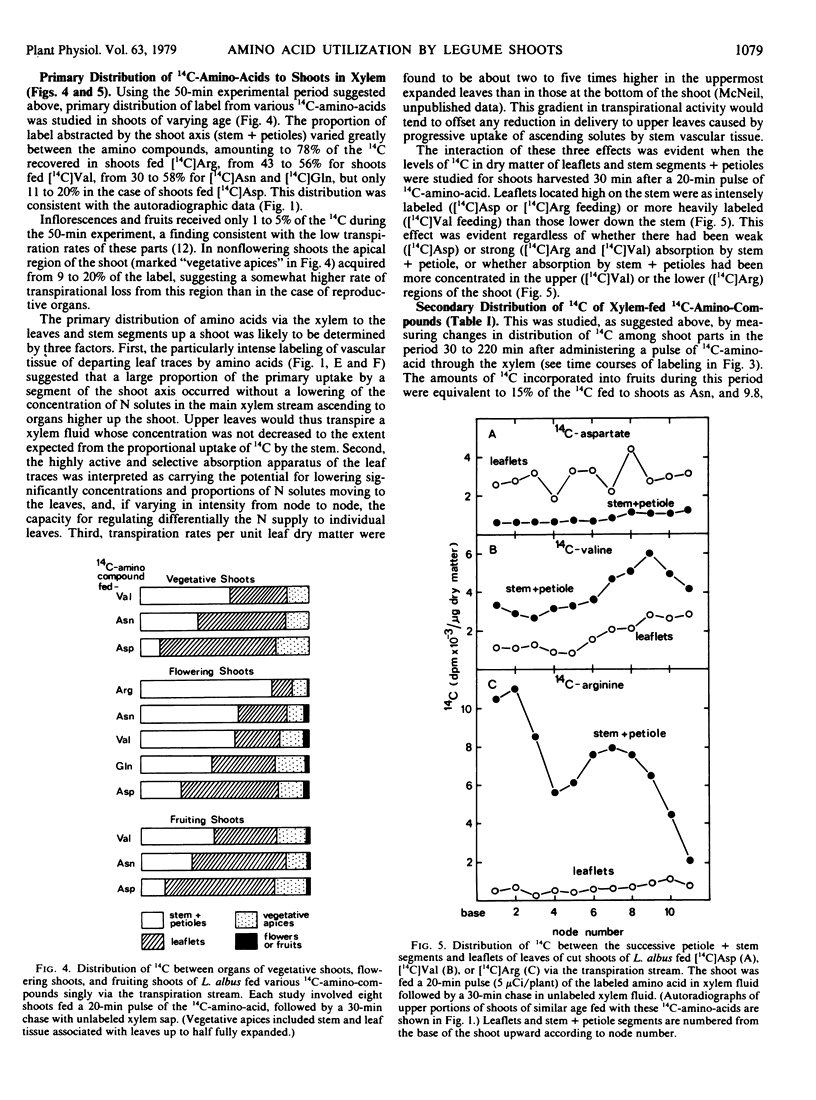
Abstract
Amino compounds representative of the major N solutes of xylem sap were pulse-fed (10 to 20 minutes) singly in 14C-labeled form to cut transpiring shoots of white lupin (Lupinus albus L.). 14C distribution was studied by autoradiography and radioassays of phloem sap, leaflet tissues, and shoot parts harvested at intervals after labeling. Primary distribution of N by xylem was simulated using a 20-minute labeling pulse followed by a 30-minute chase in unlabeled xylem sap. Shoots fed 14C-labeled asparagine, glutamine, valine, serine, or arginine showed intense labeling of leaflet veins and marked retention (35 to 78%) of 14C by stem + petioles. Shoots fed 14C-labeled aspartic acid or glutamic acid showed heaviest 14C accumulation in interveinal regions of leaflets and low uptake (11 to 20%) of 14C by stem + petioles. Departing leaf traces were major sites of uptake of all amino compounds, and the implications of this were evaluated. Fruits acquired only 1 to 5% of the fed label directly from xylem, but more than doubled their intake during the period 30 to 160 minutes after feeding through receipt of 14C transferred from xylem to phloem in stem and leaves. 14C-Labeled asparagine and valine transferred directly from xylem to phloem, but the 14C of 14C-labeled aspartic acid and arginine appeared in phloem mainly as metabolic products of the fed compound. The labeling of the soluble pool of leaflets reflected these differences. The significance of heterogeneity in distribution and metabolism of xylem amino compounds in the shoot was discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY R. G., PEETS E. A., GORDON S., BUYSKE D. A. Determination of C-14 and H3 in biological samples by Schoeniger combustion and liquid scintillation techniques. Anal Biochem. 1961 Jun;2:267–273. doi: 10.1016/s0003-2697(61)80010-9. [DOI] [PubMed] [Google Scholar]
- Pate J. S., Layzell D. B., McNeil D. L. Modeling the transport and utilization of carbon and nitrogen in a nodulated legume. Plant Physiol. 1979 Apr;63(4):730–737. doi: 10.1104/pp.63.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Sharkey P. J., Atkins C. A. Nutrition of a developing legume fruit: functional economy in terms of carbon, nitrogen, water. Plant Physiol. 1977 Mar;59(3):506–510. doi: 10.1104/pp.59.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]



