Abstract
The acquisition of reproductive competence is organized and activated by steroid hormones acting upon the hypothalamus during critical windows of development. This review describes the potential role of epigenetic processes, particularly DNA methylation, in the regulation of sexual differentiation of the hypothalamus by hormones. We examine disruption of these processes by endocrine-disrupting chemicals (EDCs) in an age-, sex-, and region-specific manner, focusing on how perinatal EDCs act through epigenetic mechanisms to reprogram DNA methylation and sex steroid hormone receptor expression throughout life. These receptors are necessary for brain sexual differentiation and their altered expression may underlie disrupted reproductive physiology and behavior. Finally, we review the literature on histone modifications and non-coding RNA involvement in brain sexual differentiation and their perturbation by EDCs. By putting these data into a sex and developmental context we conclude that perinatal EDC exposure alters the developmental trajectory of reproductive neuroendocrine systems in a sex-specific manner.
Keywords: Endocrine-disrupting chemicals, Sex differences, Hypothalamus, Epigenetics, DNA methylation, Histone modifications, Steroid hormone receptors
1. Development of reproductive neuroendocrine circuitry
1.1. Hormones, endocrine disrupting chemicals (EDCs), and brain development
The neuroendocrine systems of the brain play critical roles in the integration of information about environmental stimuli and the orchestration of appropriate responses. More specifically, the hypothalamic-anterior pituitary neuroendocrine systems provide an interface between peripheral signals and central processes involved in the control of adrenal, thyroid, reproductive, growth, metabolic, and lactational functions (Marieb, 2006). With particular regard to the acquisition of reproductive competence, hypothalamic, preoptic, and limbic regions involved in the control of reproductive physiology and behavior begin to develop in the fetus, and continue their maturation through postnatal life and puberty until adult reproductive status is attained. The hypothalamic neuropeptide, gonadotropin releasing hormone (GnRH), is obligatory for reproductive competency, and is regulated by a neuronal and glial network comprising heterogeneous inputs that signal through many different neurotransmitters, neuropeptides, and neurotrophic factors. This neural circuit mediates the influences of sex-steroid hormonal feedback onto GnRH neurons, resulting in a pattern of pulsatile GnRH release in both sexes, and the preovulatory GnRH/LH surge in females (Ebling, 2005). More broadly, GnRH neurons are regulated by a complex neural network comprising heterogeneous neural inputs from neurons signaling via many types of neurotransmitters (e.g., glutamate, GABA, norepinephrine, and neuropeptides such as kisspeptin, galanin, and others). Many of these inputs are steroid-hormone sensitive, and the anatomical organization of these neurons begins to be established during late embryonic and early postnatal life in mammals, during which developmental and sex-specific exposures to gonadal hormones act during a critical period of brain sexual differentiation to “program” permanent neuroanatomical and molecular changes. Once male- and female-typical circuits are established and organized, they are activated by the peripubertal increase in gonadal hormones and maintained by hormonal exposure throughout adulthood (Terasawa and Fernandez, 2001).
Endocrine disrupting chemicals (EDCs), as defined by an Endocrine Society statement of principles (Zoeller et al., 2012), are “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone signaling.” The Endocrine Society’s 2015 scientific statement, EDC-2, further qualified this definition by emphasizing the particular importance of developmental exposures to EDCs as most problematic (Gore et al., 2015). EDCs are ubiquitous, and exposure can occur through industrial waste that has entered the environment and the food chain, contaminated water systems, widespread use of pesticides, as well as exposures from cosmetics and personal care products, pharmaceuticals, plastic products and diet. Once these compounds are in the environment or in the food chain, depending upon their structure and properties (e.g., lipophilicity), they can bioaccumulate. Organisms that are at the top of the food chain, including humans, have the greatest body burdens [Reviewed in (Annamalai and Namasivayam, 2015)]. In the body, EDCs interfere with normal endocrine actions by a variety of mechanisms; the best-studied are by their actions as agonists or antagonists of steroid hormone receptors due to structural similarities (Fig. 1), and/or by interfering with steroid hormone synthesis and metabolism, in target tissues [Reviewed in (De Coster and van Larebeke, 2012)]. The effects of EDCs are tissue-specific and depend on timing, dose and duration of exposure. Although exposure at any life stage can cause endocrine dysfunctions, developing organisms, especially fetuses, infants, and children, are especially vulnerable. Exposures during this time, even at very low levels, may induce some alterations in the developing organism, but the phenotypic changes may not become apparent until later in development or when the organism has reached maturity. This concept, referred to as the developmental origins of health and disease (DOHaD), has been proposed to explain how the perinatal developmental period is highly sensitive to environmental perturbations and results in irreversible changes in gene expression, cellular function and morphology that predispose the organism to dysfunction later in life (Barker, 2004; Vandenberg et al., 2012; Heindel et al., 2015).
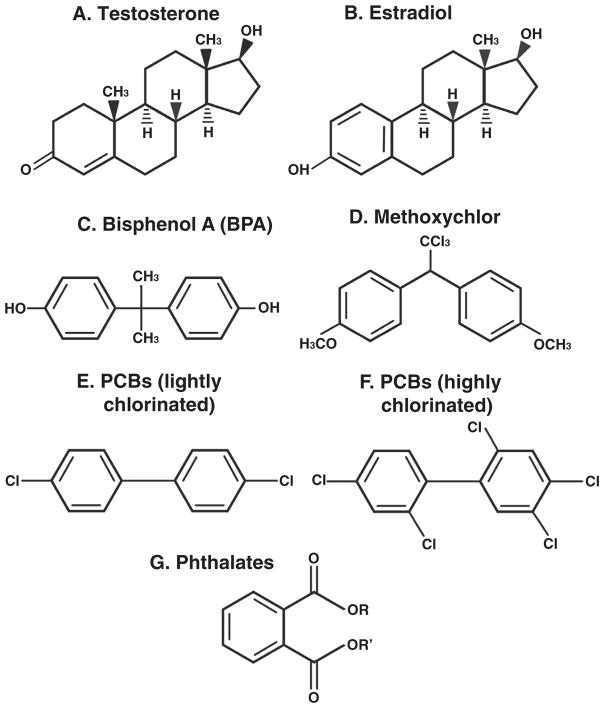
Fig. 1.
Chemical structures for steroid hormones: (A) Testosterone and (B) Estradiol. Representative endocrine disrupting chemicals (EDCs) structures are shown for (C) Bisphenol A, (D) Methoxychlor, (E) lightly chlorinated polychlorinated biphenyls (PCBs), (F) highly chlorinated PCBs, and (G) Phthalates.
Although this review article focuses solely on EDCs and the brain, it is important to point out that indirect EDC effects on the nervous system can be induced through EDCs’ actions on the gonads. There is a strong literature showing that the developing male and female gonads are highly sensitive to EDCs, with consequences for follicular development, ovulation and steroidogenesis in the ovary; and spermatogenesis, steroid synthesis in males [reviewed in (Gore et al., 2015)]. Effects of EDCs on the timing of puberty, or on subfertility/infertility, can involve any level of the hypothalamic-pituitary-gonadal axis. Therefore, the literature we will be discussing on EDC actions in the brain should be considered in this broader context of both direct and indirect mechanisms.
1.2. The brain is sensitive to hormones during prenatal and early postnatal life
The ability of the brain to respond to hormones begins extremely early in life, at the end of the first trimester in humans and in mid-embryonic development in rodents, due to the early life expression of steroid hormone receptors [Humans, (McCarthy et al., 2009); rodents, (McEwen, 1981; Forger, 2006)]. There are several sources of natural hormones to which the fetal brain is exposed, including placental hormones, maternal hormones that can cross the placenta, and the fetus’s own developing endocrine organs (Gore et al., 2014). Each hormone plays a unique role, with precise timing of hormonal exposure being absolutely critical to normal development. In the absence of the proper hormonal milieu the fetus may not survive or, if it does, it is likely to have some abnormality that may be manifested at birth, or later in life. In the developing brain, gonadal steroids alter neurogenesis, glial development, neural apoptosis (programmed cell death), and the formation and maintenance of synaptic connections (Forger, 2006; McCarthy et al., 2009). Steroids also modulate neurotransmitter synthesis, release, and actions on their target receptors (Mani et al., 1994), and control critical components of neural and glial plasticity (Arnold and Gorski, 1984; McEwen et al., 1991).
In addition to the brain’s early life responsiveness to steroid hormones is its ability to generate these hormones from precursors by steroidogenesis. The primary substrate of steroidogenesis is cholesterol, which, after a series of enzymatic reactions is converted to the major steroid hormone classes. Thus, the presence or absence of enzymes in any tissue determines if and which hormones will be synthesized. Enzymes that are critical for these processes are distributed throughout various brain regions including: cytochrome p450 side chain cleavage (p450scc) enzyme encoded by the CYP11A1 gene, which catalyzes a rate-limiting step in the initial conversion of cholesterol (Compagnone et al., 1995); steroidogenic acute regulatory protein (StAR), involved in cholesterol transport as a first step in steroid biosynthesis (Furukawa et al., 1998) and other members of the cytochrome p450 family such as 17α-hydroxylase or p450c17 (CYP17A1) and aromatase (CYP19A1) (Stromstedt and Waterman, 1995). Thus, many, if not all, of the major steroidogenic enzymes are present in the brain, with developmental-, sex-, and region-specific differences in expression. Importantly, the enzymes that interconvert testosterone to estradiol (aromatase) or to dihydrotestosterone (5α-reductase) are also abundant in the nervous systems of rodents and humans (Celotti et al., 1997; Wu et al., 2009), and expression (5α-reductase) and enzymatic activity (aromatase) coincide with the critical window for sexual differentiation of the brain in rodents (George and Ojeda, 1982; Melcangi et al., 1998). This highlights the importance of steroidogenic enzymes in the development of a male- or female-typical brain in terms of regional size, cell numbers, and neural and glial phenotypes (Balthazart et al., 2011; Panzica et al., 2012). Given the crucial role of hormones in normal brain development, it is not surprising that the brain is exceptionally sensitive to perturbations by EDC exposure during development.
1.3. Brain sexual differentiation and epigenetics
Sex determination in mammals is dependent upon the complement of sex chromosomes (XX, female; XY, male) in the fetus. Activation of a cascade of genes in males, beginning with SRY on the Y chromosome, triggers a sexual differentiation pathway that drives testicular development, whereas the absence of the Y chromosome in females results in activation/repression of genes that specify ovarian development [Reviewed in (Brennan and Capel, 2004; Bowles and Koopman, 2013)]. Information from rodent, sheep, macaques and humans, shows that the fetal testis becomes capable of secreting several hormones, including testosterone and anti-Mullerian hormone (AMH), that have profound effects on the development of male reproductive tracts, glands, and genitalia [Reviewed in (Capel, 1996; Kashimada and Koopman, 2010; Bowles and Koopman, 2013; Forger et al., 2016)]. Moreover, waves of testosterone released in the embryo and infant are important for masculine sexual differentiation of the brain [(Baum et al., 1991); Reviewed in (McCarthy, 2011; de Vries and Forger, 2015)]. The female embryo is quite different from the male. The developing ovary is relatively quiescent compared to the male testis, and the female reproductive organs and genitalia form in the absence of exposure to testosterone and AMH [Reviewed in (Capel, 1996; Kashimada and Koopman, 2010; Spiller et al., 2012)]. These differences between the sexes in normal development underscore the potential for the genitals and the brain to be highly sensitive to developmental perturbations by EDCs. Furthermore, these organs develop during overlapping but not identical phases of fetal life, underscoring the point that the critical period of sexual differentiation for one organ may not be the same as in another.
The organizational effects of steroids set the stage for numerous neurobiological processes, some of which are not manifested until childhood, adolescence, or adulthood. Our understanding of the importance of hormones in brain sexual differentiation is nearly a century old, with evidence that this is established in the embryo through early postnatal life (Phoenix et al., 1959; Barraclough and Gorski, 1961; Petrusz and Flerko, 1965; Morris et al., 2004; Kudwa et al., 2006; Sodersten et al., 2014). While most of the prior evidence was accumulated from rodent work, the human brain is also structurally and functionally sexually dimorphic (Ehrhardt and Meyer-Bahlburg, 1981; Wizemann and Pardue, 2001; Cahill, 2006). Sex differences in reproductive behavior and strategies (e.g. parental behavior, pair bonding, etc.) are necessary for the perpetuation of the species, as they increase the likelihood of successful reproduction and offspring survival. Therefore, the process of sexual differentiation of the brain is critical for species survival (Dulac and Kimchi, 2007). Even subtle changes in brain organization and activation from disruption by EDCs can have profound effects on reproductive strategies, physiology, and behaviors, with potentially devastating effects on a population living in a contaminated area (Crews and Gore, 2011, 2012).
Although not the focus of this review, it is important to acknowledge the key role of adrenal steroids. The adrenal cortex also develops during embryonic development, when it first begins to secrete cortisol or corticosterone depending upon species, as well as adrenal androgens such as DHEA that are a substrate for placental estrogen synthesis (Ishimoto and Jaffe, 2011). This placental estrogen stimulates pituitary adrenocorticotropic hormone (ACTH), which in turn stimulates adrenal steroidogenesis (Wood, 2005). Similar effects are observed in humans, in which the fetal adrenals also become active, producing progesterone, its metabolites, and cortisol (Baron-Cohen et al., 2015). Thus, the developing adrenal has the potential to affect brain glucocorticoid receptors through cortisol production, as well as to indirectly influence estrogen receptors through the placenta. This is an understudied yet intriguing mechanism for EDCs to alter the neuroendocrine stress axis as well. However, due to a dearth of evidence in this field, the remainder of this review article will focus on gonadal steroid hormones and EDCs.
As mentioned above, the organizational and activational effects of gonadal hormones in sexual differentiation of the brain is well established. However, recent evidence suggests that sex chromosomes also play a role in sexual differentiation of the brain. The development of four core genotype mice, transgenic animals in which the SRY gene is knocked out on the Y chromosome and introduced on an autosomal chromosome, has contributed to our understanding. Mating these animals yields gonadally similar males and females (XXM vs XYM with testes, and XXF and XYF with ovaries). This model provides the opportunity to investigate the contribution of sex chromosomes to sexual differentiation of the brain as well as interactions of sex chromosomes and gonadal hormones [Reviewed in (Arnold and Chen, 2009)]. These animals have revealed that some sex differences in the brain, e.g., differences in mesencephalic tyrosine hydroxylase, develop before the gonadal hormone surge in rodents and are dependent on sex chromosomes (Sibug et al., 1996). Furthermore, sexually dimorphic behaviors such as aggression and maternal behaviors are influenced by interactions of sex chromosomes and gonadal hormones (Gatewood et al., 2006). Future work is needed to determine whether and how EDCs may affect these processes.
Because brain sexual differentiation produces robust and enduring alterations in brain and behavior, epigenetic mechanisms have been proposed as regulators of both the phenotypic priming and activation of male- and female-typical behavior and reproductive function. Although there are many definitions of epigenetics, here we define a molecular epigenetic change as one that is transmitted to daughter cells through cell division, and does not involve changes to the DNA sequence itself, but rather, occurs through modifications that influence production of RNA or protein from the DNA template. These mechanisms include DNA methylation, histone modifications and post-transcriptional regulators such as non-coding RNAs. Work to date on epigenetic regulation of sexual differentiation of the brain or perturbations by EDCs has focused on sexually dimorphic hypothalamic nuclei as targets, notably the anteroventral periventricular nucleus (AVPV) and medial preoptic area (mPOA) (Table 1), and this will be the main focus of the sections below. When available, data from limbic and reward systems, as well as neocortical brain regions, have been included (Table 1). However, it should be noted that sex differences and sexual differentiation of the brain are wide ranging and much more work is necessary to determine if and how epigenetic mechanisms may contribute to the effects of EDCs throughout the brain.
Table 1.
Sexually dimorphic brain regions.
| Brain region | Abbreviation | Sexually dimorphic in size | Behaviors and functions regulated | References | |
|---|---|---|---|---|---|
| Anterior hypothalamus and Preoptic Area (POA) | Anteroventral periventricular nucleus | AVPV | Yes (F > M) | Regulates preovulatory LH surge in females and may play a role in the circadian regulation of GnRH in both males and females | Wiegand and Terasawa (1982), Petersen and Barraclough (1989), Davis et al. (1996), Smith et al. (2006), Robertson et al. (2009) |
| Medial preoptic nucleus/medial preoptic area | mPOA | Yes (M > F, within the sexually dimorphic nucleus of the POA, SDNPOA) | Regulates male reproductive behavior and female proceptive behavior. Also plays a role in maternal behavior | Lauber et al. (1991), Brailoiu et al. (2007), Graham and Pfaus (2013), Hull and Dominguez (2015) | |
| Posterior hypothalamus, Medial Basal Hypothalamus (MBH) | Arcuate nucleus | ARC | No | Regulates feeding behavior and negative feedback of sex steroid hormones onto GnRH neurons in both males and females | Reviewed in Myers et al. (2009), Navarro (2012), Sternson (2013), Cornejo et al. (2016) |
| Ventral medial hypothalamus | VMH | Yes (M > F) | Regulates aggression in males and receptive (lordosis) behavior in females; metabolic homeostasis and female-specific energy expenditure | Pfaff and Sakuma (1979), King and Frohman (1985), Meisel and Pfaff (1985), Majdic et al. (2002), Dugger et al. (2007), Falkner and Lin (2014), Correa et al. (2015) | |
| Extra-hypothalamic regions | Prefrontal cortex | PFC | Yes (M > F in ventral-medial PFC) and are strain specific | Involved in inhibition of several limbic regions; undergoes vast developmental changes during puberty | Grace (2000), Markham et al. (2007), Koss et al. (2012), Koss et al. (2014), Keeley et al. (2015), Drzewiecki et al. (2016) |
| Bed nucleus of the stria terminalis | BNST | Yes (M > F) | Limbic structure thought to regulate aversion related behaviors including stress, fear, and anxiety; and appetitive responses such as maternal behavior and reward | Hines et al. (1985), van Leeuwen et al. (1985), Numan and Numan (1996), Hisasue et al. (2010), Haufler et al. (2013). Reviewed in Kash et al. (2015), McHenry et al. (2015) | |
| Amygdala | AMY | Yes (depending on the subregion) | Limbic region that regulates social behaviors including aggression and anxiety | Pinel et al. (1977), Hitchcock and Davis (1986), Shibata et al. (1986) |
While epigenetic molecular mechanisms have been proposed as a mechanism for brain sexual differentiation and disruption for over a decade, empirical evidence has been lacking until recently [Reviewed in (Forger, 2016)]. We are only now beginning to identify how these mechanisms may work in concert to program the brain in male- and female-typical patterns. Because developmental exposures to EDCs disrupt hormone actions and sexual differentiation of the brain and behavior (Walker and Gore, 2007), epigenetic mechanisms may underlie some of the effects of EDCs that have been observed after perinatal exposure. In fact, this has been shown in other tissues, including reproductive organs, and has been investigated widely as a potential mechanism for the developmental programming of numerous reproductive cancers [Reviewed in (Singh and Li, 2012; Seachrist et al., 2016)]. However, few studies have specifically investigated if EDCs alter the epigenetic machinery or mechanisms in the brain. To follow is an overview of three well-characterized epigenetic molecular mechanisms, their roles in sexual differentiation of the brain, and evidence of epigenetic regulation of altered gene expression due to perinatal EDC exposure.
2. DNA methylation, sexual differentiation of the brain, and EDCs
2.1. Mechanisms of DNA methylation
DNA methylation is the process of adding a methyl group to the C5 position of a cytosine (5-mC) often adjacent to a guanine at the 5′ site, referred to as CpG (Fig. 2). DNA methylation has traditionally been considered to be a relatively stable epigenetic mark playing a pivotal role in development, the regulation of tissue-specific gene expression, X inactivation, imprinting of parental alleles, cellular differentiation, and repetitive element silencing. DNA methylation at gene promoters is generally associated with transcriptional repression, whereas in many species, including humans, methylation in gene bodies is associated with active transcription [Reviewed in (Suzuki and Bird, 2008)] and often occurs as the oxidized form of 5-mC, 5-hydroxy-mC (5-hmC). 5-hmC appears to be concentrated in gene bodies (Tahiliani et al., 2009), is associated with active transcription, and may be a transitional epigenetic state marking a site for future demethylation (Szulwach et al., 2011) (Fig. 2). Importantly, 5-hmC is enriched in the brain (Kriaucionis and Heintz, 2009). Thus the identification of this mark indicates that DNA methylation, especially in the brain, may be more dynamic than previously thought (Mikaelsson and Miller, 2011). What is clear is that DNA methylation and histone modifications (see below) participate in an “epigenetic conversation” to regulate transcription (Fig. 3). For example, DNA methylation can recruit methyl-binding proteins, which in turn recruit histone deacetylases (HDACs), leading to chromatin condensation and transcriptional repression (Hashimoto et al., 2010).
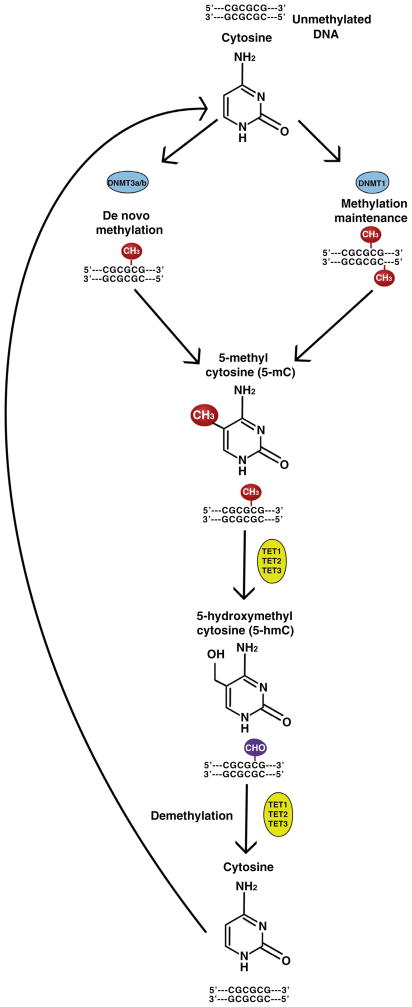
Fig. 2.
Model of DNA methylation machinery. During DNA methylation a methyl group is added to the 5C position of the cytosine nucleotide. A family of enzymes called DNA methyltransferases (DNMTs) catalyze the reaction. This can take place as de novo methylation (adding a new methyl group to an unmethylated cytosine) by DNMT3a/3b, or hemi-methylation (adding a methyl group to the unmethylated strand of DNA, often referred to as methylation maintenance), catalyzed by DNMT1. Cytosines are thought to be unmethylated through the actions of the Tet proteins, which catalyze conversion of 5-mC to 5-hmC, and ultimately result in the removal of the methyl group from the DNA molecule.
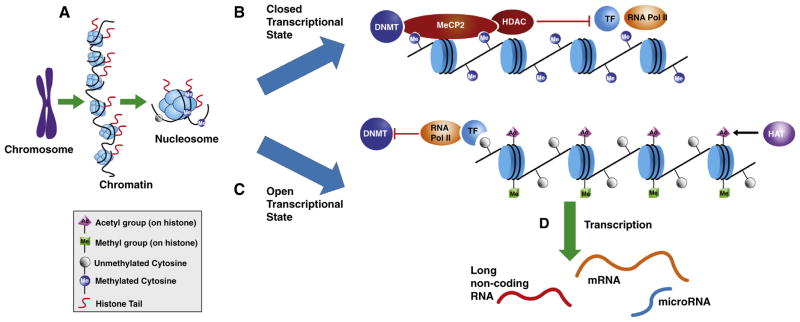
Fig. 3.
Epigenetic mechanisms can increase or decrease the likelihood of transcription through an “epigenetic conversation.” DNA is condensed in the nucleus as chromatin, which consists of the DNA strands wrapped around histones octamers consisting of H2A, H2B, H3 and H4. (A) Each histone protein can be modified through the addition of different functional groups that influence how tightly the DNA strands interact with the histone proteins. Histone modifications are a relatively transient event as “writers” and “erasers,” acting by adding or removing functional groups in response to external stimuli. (B) Histone deacetylases (HDACs) remove the acetyl mark from a histone tail. In a closed state, MeCP2 and other methyl-binding proteins bind methylated cytosines on the DNA strand, which in turn, recruit HDACs to the histones to remove acetyl groups from the histone tails. This, in turn, increases the interaction of the DNA with the histones. DNMTs also play a role in partnering with MeCP2 with a net effect of decreasing the likelihood of transcription factor (TF) binding and transcription. (C) Histone acetyltransferases (HATs) add acetyl marks to a histone tail, resulting in an open chromatin state. By this mechanism, HATs and histone methyltransferases (not shown) add functional groups to the histone tails to increase access of transcription factors and RNA polymerase II to the DNA, thereby blocking DNMTs from methylating the DNA, and facilitating gene transcription. (D) The closed or open status of chromatin affects transcription of mRNAs as well as non-coding RNAs such as microRNAs and long non-coding (lnc) RNAs, that in turn regulate expression, stability, and organization of gene expression and chromatin structure.
Initially, DNA methylation was hypothesized as an important epigenetic mechanism regulating brain sexual differentiation, as well as for mediating the effects of EDCs, because the permanent effects of early life hormone (and EDC) on brain structure and subsequent behavior presupposed an irreversible programming event early in life. This hypothesis has had to evolve because of the more recent evidence for the greater transience of DNA methyl marks than originally thought. In the next sections, we discuss the evidence that DNA methylation is an important mechanism for brain sexual differentiation, followed by information on the actions of two classes of EDCs, bisphenol A (BPA) and polychlorinated biphenyls (PCBs), on this molecular pathway.
2.2. DNA methyltransferases (DNMTs) and brain sexual differentiation
Sex differences in global DNA methylation have been observed in several brain regions in rodents including the POA (Nugent et al., 2011; Ghahramani et al., 2014), bed nucleus of the stria terminalis (BNST) and striatum (Ghahramani et al., 2014) (Table 2). The degree and directionality of these differences is dependent on the developmental time point of evaluation, but generally speaking, global methylation increases with increasing age in both male and female mice (Ghahramani et al., 2014). Importantly, global sex differences in DNA methylation in the rat POA were reversed by a masculinizing dose [Postnatal day (P) 0; 100 μg] of estradiol in females (Nugent et al., 2011). However in the POA/BNST combined and striatum of mice, sex differences in global DNA methylation were not observed, but a male-specific increase in DNA methylation of autosomal genes was induced in females exposed to a masculinizing dose of testosterone (100 μg) when measured on P60 but not P4 (Ghahramani et al., 2014). This suggests that perinatal exposure to gonadal hormones regulates DNA methylation and results in species-, sex-, age- and region-specific adult methylation patterns. In further support of this, recent evidence suggests that activity of a family of enzymes that are part of the machinery regulating DNA methylation, DNA methyltransferases (DNMTs), is sexually dimorphic in rats and altered by perinatal treatments with exogenous steroid hormones (Nugent et al., 2015).
Table 2.
Perinatal EDC and hormonal effects on sex differences in DNA methylation/methylation machinery.
| Gene | Endpoint | Species | brain region | Embryonic E0–E21 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood ≤ P60 | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Global methylation | Baseline sex differences | Rat | POA | F > M (P1) | Nugent et al. (2015) | |||||
| Mouse | POA/BNST | F = M (P4) | Global me: F = M (P60) Autosomal me: M > F (P60) |
Ghahramani et al. (2014) | ||||||
| Mouse | Striatum | F = M (P4) | Global me: F = M (P60) Autosomal me: M > F (P60) |
Ghahramani et al. (2014) | ||||||
| Perinatal E2/T exposure | Rat | POA | F + E2 = M (P1) | Nugent et al. (2015) | ||||||
| Mouse | POA/BNST | No Δ (P4) | Global me: No Δ (P60) Autosomal me: F + T = M (P60) |
Ghahramani et al. (2014) | ||||||
| Mouse | Striatum | No Δ (P4) | Global me: No Δ (P60) Autosomal me: F + T = M (P60) |
Ghahramani et al. (2014) | ||||||
| Perinatal BPA exposure | Mouse | Forebrain | =methylation & demethylation (E12.5) | Yaoi et al. (2008) | ||||||
| Dnmt enzymatic activity | Baseline sex differences | Rat | POA |
F > M (P0 P2) F = M (P4, P7) |
Nugent et al. (2015) | |||||
| Perinatal E2/T exposure | Rat | POA |
F + E2 = M (P0, P2) No Δ (P4, P7) |
Nugent et al. (2015) | ||||||
| Dnmt1 mRNA/protein expression | Baseline sex differences | Mouse | Whole brain/hypo | Brain: F = M (E18.5) | Hypo: F > M (P28) | Wolstenholme et al. (2012) and Kundakovic et al. (2013) | ||||
| Rat | POA | F = M (P0, P1, P2, P4, P7) | F = M (P10) | Kolodkin and Auger (2011) and Nugent et al. (2015) | ||||||
| AVPV | F > M (P15) | F = M (P30) | F = M (P45) | M > F (P90) | Walker et al. (2014) | |||||
| MBH | F = M (P1) | F = M (P10) | Kolodkin and Auger (2011) | |||||||
| ARC | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P90) | Walker et al. (2014) | |||||
| AMY | F = M (P1) | F = M (P10) mRNA and protein | Kolodkin and Auger (2011) | |||||||
| Mouse | PFC | F = M (P28) | Kundakovic et al. (2013) | |||||||
| Perinatal E2/T exposure | Rat | POA | No Δ (P0, P1, P2, P4, P7) | Nugent et al. (2015) | ||||||
| AVPV | F + E2 = M (P15) | No Δ (P30) | No Δ (P45) | F + E2 = M (P90) | Walker et al. (2014) | |||||
| ARC | F = M (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Walker et al. (2014) | |||||
| AMY | No Δ (P2) mRNA and protein | Kolodkin and Auger (2011) | ||||||||
| Perinatal BPA exposure | Mouse | Whole brain/hypo | Brain: No Δ (E18.5) |
Hypo: M + BPA = F F + BPA = M (P28) |
Wolstenholme et al. (2012) and Kundakovic et al. (2013) | |||||
| Rat | Amy (BLA) | F + BPA > F (P45) | Zhou et al. (2013) | |||||||
| Mouse | PFC | F = M (P28) | Kundakovic et al. (2013) | |||||||
| Perinatal PCB exposure | Rat | Hypo | F + PCBs < F (P21) | Desaulniers et al. (2005) | ||||||
| AVPV | F + A1221 = M (P15) | No Δ (P30) | No Δ (P45) | F + A1221 = M (P90) | Walker et al. (2014) | |||||
| ARC | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Walker et al. (2014) | |||||
| Dnmt3a mRNA/protein expression | Baseline sex differences | Mouse | Whole brain/hypo | Brain: F = M (E18.5) | Hypo: F = M (P28) | Wolstenholme et al. (2012) and Kundakovic et al. (2013) | ||||
| Rat | POA | F = M (P0, P1, P2, P4, P7) | F = M (P10) | Kolodkin and Auger (2011) and Nugent et al. (2015) | ||||||
| AVPV | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P90) | Walker et al. (2014) | |||||
| MBH | F = M (P1) | F = M (P10) | Kolodkin and Auger (2011) | |||||||
| ARC | F > M (P15) | F = M (P30) | F = M (P45) | M > F (P90) | Walker et al. (2014) | |||||
| AMY | F > M (P1) mRNA and protein | F = M (P10) mRNA and protein | Kolodkin and Auger (2011) | |||||||
| Mouse | PFC | F > M (P28): | Kundakovic et al. (2013) | |||||||
| Perinatal E2/T exposure | Rat | POA | No Δ (P0, P1, P2, P4, P7 P10) | Kolodkin and Auger (2011) and Nugent et al. (2015) | ||||||
| AVPV | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Walker et al. (2014) | |||||
| ARC |
F + E2 = M M + E2 = F (P15) |
No Δ (P30) | No Δ (P45) | F + E2 = M (P90) | Walker et al. (2014) | |||||
| AMY | F + E2 or T < F (P2) mRNA and protein | Kolodkin and Auger (2011) and Nugent et al. (2015) | ||||||||
| Perinatal BPA exposure | Mouse | Whole brain/hypo | Brain: No Δ (E18.5) | Hypo: M + BPA < M & F (P28) | Wolstenholme et al. (2012) and Kundakovic et al. (2013) | |||||
| PFC |
F + BPA = M M + BPA = F (P28) |
Kundakovic et al. (2013) | ||||||||
| Perinatal PCB exposure | Rat | AVPV | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Walker et al. (2014) | |||
| ARC |
F + A1221 = M M + A1221 = F (P15) |
No Δ (P30) | No Δ (P45) | F + A1221 = M (P90) | Walker et al. (2014) | |||||
| Dnmt3b mRNA/protein expression | Baseline sex differences | Mouse | Whole brain/hypo | Brain: F = M (E18.5) | Wolstenholme et al. (2012) | |||||
| Rat | POA | F = M (P0, P2, P4, P7) | Nugent et al. (2015) | |||||||
| Perinatal E2/T exposure | Rat | POA | No Δ (P0, P2, P4, P7) | Nugent et al. (2015) | ||||||
| Mouse | Brain: No Δ (E18.5) | Wolstenholme et al. (2012) | ||||||||
| MeCP2 mRNA/protein expression | Baseline sex differences | Rat | POA | F = M (P1) | F = M (P10) | Kurian et al. (2007) | ||||
| VMH | F > M (P1) | F = M (P10) | ||||||||
| AMY | F > M (P1) | F = M (P10) |
Effects of perinatal EDC or hormone exposure on sex differences in DNA methyation and methylatin machinery are age- and region- specific. Known baseline sex differences (bold) in methylation and expression of methylation machinery are indicated across brain regions and across developmental timepoints (from embryos through adults). Effects of EDCs and hormones for each endpoint are indicated at developmental timepoints when data are available. (bolded) No change is indicated as No Δ. In most cases, only mRNA was measured. Changes in protein are indicated when information is available. For information regarding differences in dose and timing/route of exposure please see text. Abbreviations: A1221 = aroclor 1221, a PCB mixture; global me = global methylation; autosomal me = autosomal methylation; E2 = estradiol; T = testosterone; Hypo = whole hypothalamus; F = females; M = males.
DNMTs are a family of methyltransferases that catalyze the addition of a methyl group to CpGs. There are five known DNMTs in the brain: DNMT1, DNMT2, DNMT3a, DNMT3b and DNMT3L. In general, DNMT1 regulates DNA methylation maintenance, meaning it preferentially methylates hemi-methylated DNA or DNA on which only one strand is methylated, usually after replication (Fig. 2). DNMT3a and 3b regulate de novo DNA methylation and preferentially methylate unmethylated CpGs, especially during development. Finally, while DNMT3L does not seem to have catalytic activity, it assists in de novo DNA methylation by increasing the ability to bind a methyl donor [Reviewed in (Kareta et al., 2006; Subramaniam et al., 2014)]. While it is apparent that each enzyme catalyzes specific types of DNA methylation events, there is mounting evidence that they work in concert. For example, DNMT1, while best-studied for its role in the maintenance of DNA methylation, is also required for de novo DNA methylation. Furthermore, DNMT3a and 3b both play a role in DNA methylation maintenance, suggesting that there is a complex interplay between these enzymes during DNA methylation [Reviewed in (Jin et al., 2011)].
There are sex differences in expression and activity of DNMTs in rodents (Table 2). During the neonatal period, DNMT enzymatic activity was higher in female than in male rats, and was decreased to male-typical levels in females by neonatal exposure to estradiol. Blocking DNMT activity in the neonatal female rat via a DNMT antagonist or conditional gene knock-out in mouse POA (specifically Dnmt3a), masculinized neonatal gene expression and adult reproductive behavior (Nugent et al., 2015). These data suggest that brain masculinization is actively repressed by DNA methylation in females, and that brain feminization is an active process and not simply the default state of brain development.
Further supporting the importance of DNA methylation in brain sexual differentiation, several groups reported sex differences in expression of DNMT mRNA and protein levels in the hypothalamus and other limbic regions (Table 2). Most of these studies focused on DNMT1 and DNMT3a. Sex differences in Dnmt3a expression in rats were observed in the amygdala as early as P1 (females > male) (Kolodkin and Auger, 2011), but in general, sex differences in gene and protein expression of DNMT1 and DNMT3a in rats and mice were not apparent until P15 (measured on E18.5, P0 – 8, P10, and P15) (Kolodkin and Auger, 2011; Wolstenholme et al., 2012; Walker et al., 2014; Nugent et al., 2015). From P15 through adulthood (see Table 2 for details), sex differences in both Dnmt1 and Dnmt3a expression emerged in a region-specific manner in the mouse hypothalamus (Kundakovic et al., 2013; Walker et al., 2014).
Regarding Dnmt1, gene expression was greater in the female than the male rat AVPV (Table 2) when measured at P15 (Walker et al., 2014) and higher in peri-pubertal (P28) females than males when measured in whole hypothalamus of mice (Kundakovic et al., 2013). However, by adulthood (P90) a reversal of the sex difference was observed in the AVPV whereby Dnmt1 expression was greater in the male than female, underscoring the point that the age at which gene expression is measured has an important influence on the outcome. As mentioned above, sex differences in Dnmt1 expression are also region specific, as no sex differences were identified across development (P15 – P90) in the arcuate (ARC) nucleus of the hypothalamus of rats (Table 2) (Walker et al., 2014). Interestingly, if female rats were exposed to a low dose (50 μg) of estradiol benzoate (EB) during the critical period of brain sexual differentiation (E16 and E18), they displayed a male-typical expression pattern of Dnmt1 in the AVPV from P15 to P90 (Walker et al., 2014) suggesting that the developmental trajectory of Dnmt1 expression in the AVPV is organized by gonadal hormones during the critical period. Further research is necessary to determine if this altered developmental profile yields sex-specific alterations to DNA methylation in the developing AVPV.
Unlike Dnmt1, Dnmt3a expression is sexually dimorphic in the ARC but not the POA or medial basal hypothalamus (MBH) of rats on P1 and P10 (Kolodkin and Auger, 2011), the AVPV of rats (P15 through P90) (Walker et al., 2014), or whole hypothalamus in mice on P28 (Kundakovic et al., 2013). For example, our lab reported that sex differences in the rat ARC were age-specific with greater expression in females than males at P15, and a reversal of that sex difference (M > F) in adulthood (P90) (Walker et al., 2014). Additionally, a low dose of EB (50 μg) on E16 and E18 resulted in a reversal of the sex difference on in the male and female ARC on P15. However, this effect was not persistent in males, as only the females exposed to EB displayed a male-typical expression profile on P90 (Walker et al., 2014). Interestingly, while the AVPV is sexually dimorphic, the ARC is thought to be structurally similar between males and females (Table 1), suggesting that DNA methylation may play a region-specific role in brain sexual differentiation, by driving sex differences in the case of the AVPV and repressing them in the case of the ARC.
Unlike Dnmt1, Dnmt3a is sexually dimorphic in extrahypothalamic brain regions. As mentioned above, in the juvenile amygdala, a limbic region that regulates social behaviors including aggression (Pinel et al., 1977) and anxiety (Hitchcock and Davis, 1986; Shibata et al., 1986) (Table 2), DNMT3a mRNA and protein expression was greater in female than male rats on P1 but not P10. A masculinizing dose of EB (100 μg) or dihydrotestosterone (250 μg) on P1 reduced Dnmt3a mRNA expression on P2 in female rats when compared to the vehicle treated controls (Kolodkin and Auger, 2011), suggesting that the female amygdala is sensitive to disruption by gonadal hormones during the critical period of sexual differentiation of the brain. Additionally, in the peripubertal (P28) prefrontal cortex (PFC) of mice, a region of the brain involved in inhibition of several limbic regions (Grace, 2000) that undergoes vast developmental changes during puberty (Koss et al., 2012; Koss et al., 2014; Drzewiecki et al., 2016), Dnmt3a expression was greater in the female than the male PFC (Kundakovic et al., 2013). Finally, in the mouse cortex, Dnmt3a expression increases from P1 – P10, peaks on P10 and decreases to early postnatal levels by P25 in both males and females and Dnmt1 expression increases from P1 – P25 (direct sex differences not analyzed) (Westberry et al., 2010). Taken together these data suggest that DNA methylation may play a role in sexual differentiation of extra-hypothalamic regions, presumably through the actions of DNMT3a.
2.3. EDCs and DNA methylation machinery
2.3.1. 3a Bisphenol A (BPA)
BPA is an EDC that is widely used in the production of polycarbonate plastics, epoxy resins used to line metal cans, and numerous plastic consumer products including toys, medical equipment and electronics [Fig. 1; (Suzuki et al., 2000; Kang et al., 2003; Vandenberg et al., 2007)]. Although primarily studied for actions on estrogen receptor-mediated pathways (Cao et al., 2013; Naule et al., 2014), BPA also perturbs thyroid hormone, androgen receptor, and aromatase systems (Pelayo et al., 2012; Picot et al., 2014; Kinch et al., 2015). Human exposure to BPA occurs through everyday exposure to food sources and plastics containing BPA, and through handling of thermal receipts, among other routes. Serum concentrations in the human population are estimated to be between ~0.2–20 ng/ml (Vandenberg et al., 2007). Currently, the no-observed-adverse-effect-level (NOAEL) that has been extrapolated for BPA is considered to be 50 mg/kg/day; however, over 150 peer-reviewed studies have reported detrimental effects of BPA exposure far below the estimated NOAEL (Vandenberg et al., 2007). Gestational and/or early life exposure to BPA in animal models causes long-term alterations in gene expression, protein expression, behavior, brain morphology, cellular function, reproductive physiology, genital abnormalities, reproductive cancers and infertility [Reviewed in (Kundakovic and Champagne, 2011; Seachrist et al., 2016)]. Although it is not possible to infer such direct linkages between early life BPA exposures and disease in humans, epidemiological evidence shows that humans with higher BPA concentrations, compared to those with low levels, have increased incidence of prostate cancer, cardiovascular disease, reproductive impairments, and other endocrine and chronic diseases [Reviewed in (Gore et al., 2015)]. However, few studies have investigated the epigenetic mechanisms in the brain that may be affected by BPA, nor determined consequences for reproductive and behavioral outcomes that may relate to actions on neuroendocrine or neurodevelopmental systems.
BPA was first shown to directly alter DNA methylation in the variable yellow agouti (Avy) mouse containing a retrotransposon that is methylated to different degrees, and in which DNA methylation status is directly related to coat color and obesity, phenotypic features that are driven by the agouti gene. In this mouse, maternal exposure to BPA (50 mg/kg to the dam beginning 2 weeks prior to mating through the end of lactation) decreased methylation and resulted in altered coat color, an effect that was prevented by supplementing with a methyl donor (folic acid) during BPA exposure throughout gestation and lactation (Dolinoy et al., 2007). There is also evidence that BPA affects the epigenome through DNA methylation in reproductive tissues and may underlie the development of reproductive cancers, especially prostate (Prins, 2008; Prins et al., 2014; Calderon-Gierszal and Prins, 2015; Wong et al., 2015) and breast cancer [Reviewed in (Singh and Li, 2012; Seachrist et al., 2016)]. To our knowledge, only one study has explicitly investigated genome-wide changes in DNA methylation in the mouse forebrain associated with gestational BPA exposure (20 μg/kg maternal body weight) (Yaoi et al., 2008). Using restricted landmark genomic scanning, Yaoi et al. determined that BPA altered both methylation and demethylation to roughly equivalent degrees in the mouse forebrain on embryonic day 12.5 and 14.5.
Several studies have measured BPA induced expression of DNA methylation machinery in the brain as a proxy for DNA methylation changes (Table 2). BPA exposure (5 mg/kg in the maternal diet) during gestation had no effect on Dnmt expression in whole mouse brain at embryonic day 18.5 (Wolstenholme et al., 2012). However, by P28, dose and sex-specific effects emerged in the whole hypothalamus and PFC of male and female mice (Kundakovic et al., 2013). In the female hypothalamus, Dnmt1 but not Dnmt3a displayed a U-shaped dose-response curve, whereas in males both Dnmt1 and Dnmt3a had this effect. In the PFC of these animals, both males and females displayed a dose-dependent decrease in Dnmt1 expression. However, gestational BPA exposure in mice resulted in opposing expression patterns of Dnmt3a between the sexes, with males having an inverted U-shaped dose-response curve and females displaying a U-shaped dose response curve (Kundakovic et al., 2013). Such non-monotonic dose response curves (i.e. U-shaped and inverted-U shaped dose response curves) are common in response to both natural hormones as well as EDCs (Vandenberg et al., 2012). Consistent with this, inverted U-shaped dose-response curves were observed for both Dnmt1 and Dnmt3a (but not Dnmt3b) in an embryonic hypothalamic cell line (mHypoE-N44) treated with BPA for 3 h (Warita et al., 2013). Finally, BPA exposure (2 μg/kg/day) of rats from E0 through P21 resulted in an increase in Dnmt1 expression in the basolateral amygdala, an effect that was associated with differences in anxiety-related behaviors and GABA function in rats (Zhou et al., 2013). These data suggest that BPA alters DNMT expression in a sex- and brain region-specific manner however, the studies discussed above only show that BPA can influence Dnmt expression in the brain. To our knowledge, no study has conclusively shown that BPA altered Dnmt expression after BPA exposure influences sexual differentiation of the brain in adulthood. This is a major gap in the literature and is necessary for providing amechanism by which BPA may influence sexually dimorphic behaviors and physiology in adulthood. While more research is necessary, these data do lend support to the hypothesis that gestational exposure to BPA might alter the epigenome in brain by targeting the enzymes that catalyze DNA methylation and suggest that DNA methylation may play a role in maintenance of sexually dimorphic gene expression in the brain.
2.3.2. 3b Polychlorinated biphenyls (PCBs)
PCBs (Fig. 1) are industrial contaminants that were used in a variety of applications including, but not limited to, electrical transformers, lubricants, and carbonless paper from the 1930s through the 1970s. Even though their synthesis and use has largely been banned for decades, they persist in the environment because they are stable lipophilic compounds that can bioaccumulate up the food chain. Depending on their structure, PCBs can interact with various receptors and enzyme systems in the body including those involved in thyroid and reproductive function and neurotransmission [Reviewed in (Zoeller et al., 2002)].
To date, there have not been any studies investigating the effects of PCBs on global methylation patterns in brain. However, in the human literature, serum concentrations of a mixture of persistent organic pollutants, including PCBs, was associated with global hypomethylation in the blood of people exposed to high [Greenland Inuit; (Rusiecki et al., 2008)] and low [Koreans; (Kim et al., 2010)] levels of PCBs. A similar effect was found in sperm (Consales et al., 2016). Interestingly, in elderly humans an opposite effect was observed whereby high levels of serum PCBs were associated with global hypermethylation (Lind et al., 2013). These discrepancies may be due to the age of the subjects, as global DNA methylation has been shown to decrease with aging in numerous mammalian species across a number of tissues, including brain, [Reviewed in (Lardenoije et al., 2015)].
In the rat brain, PCBs alter sexual differentiation of the AVPV and mPOA (Dickerson et al., 2011a; Dickerson et al., 2011b), reproductive senescence [aging; (Walker et al., 2013)], behavior [(Steinberg et al., 2007; Reilly et al., 2015; Bell et al., 2016b); reviewed in (Walker and Gore, 2007)] and cognition [(Widholm et al., 2001); Reviewed in (Schantz and Widholm, 2001; Boucher et al., 2009; Dzwilewski and Schantz, 2015)], outcomes that are influenced by changes in DNA methylation. Additionally, there is some evidence that perinatal exposure to PCBs altered expression of the methylation enzymes, DNMT1 and DNMT3a. In general, perinatal PCB exposure decreased sexually dimorphic mRNA expression of Dnmt1 in the female hypothalamus prior to puberty (Table 2). Specifically, exposure of female rats to a high dose (~1000X highest human exposure) of a reconstituted mixture of aryl hydrocarbon agonists (including PCBs) from P0 to P21, resulted in mRNA levels of Dnmt1 in the whole hypothalamus that were reduced by 38% compared to controls on P21 (Desaulniers et al., 2005). Similarly, a low dose of Aroclor 1221 (A1221), a mixture of approximately 45 lightly chlorinated PCB congeners, decreased expression of Dnmt1 in the female rat AVPV on P15, comparable to levels in males in which expression of this gene is normally lower than in females (Walker et al., 2014). Interestingly, by comparing the developmental profiles between males and females from P15 through P90 in that study, it becomes apparent that A1221 exposure on E16 and 18 (1 mg/kg) induced a male-typical expression pattern throughout development of the AVPV of females. In the rat ARC, Dnmt1 expression was not sexually dimorphic or altered by perinatal exposure to A1221 (Walker et al., 2014).
We also identified both sex and region-specific effects of perinatal A1221 exposure on Dnmt3a expression (Walker et al., 2014). While no effects were observed in the AVPV, in the ARC, male and female rats exposed to A1221 on E16 and 18 displayed a reversal of baseline sex difference (F > M) in expression on P15. Additionally, a persistent effect on expression was observed in females, who displayed male-typical expression on P90. This effect was not observed in adult males (P90) exposed to A1221. In both the AVPV and the ARC, A1221 had similar effects as prenatal exposure to EB, suggesting that the results may be attributable to the estrogenic activity of these specific congeners of lightly chlorinated PCBs.
Taken together, these data suggest that DNMT expression in the brain is altered by early life exposures to PCBs. However, whether the changes in genes for the DNMTs occur in parallel with protein expression or activity of the enzymes, is unknown. Additionally, other epigenetic mechanisms may be influenced by PCBs and other EDCs, so data on DNMTs should also be considered in the context of these other processes that have not yet been investigated.
2.4. Methyl-CpG-binding protein 2 (MeCP2) and brain sexual differentiation
Methyl binding proteins play a crucial role in the “epigenetic conversation” between DNA methylation, histone modifications, and transcriptional repression. Methyl binding proteins are recruited to methylated CpGs, which in turn recruit histone deacetylases (HDACs) that remove acetyl groups from histone tails (Fig. 3). The deacetylation of histones leads to transcriptional repression by increasing the interaction of DNA with histones, as will be discussed in more detail below [Reviewed in (Jaenisch and Bird, 2003)]. While there are a number of methyl binding proteins, the only one investigated as a potential regulator of sexual differentiation of the brain is methyl-CpG-binding protein 2 [MeCP2; (Kurian et al., 2007; Kurian et al., 2008; Forbes-Lorman et al., 2012)]. MeCP2 is an X-linked gene commonly associated with the neurodevelopmental disorder, Rett’s syndrome (Amir et al., 1999; Samaco et al., 2005; Chahrour and Zoghbi, 2007). In rats, males expressed less MeCP2 mRNA and protein than females in the amygdala and ventral medial hypothalamus (VMH; Table 1) on P1 but not P10; this effect was not seen in the POA (Kurian et al., 2007). Transient knock-down of MeCP2 using siRNAs infused into the amygdala of rats on P0 to P2 reduced sex differences in play behavior (decreased in male to female levels) assessed on P25 to P29 (Kurian et al., 2008) and eliminated sex differences in gene expression of vasopressin (Avp), galanin (Gal), and androgen receptor (Ar) by decreasing expression in males to female levels on P14. Transient knock-down of MeCP2 on P0 to P2 in rats resulted in prolonged alteration in protein levels in the adult limbic circuitry. Specifically, sex differences in AVP-immunoreactive cells (males > females) in the central amygdala, BNST, and projection fibers in the lateral septum were abolished and male levels were reduced to female levels (Forbes-Lorman et al., 2012), suggesting that MeCP2 plays a role in sexual differentiation of the limbic circuitry.
2.5. BPA and MeCP2
A recent study in embryonic hypothalamic cells (mHypoE-N44) reported a dose-dependent increase in Mecp2 mRNA expression 3 h after BPA exposure (used at 0.02, 0.2, 2, 20, or 200 μmol), with the highest two doses having significant effects (Warita et al., 2013). While more research is necessary, these data provide the first intriguing evidence that BPA affects the “epigenetic conversation” between DNA methylation and histone modification and/or chromatin silencing by altering Mecp2 expression.
2.6. Gene specific DNA methylation of sex steroid hormone receptors and brain sexual differentiation
Sex steroid hormone receptors are transcription factors that are members of the nuclear hormone receptor superfamily. Among their members are the gonadal steroid hormone receptors, estrogen receptor alpha and beta (Esr1, Esr2 genes), progesterone receptor (Pgr) and androgen receptor (Ar). Nuclear sex steroid hormone receptors are activated by ligand binding in the cytoplasm and/or nucleus, followed by receptor-ligand dimerization, and translocation into the nucleus. There, they bind specific DNA sequences, referred to as response elements, in promoter regions of steroid-sensitive genes. Depending on the presence of other cell-specific cofactors and transcription factors on the target gene promoter, gonadal steroid hormones can activate or repress transcription. Interestingly, the binding of sex steroid hormone receptors to DNA can produce a “molecular memory,” presumably through altered DNA methylation at the promoter, which results in longterm alterations in expression of hormone sensitive genes and regulation of the genes by nuclear hormone receptors [(Thomassin et al., 2001); Reviewed in (Holterhus, 2011)].
Sex steroid hormone receptors are expressed in the brain beginning in embryonic development; this is necessary to coordinate the timing of sexual differentiation of the brain with hormonal changes during critical periods of organization, and to enable the subsequent activation of these organized pathways by pubertal hormones to trigger sex-typical adult reproductive physiology and behavior. ESR1, PGR, and AR are well-studied for their influences on the sexual differentiation of the brain; of these, the estrogen receptor α has been extensively studied as a possible target of DNA methylation during the process of brain sexual differentiation (Tables 3–5) [Reviewed in (Matsuda, 2014)]. There is a CpG-rich region of Exon 1b of the Esr1 gene; and methylation of this region has been shown to be negatively correlated with altered Esr1 expression in several brain regions in females (Champagne et al., 2003; Champagne et al., 2006; Prewitt and Wilson, 2007; Kurian et al., 2010; Schwarz et al., 2010; Westberry et al., 2010; Pena and Champagne, 2015) and males (Prewitt and Wilson, 2007; Kurian et al., 2010; Schwarz et al., 2010; Westberry et al., 2010). Furthermore, both DNA methylation and Mecp2 were associated with the ESR1 promoter on P10 and P18 in the cortex of male and female mice (Westberry et al., 2010), a time when Esr1 expression was decreased (Prewitt and Wilson, 2007) suggesting that epigenetic mechanisms regulate Esr1 expression throughout development. Few studies have made direct comparisons between the sexes of DNA methylation at the ESR1 promoter (Kurian et al., 2010; Schwarz et al., 2010). At P1, two of seven CpGs in Exon1b showed sex-specific methylation in the POA and MBH [females > males (Champagne et al., 2003; Champagne et al., 2006; Schwarz et al., 2010; Pena and Champagne, 2015)]. In the POA but not the MBH of rats, this effect was reversed to male-typical levels when females were exposed to a masculinizing dose (100 μg) of estradiol at P0, suggesting that methylation of Esr1 contributes to sexual differentiation of the POA (Schwarz et al., 2010). Furthermore, and contrary to the dogma that DNA methylation is static, methylation of this region in Esr1 was dynamic across development; in fact, the sex difference in methylation seen at birth (female > male) (Schwarz et al., 2010) was reversed at P8 (male > female) (Kurian et al., 2010), abolished at P20, and emerged again at P60 (female > male) in rats (Schwarz et al., 2010).
Table 3.
Sex differences in sex steroid hormone receptor expression and methylation in the brain.
| Esr1 - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood ≥P60 | Species, References |
|---|---|---|---|---|---|---|---|
| DNA methylation – POA | F > M (P1) | M > F (P8) | F = M (P20) | F > M (P60) | Rat (Kurian et al., 2010; Schwarz et al., 2010) | ||
| mRNA/protein expression - POA | F > M (P 1 & 5) |
F > M (P8, P10, P15) F > M (P10) |
F = M(P21, P30) | F > M(P45) | F = M (P60) | (Monje et al., 2007; Kurian et al., 2010; Walker et al., 2014) | |
| mRNA/protein expression - AVPV |
F > M(P0, P2, P4) F = M (P1 & P7) |
F = M (P15 & P19) |
F > M (P21) F = M (P30) |
M > F (P45) | M > F (P90) | Rat (Cao and Patisaul, 2011; Cao et al., 2013; Rebuli et al., 2014; Walker et al., 2014) | |
| F > M (E19) | F = M (P5) |
F > M (P 8) F = M (P15) Esr1 and TH coexpression: F > M (P19) |
F > M (P21) F = M (P25) |
F > M (P60) F = M (P90) |
Mouse and Rat (Patisaul et al., 2006; Monje et al., 2007; Brock et al., 2015) | ||
| mRNA/protein expression - mPOA | F = M (P0) F > M (P1, P2, P4, P7) |
F > M (P10 & P19) | F = M (P120) | Mouse and Rat (Takagi et al., 2005; Cao and Patisaul, 2011; Cao et al., 2013; Faass et al., 2013; Bell et al., 2016a) | |||
| F = M (E19) |
F = M (P1) M > F (P5) |
F > M (P15) | M > F (P25) | F > M (P90) | (Dickerson et al., 2011a; Brock et al., 2015) | ||
| DNA methylation - MBH | F > M (P1) | F = M (P20) | F = M (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - MBH | F > M (P 1 & 5) | F > M (P15) | F > M (P30) | F = M (P45) | F > M (P60) | Rat (Walker et al., 2012) | |
| mRNA/protein expression - ARC | F = M (P0) F > M (P1) F = M (P2, P4, P7 rostral) F > M (P2, P4, P7 caudal) |
F > M (P15 & 19 (Caudal) | F = M (P30) | F = M (P45) | F = M (P90) | Rat (Cao and Patisaul, 2011; Cao et al., 2013; Walker et al., 2014) | |
| F = M (E19) | F = M (P5) | F = M (P15) | F = M (P25) | F = M (P90) | Mouse (Brock et al., 2015) | ||
| mRNA/protein expression - VMH | F = M (P0) F > M (P1, P2, P4, P7) |
F > M (P19) | F = M (P120) | Rat (Cao and Patisaul, 2011; Cao et al., 2013; Faass et al., 2013) | |||
| F = M (E19) | F = M (P5) | F = M (P15) | F = M (P25) | F = M (P90) | Mouse (Brock et al., 2015) | ||
|
| |||||||
| Esr2 - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | Species, References |
|
| |||||||
| DNA methylation - POA | M > F (P1) | M > F (P20) | M > F (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - POA | F = M (P 1 & 5) | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P60) | Rat (Walker et al., 2012) | |
| mRNA/protein expression - AVPV |
M > F (P0, P1) F = M (P2, P4, P7) |
F = M (P15, P19) | F = M (P30) | F = M (P45) |
F > M (P90) F = M (P90) |
Rat (Cao and Patisaul, 2011; Cao et al., 2013; Rebuli et al., 2014; Walker et al., 2014) | |
| mRNA/protein expression - mPOA | F = M (P0, P1, P2, P4, P7) | F = M (P10) F > M (P19) |
F > M (P90) F = M (P120) |
Rat (Takagi et al., 2005; Cao and Patisaul, 2011; Cao et al., 2013; Faass et al., 2013; Rebuli et al., 2014) | |||
| DNA methylation - MBH | F = M (P1) | M > F (P20) | F > M (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - MBH | F = M (P1 & 5) | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P60) | Rat (Walker et al., 2012) | |
| mRNA/protein expression - ARC | Not Detected P0, P2, P4 F = M (P7) |
F = M (P15, P19) | F = M (P30) | F = M (P45) | F = M (P90) | Rat (Cao and Patisaul, 2011; Walker et al., 2014) | |
| mRNA/protein expression - VMH | F > M (P0, P1; caudal VMH only P2, P4, & P7) | F = M (P19) | Rat (Cao and Patisaul, 2011; Cao et al., 2013) | ||||
|
| |||||||
| Ar - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | Species, References |
|
| |||||||
| mRNA/protein expression - POA | F = M (P1, P5) | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P60) | Rat (Walker et al., 2012) | |
| DNA methylation - AVPV | F > M (P15) | F > M (P30) | F > M (P45) | F > M (P90) | Rat (Walker et al., 2014) | ||
| mRNA/protein expression - AVPV | F > Male (P15) | F = M (P30) | F = M (P45) | M > F (P90) | Rat (Walker et al., 2014) | ||
| M > F (P5) | M > F (P90) | Mouse (Brock et al., 2015) | |||||
| DNA methylation - mPOA | F > M (P120) | Rat (Bell et al., 2016a) | |||||
| mRNA/protein expression - mPOA | F = M (P120) | Rat (Bell et al., 2016a) | |||||
| F = M (P5) | F = M (P15) | M > F (P25) | M > F (P90) | Mouse (Brock et al., 2015) | |||
| mRNA/protein expression - MBH | F = M (P1, P5) | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P60) | Rat (Walker et al., 2012) | |
| mRNA/protein expression - ARC | F = M (P15) | F = M (P30) | F > M (P45) | M > F (P90) | Rat (Walker et al., 2014) | ||
| M > F (P5) | F = M (P15) | M > F (P25) | M > F (P90) | Mouse (Brock et al., 2015) | |||
| mRNA/protein expression - VMH | M > F (P5) | F = M (P15) | F = M (P25) | M > F (P90) | Mouse (Brock et al., 2015) | ||
|
| |||||||
| Pgr - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | Species, References |
|
| |||||||
| DNA methylation - POA | F = M (P1) | F = M (P20) | F = M (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - POA | F = M (P1, P5) | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P60) | Rat (Walker et al., 2012) | |
| mRNA/protein expression - AVPV | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P90) | Rat (Walker et al., 2014) | ||
| M > F (E22) | Rat (Quadros et al., 2002b) | ||||||
| mRNA/protein expression - mPOA | M > F (P10) | F = M (P120) | Rat (Takagi et al., 2005; Faass et al., 2013) | ||||
| M > F (E22) | F > M (P7) | F > M (P14) | Rat (Quadros et al., 2002b; Quadros and Wagner, 2008) | ||||
| DNA methylation - MBH | F = M (P1) |
M > F (3 sites) F = M (1 site) (P20) |
F = M (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - MBH | F = M (P1, P5) | F = M (P15) | F = M (P30) | F = M (P45) | F = M (P60) | Rat (Walker et al., 2012) | |
| mRNA/Protein Expression - ARC | F = M (P15) | F = M (P30) | F > M (P45) | F > M (P90) | Rat (Walker et al., 2014) | ||
| mRNA/protein expression - VMH | F > M (P120) | Rat (Faass et al., 2013) | |||||
| F > M (P7) | F > M (P14) | Rat (Quadros and Wagner, 2008) | |||||
Sex differences in expression and DNA methylation of sex steroid hormone receptors are shown across development and in different brain regions. Significant baseline sex differences in methylation and expression are shown (bolded). When mRNA and protein levels are available, protein changes are underlined. For information regarding differences in dose and timing/route of exposure please see text. Abbreviations: E2 = estradiol; T = testosterone; F = females; M = males.
Table 5.
EDC effects on sex steroid hormone receptor expression and methylation in the brain.
| Esr1 - Brain region | EDC | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | Aging >12 mo | References (All in rat unless indicated otherwise) |
|---|---|---|---|---|---|---|---|---|
| mRNA/protein expression – POA | BPA |
F + BPA > F (P8) F + BPA = M (P8) |
F + BPA > F (P21) No Δ (P30) |
No Δ (P120) | Rat (Ramos et al., 2003; Monje et al., 2007) | |||
| MXC | F + MXC > F (~16 mo) | Rat (Gore et al., 2011) | ||||||
| PCB | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | |||||
| mRNA/protein expression - AVPV | BPA | No Δ (P1) | F + BPA = M (P21) | No Δ (P90) | Rat (Cao et al., 2013; Rebuli et al., 2014) | |||
|
F + BPA > F (P8) F + BPA = M (P8) ERα co-expression with tyrosine hydroxylase: F + BPA = M (P19) |
F + BPA > F (P21) F + BPA = M (P21) |
Rat (Patisaul et al., 2006; Monje et al., 2007) | ||||||
| PCB | F + A1221 = M (P60) | Rat (Dickerson et al., 2011b) | ||||||
| mRNA/protein expression - mPOA | BPA | No Δ (P1) | No Δ (P21) | No Δ (P90) | Rat (Cao et al., 2013; Rebuli et al., 2014) | |||
| No Δ (P90) | Mouse (Naule et al., 2014) | |||||||
| MXC | No Δ (P10) | Rat (Takagi et al., 2005) | ||||||
| PCB | No Δ + A1254 (P120) F + A1221 < F (P120) |
Rat (Faass et al., 2013; Bell et al., 2016a) | ||||||
| No Δ (P1) | Rat (Dickerson et al., 2011a) | |||||||
| mRNA/protein expression - MBH | BPA | F + BPA > F; No Δ in M (P30) | Rat (Khurana et al., 2000) | |||||
| mRNA/protein expression - ARC | BPA | No Δ (P1) | No Δ (P21) | No Δ (P90) | Mouse, Rat (Cao et al., 2013; Rebuli et al., 2014) | |||
| No Δ (P90, ~P100, during LH surge) | Mouse, Rat (Monje et al., 2010; Naule et al., 2014) | |||||||
| PCB | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P60) | Rat (Walker et al., 2014) | |||
| mRNA/protein expression - VMH | BPA | No Δ (P1) | No Δ (P21) | No Δ (P90 & P120) | Rat (Cao et al., 2013; Faass et al., 2013; Rebuli et al., 2014) | |||
| No Δ (P90) | Mouse (Naule et al., 2014) | |||||||
|
| ||||||||
| Esr2 - Brain region | EDC | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | Aging >12 mo | |
|
| ||||||||
| mRNA/protein expression - POA | BPA | M + BPA < M (P30) | M + BPA < M (P120) | Rat (Ramos et al., 2003) | ||||
| MXC | No Δ (~16 mo) | Rat (Gore et al., 2011) | ||||||
| PCB | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | |||||
| mRNA/protein expression - AVPV | BPA | No Δ (P1) | No Δ (P21) | F + BPA = M (P90) | Rat (Cao et al., 2013; Rebuli et al., 2014) | |||
| F + BPA > F (~P100, during LH surge) | Rat (Monje et al., 2010) | |||||||
| PCB | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Rat (Walker et al., 2014) | |||
| mRNA/protein expression - mPOA | BPA | No Δ (P1) | No Δ (P21) | F + BPA = M (P90) | Rat (Cao et al., 2013; Rebuli et al., 2014) | |||
| MXC | F + MXC < F; No Δ (M) (P10) | Rat (Takagi et al., 2005) | ||||||
| PCB | F + A1254 > F (P120) M + A1254 > M (P120) |
Rat (Faass et al., 2013) | ||||||
| mRNA/protein expression - MBH | BPA | M + BPA > M (P30) | M + BPA > M (P120) | Rat (Ramos et al., 2003) | ||||
| mRNA/protein expression - ARC | BPA | No Δ (P21) | No Δ (P90) | Rat (Rebuli et al., 2014) | ||||
| PCB | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Rat (Walker et al., 2014) | |||
| mRNA/protein expression - VMH | BPA | No Δ (P1) | No Δ (P21) | No Δ (P90) | Rat (Rebuli et al., 2014) | |||
| PCB | No Δ (P120) | Rat (Faass et al., 2013) | ||||||
|
| ||||||||
| Ar - Brain region | EDC | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | Aging >12 mo | |
|
| ||||||||
| mRNA/protein expression - POA | PCB | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | ||||
| DNA methylation - AVPV | PCB | No Δ (P15) | No Δ (P30) | M + A1221 < M (P45) | M + A1221 < M (P90) | Rat (Walker et al., 2014) | ||
| mRNA/protein expression - AVPV | F + A1221 = M (P15) | No Δ (P30) | No Δ (P45) | F + A1221 = M (P90) | ||||
| DNA methylation - mPOA | PCB | No Δ (P120) | Rat (Bell et al., 2016a) | |||||
| mRNA/protein expression - mPOA | M + A1221 < M (P120) | |||||||
| mRNA/protein expression - ARC | PCB | No Δ (P15) | No Δ (P15) | M + A1221 = F (P45) | No Δ (P90) | Rat (Walker et al., 2014) | ||
|
| ||||||||
| Pgr - Brain region | EDC | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | Aging >12 mo | |
|
| ||||||||
| mRNA/protein expression - POA | PCB | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | ||||
| mRNA/protein expression - AVPV | PCB | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Rat (Walker et al., 2014) | ||
| mRNA/protein expression - mPOA | BPC | F + BPA < F (~P100, during LH surge) | Rat (Monje et al., 2010) | |||||
| MXC | F + MXC > F; M + MXC < M (P10) | Rat (Takagi et al., 2005) | ||||||
| PCB | No Δ (P120) | Rat (Faass et al., 2013) | ||||||
| mRNA/protein expression - ARC | BPA | No Δ (~P100, during LH surge) | Rat (Monje et al., 2010) | |||||
| PCB | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Rat (Walker et al., 2014) | |||
| mRNA/protein expression - VMH | PCB | F + A1254 = M (P120) | Rat (Faass et al., 2013) | |||||
Effects of perinatal EDCs on sex differences in expression and DNA methylation of sex steroid hormone receptors. Significant effects of EDCs are shown (bolded) across brain regions and across developmental timepoints, from embryos through adults. When mRNA and protein levels are available, protein changes are underlined. For information regarding differences in dose and timing/route of exposure please see text. No change is indicated as No Δ. Abbreviations: A1221 = aroclor 1221, a PCB mixture; A1254 = aroclor 1254, a PCB mixture; E2 = estradiol; T = testosterone; F = females; M = males; LH = luteinizing hormone.
On the other hand, Esr2 and Pgr, but not Esr1, seem to play a role in sexual differentiation of the MBH (Tables 3–5). Additionally, as observed for global methylation patterns described previously (Ghahramani et al., 2014), these methylation changes did not become apparent until later in life. In the case of Esr2, the sex differences in methylation (male > female) were observed at P20 and P60 but not P1 in rats, and were increased to male-typical levels in masculinized females. Regarding Pgr, sex differences in methylation were only observed at P20 (male > female) and were increased to male-typical levels in masculinized female rats at P20, when sex differences can normally be observed (Schwarz et al., 2010). These data suggest that DNA methylation of the regulatory regions of sex steroid hormone receptors may influence the expression of these receptors in a region-specific manner, and that many of these effects may be influenced by the perinatal surge in gonadal hormones. The strongest evidence for this hypothesis is supported by the extensive data investigating ESR1 regulation where DNA methylation is consistently negatively correlated Esr1 expression in numerous brain regions across development [Reviewed in (Matsuda et al., 2012)]. However, evidence for other sex steroid hormone receptors is lacking and to our knowledge, no study has conclusively shown that altering DNA methylation at sex steroid hormone receptors, potentially through genome editing technics (Crispr/Cas9), alters sexual differentiation of the brain and influences long-term changes in sexually dimorphic behaviors and physiology. However, these findings do lend validity to the hypothesis that DNA methylation is an important epigenetic mechanism underlying sexual differentiation of the brain.
2.7. EDCs and gene specific methylation of sex steroid hormone receptors
A number of studies have investigated how developmental exposure to EDCs alters the expression of steroid hormone receptors in hypothalamic regions. While few have attempted to elucidate the epigenetic mechanisms underlying the observed expression changes, we will discuss the literature on effects of EDCs on the expression of sex steroid hormone receptors and, when available, provide evidence for the involvement of DNA methylation.
2.7.1. 7a BPA
The effects of BPA on sex steroid hormone receptor expression vary depending upon brain region analyzed and developmental time point investigated (Tables 3–5), as well as the dose, route, and timing of exposure. Despite these experimental differences, some patterns are beginning to emerge that may help inform the molecular mechanisms underlying the effects of BPA on altered hypothalamic-pituitary-gonadal physiology and reproductive behavior. In whole hypothalamus of mice, BPA induced a complex regulatory pattern of both mRNA and protein expression of the nuclear estrogen receptors. In peripubertal mice (P28), gestational exposure to BPA resulted in an inverted U-shaped dose-response curve in males and a U-shaped dose-response curve (2, 20 and 200 μg/kg/d) in female for both Esr1 and Esr2 (Kundakovic et al., 2013). In females, exposure to 20 μg/kg/day of BPA decreased expression of Esr1, and decreased DNA methylation of in the 5′ untranslated region referred to as Exon A of the Esr1 gene (Kundakovic et al., 2013). The positive correlation of gene expression and DNA methylation in this region is surprising as an inverse relationship between methylation in this region expression of Esr1 is observed in other models [Reviewed in (Matsuda, 2014)]. These differences may be attributable to transcription factor binding, other epigenetic mechanisms, or timing of the insult/stimulus (Kundakovic et al., 2013). No effects were observed in males in that study, showing the sex-specificity of epigenetic mechanisms of long-term regulation of sex steroid hormone receptor expression in brain. Furthermore, results are consistent with the possibility that methylation status of the Esr1 gene may regulate sex-specific alterations in Esr1 mRNA expression in the peripubertal hypothalamus (Kundakovic et al., 2013). Finally, the inverted U- and U-shaped dose response curves for BPA effects on Esr1 and Esr2 expression in the hypothalamus are consistent with non-monotonic dose-response effects of environmental EDCs.
In further support of a role for BPA in the regulation of estrogen receptors, in a different study in rats, a high dose of BPA (150 mg/kg/day), increased ERβ but not ERα protein levels in P12 hypothalamus, but no effects were observed at P70 (Yu et al., 2010). On the other hand, a low dose of BPA (2 μg/kg/day) increased Esr1 expression in whole hypothalamus of male and female rats, and decreased Esr2, an effect that was seen only in males (Chen et al., 2014). Taken together, these data suggest that the hypothalamus is sensitive to perturbation of perinatal BPA exposure in a dose- and sex-dependent manner.
These prior studies were conducted using whole hypothalamic dissections. However, the hypothalamus is composed of heterogeneous nuclei that have disparate functions (Table 1). Therefore, other reports have been published on sub-regions of the hypothalamus together with the preoptic area (POA) to elucidate how changes in gene expression in specific regions might contribute to the long-term alterations in behavior (Kundakovic and Champagne, 2011). In the POA, effects of EDCs on Esr1 expression were developmental age-dependent (Tables 3–5). More specifically, in juvenile female rats, a low dose of BPA (0.05 mg/kg) given on P1–P7 increased Esr1 expression on both P8 and P21. However, a high dose (20 mg/kg) from P1 to P7 increased Esr1 expression on P8, but decreased expression on P21 (Monje et al., 2007). BPA (25 or 250 μg/kg/day) administered during gestation had no effect on Esr1 expression in the adult male rat POA, but increased male Esr2 expression when measured on either P30 and P120 (Ramos et al., 2003). In the medial POA/medial preoptic nucleus (mPOA/MPN; Table 1), gestational exposure to BPA (5 mg/kg/day) increased Esr1 and decreased Esr2 mRNA expression in sheep (Mahoney and Padmanabhan, 2010), but had no effect on Esr1 expression in rats or mice (measured on P1, P21, P60 and P90) (Cao et al., 2013; Naule et al., 2014; Rebuli et al., 2014). In the AVPV, the effects of BPA on Esr1 and Esr2 expression were age-specific, and affected females to a greater extent than males (Tables 3–5). Gestational exposure of rats to low doses of BPA (2.5 or 25 μg/kg/day) but not to higher dosages decreased Esr1 expression on P21 and decreased expression of Esr2 at P90 (Rebuli et al., 2014). Additionally, perinatal BPA altered sensitivity to estradiol in the AVPV, as indicated by an increase in ERα and dose-dependent decrease in PGR protein levels after an E2 induced LH surge in ovariectomized female rats (Monje et al., 2010). Thus, the effects of BPA on the developing anterior hypothalamus depend upon age at analysis and are sex-specific.
The posterior hypothalamus, on the other hand, is less sensitive to perturbations by BPA. The MBH contains a number of sub-nuclei, including the ARC and VMH (Table 1). Neonatal exposure to BPA (100 μg/day from P1 to 5) increased Esr1 expression in the female but not the male rat MBH on P30 (Khurana et al., 2000). Consistent with these findings, prenatal exposure to a low (25 μg/kg) or high (250 μg/kg) dose of BPA had no effect on Esr1 or Esr2 expression in male rats on P30 or P120 as well (Ramos et al., 2003), suggesting that the female MBH may be more sensitive to developmental perturbations than males. Both the ARC and VMH, two subregions of the MBH (Table 1), displayed a similar response to BPA with regard to Esr1 and Esr2 expression (Tables 3–5). On P1 (~24 h after the last exposure), Esr1 and Esr2 were increased in the ARC, and Esr2 was increased in the VMH, of both male and female rats exposed to BPA (2.5 μg/kg/day and 25 μg/kg/day E6–E21) when compared to naïve but not vehicle treated animals. It should be noted that expression of both Esr1 and Esr2 were increased by the route of exposure (gavage), so these results should be interpreted with caution (Cao et al., 2013). In any case, these effects did not persist in female rats, as no effects of gestational exposure were observed on P21 or P90 independent of dose (2.5–2700 μg/kg/day E6–E21) [males not measured; (Rebuli et al., 2014)]. Finally, there is evidence that neonatal exposure of rats to BPA results in altered sensitivity to estradiol in the ARC. While estradiol increased ERα protein levels in the AVPV, it decreased ERα in the ARC, suggesting hypersensitivity of the hypothalamus to changes in serum estradiol (Monje et al., 2010).
While only one study has investigated the epigenetic mechanisms underlying BPA induced alterations in sex steroid hormone receptor expression (Kundakovic et al., 2013), the persistent and sex- and region-specific alterations in mRNA expression provide compelling evidence that epigenetic mechanisms are regulating these long-term effects. Therefore, it is plausible to hypothesize that DNA methylation is a candidate for EDC actions given this molecular mechanism’s role in sexual differentiation of the brain.
2.7.2. 7b Methoxychlor (MXC)
Methoxychlor (MXC; Fig. 1) is an organochlorine widely used as pesticide, developed as a replacement for DDT and a derivative of the original compound. Initially, it was thought to be less toxic and less persistent but has recently been banned because of its EDC activity and toxicity. Gestational exposure to MXC has been consistently linked to the advancement of puberty in females and delayed puberty in males in rodents (Gray et al., 1989a, 1989b; Masutomi et al., 2003; Armenti et al., 2008) and humans (Ozen et al., 2012). It is also associated with impaired reproductive function (Masutomi et al., 2003; Savabieasfahani et al., 2006; Armenti et al., 2008), infertility, and early reproductive senescence (Armenti et al., 2008). With reference to the POA, perinatal exposure to high (100 mg/kg/day) but not low (20 μg/kg/day) dosages of MXC advanced reproductive senescence in female rats, and increased expression of Esr1 but not Esr2 when measured at 17 months of age. The change in Esr1 gene expression was not associated with changes in DNA methylation of Esr1 in the Exon 1b promoter region, suggesting that other epigenetic mechanisms may underlie the gene expression changes observed (Gore et al., 2011). By contrast, a similar regimen of treatment with estradiol benzoate at high doses [100 mg/kg/day] in that same study not only increased Esr1 expression; it also increased DNA methylation in the Exon 1b promoter (Gore et al., 2011). Thus, DNA methylation in the aging POA can be modified by early life exposure to estradiol, but this effect is not caused by MXC despite the chemical’s effect on Esr1 gene expression.
While gestational exposure to MXC resulted in profound effects on gene expression after reproductive senescence, few effects of any dose (24, 240, or 1200 ppm) of MXC were observed in the mPOA, a sub-region of the POA, on P10 in male and female rats (Masutomi et al., 2003). These data suggest that the physiological and gene expression effects of gestational exposure to MXC may not manifest until after the pubertal transition or later. However, it should be noted that differences in experimental conditions (dose, treatment period, age at analysis) undoubtedly contribute to the different outcomes of the studies.
2.7.3. 7c PCBs
PCBs interact with a variety of steroid hormone receptors, including the two estrogen receptors, progesterone receptors, and the androgen receptor in the hypothalamus (Tables 3–5).
The androgen receptor (AR) is emerging as an important target of perinatal PCB exposure in both sexes and in several brain regions. There are over 200 possible PCB structures, and their chemical properties confer different mechanisms of action (Fig. 1). For example, lightly chlorinated congeners are more likely to display estrogenic/antiestrogenic effects whereas the more persistent and highly chlorinated PCBs are more likely to interact with neurotransmitter systems or aryl hydrocarbon receptors [Reviewed in (Tilson and Kodavanti, 1998)]. Although less well-studied, PCBs can also act as anti-androgens in cell lines or in assays of AR transcriptional activation/repression (Bonefeld-Jorgensen et al., 2001; Portigal et al., 2002; Schrader and Cooke, 2003), and as discussed below, modulate expression of ARs and steroidogenic enzymes in vivo.
Persistent effects of PCBs on Ar expression in the brain are relatively consistent and interestingly, occur independently of the composition of the PCB mixture. Like ERs, the AR plays a role in sexual differentiation of the brain and behavior [Reviewed in (Zuloaga et al., 2008)] and its actions as a transcription factor in development is thought to impart a “molecular memory” on its targets, presumably through epigenetic mechanisms [Reviewed in (Holterhus, 2011)]. Therefore, the finding that the AR is a target of perinatal EDC exposure is not unexpected (Tables 3–5). In the rat embryonic hypothalamus on E20, Ar expression was decreased in females but not males after exposure on E15 to E19 to A1254 (25 mg/kg), a mixture of persistent and highly chlorinated PCBs (Colciago et al., 2006). Our lab has conducted a number of studies investigating the effects of gestational exposure to A1221, which is a more lightly chlorinated mixture (Matthews and Dedrick, 1984), on gene expression throughout the hypothalamus. The results showed that Ar expression was consistently altered in an age-, sex- and region-specific manner. In the rat POA, Ar was decreased in adult female rats but not males after gestational exposure (E16 and E18) to A1221 (1 mg/kg) (Dickerson et al., 2011b). In the AVPV, Ar expression was altered in females but not males by gestational A1221 (E16 and E18), with females displaying a male-typical expression pattern across development when assessed at four ages from P15 through P90 (Walker et al., 2014). This dosing paradigm did not alter Ar expression in the male rat MPN at these same developmental ages (Topper et al., 2015). Interestingly, altering the dosing paradigm slightly (A1221 administered on E16, E18 and E20, rather than just on E16 and E18) decreased Ar expression in the mPOA of adult males (~P100) but not females, suggesting that even slight changes in the window of exposure and/or age at analysis result in sex and/or region specific alterations on gene expression in adulthood (Bell et al., 2016a). In the latter experiment, adolescent rats were exposed to A1221 on P24, P26, and P28 (1 mg/kg), resulting in a similarly decreased Ar expression in the mPOA of adult males (Bell et al., 2016a). Finally, in the ARC, gestational exposure to A1221 (E16 and E18) increased Ar expression in pubertal males (P45), but had no effects on females (Walker et al., 2014). Taken together, these data provide compelling evidence that the AR is an important gene target of developmental exposures to PCBs, and that even small differences in the timing of exposure result in different outcomes on this endpoint.
In an effort to identify potential epigenetic mechanisms underlying the long-term changes in Ar expression, DNA methylation was measured in the rat AVPV (Walker et al., 2014) and mPOA (Bell et al., 2016a) of the same animals used for gene expression assays. Contrary to our hypothesis, few effects of DNA methylation were observed in the female AVPV and none were detectable in the male mPOA (Bell et al., 2016a). Thus, other as yet-to-be-determined epigenetic mechanisms appear to be regulating the long-term expression changes in Ar in the AVPV (Walker et al., 2014) and mPOA (Bell et al., 2016a).
By contrast to these consistent effects of PCBs and A1221 on the AR, their effects on estrogen receptors are generally more subtle and less consistent. Our lab found that gestational exposure to A1221 (1 mg/kg on E16 and E18) had no effect on Esr1 or Esr2 gene expression in the POA of neonatal (P1) (Dickerson et al., 2011a) or adult rats (P60) (Dickerson et al., 2011b), or in the AVPV and ARC of rats across development (P15 – P90). Despite this lack of effect on gene expression, exposures to A1221 or a reconstituted mixture of PCBs decreased immunoreactive ERα (Dickerson et al., 2011b) and ERβ (Salama et al., 2003) cell numbers in adult females (Salama et al., 2003; Dickerson et al., 2011b) but not males (Dickerson et al., 2011b). These results suggests the possibility that perinatal exposure to PCBs may have lasting effects on posttranscriptional or posttranslational properties of estrogen receptors in the AVPV. In the rat mPOA, gestational and adolescent A1221 exposure decreased Esr1 expression in adult males but not females (Bell et al., 2016a). Conversely, polybrominated diphenyl ethers (PBDEs, 1 or 10 mg/kg given from E10–18) increased Esr1 in the mPOA of adult male and female rats, and A1254 (10 mg/kg, same days), a more highly chlorinated industrial mixture of PCBs than A1221, increased Esr2 expression in adulthood in both sexes (Faass et al., 2013). Differences are more than likely due to the mechanisms of action of the different compounds, dose and duration of exposure as well as other experimental differences. Finally, in the VMH of these same rats, PBDEs increased Esr1 expression in females and males (1 mg/kg dose only) and decreased Esr2 expression (Faass et al., 2013). Taken together, these data suggest that perinatal exposure to PCBs can alter estrogen receptor gene expression and protein levels in the hypothalamus but effects are region-specific.
While there is evidence that the progesterone receptor (PGR) plays a role in sexual differentiation of the brain (Wagner et al., 1998; Lonstein et al., 2001; Quadros et al., 2002a), it is understudied in the field of endocrine disruption (Gore, 2015). Our lab reported that gestational exposure to A1221 did not affect Pgr expression in the POA (P1 or P60) (Dickerson et al., 2011a; Dickerson et al., 2011b), or the AVPV or ARC of rats (P15 through P90) (Walker et al., 2014). In the rat VMH, adult sex differences (F > M) in Pgr expression were abolished by both high and low doses of A1254 and PBDEs in gestation, with Pgr expression decreasing in females and increasing in males (lower dosage only) (Faass et al., 2013). These effects may be due to differences in the properties and mechanisms of action of A1221 and A1254.
Taken together, these data suggest that sex steroid hormone receptors are sensitive targets for early life programming of the brain. In some cases DNA methylation is a candidate for the regulation of such programming, but it is probable that other mechanisms are involved, especially because most of the gene expression changes were not associated with alterations in methylation. It is important to note that any interpretation of changes (or the lack of changes) in DNA methylation in the hypothalamus after exposure to EDCs must be done cautiously for a number of reasons. First, the brain is highly heterogeneous. Even neighboring neurons can have entirely different functions and phenotype, and express different receptors and other proteins. Second, technical advances are needed to determine cell specific DNA methylation changes on a genome-wide scale to get a comprehensive view of how EDCs affect this mechanism. Third, most methylation studies assay either global methylation, or methylation on just a few sites of a promoter, neither of which may representing the relationship between complex permutations and combinations of many sites that contribute to the overall contribution of methylation to gene regulation. Of relevance to this point, the studies presented herein mainly focused on the promoter regions of genes, or in a limited number of intragenic sites such as Exon 1a of Esr1. In the future, it will be necessary to conduct genome-wide studies to account for changes in methylation across gene bodies and other genomic regions, and to account for both 5-mC and 5-hMC as each of these modifications are associated with opposing functional outcomes, the balance of which causes gene expression changes in adulthood.
3. Histone modifications, sexual differentiation of the brain, and EDCs
3.1. Molecular mechanisms of histone modifications
DNA is condensed in the nucleus in a highly organized and compact manner as chromatin (Fig. 3). The nucleosome, the functional unit of chromatin, is composed of ~147 base pairs wrapped around core histone octamers consisting of 2 copies of each of the following proteins: H2A, H2B, H3 and H4. Post-translational modifications to each histone protein, including acetylation, methylation, phosphorylation, SUMOylation, ubiquitination, citullination, ADP-ribosylation, and others, occur when different functional groups are covalently added to amino acid residues of their N-terminal tails. These modifications alter chromatin structure and change the interaction of the DNA with associated histones, thus increasing or decreasing the likelihood of transcription at a given locus. Histone modifications are diverse and we are only beginning to understand how various combinations of histone post-translational modifications interact to influence or indicate different transcriptional states [Reviewed in (Maze et al., 2014)]. Moreover, histone modifications are exceptionally dynamic and reversible. They are added and removed by a large family of enzymes referred to as “writers” and “erasers,” respectively [Reviewed in (Jaenisch and Bird, 2003)]. Efforts are underway to understand how histone modifications can influence such long-term processes as sexual differentiation of the brain and whether they are disrupted by EDCs.
3.2. Histone modifications and sexual differentiation of the brain
While there are a number of known chromatin modifications, the two that are the most studied and best-understood are histone acetylation and methylation (Fig. 3). Histone acetyltransferases (HATs) catalyze the addition of acetyl groups to lysine residues on the histone tails and increase the likelihood of transcription through the addition of a negative charge to histones, thus causing a repulsion between the DNA and histone proteins resulting in an open chromatin state and allowing for transcription factors and co-factors to interact with the DNA. This process can be reversed through the action of histone deacetylases (HDACs), which remove the acetyl mark and change the chromatin conformation. Deacetylation is associated with a closed chromatin state and reduced transcription. Histone methylation is much more complicated and can result in active or repressed states depending on the number and location of methyl groups added to the chromatin [Reviewed in (Jenuwein and Allis, 2001)].
Few studies have investigated the role of histone acetylation in sexual differentiation, likely because of its relative transience. One group demonstrated sex differences in both H3K9 and H3K14Ac (males > females), two marks associate with transcriptional activation, in the cortex and hippocampus on E18 and P0 of mice; however this difference was no longer evident by P6 (Tsai et al., 2009). Interestingly, treating females with a masculinizing dose of testosterone propionate (2 μg) on E16 through E21 resulted in male-typical H3K9/14Ac expression on P0 (Tsai et al., 2009), suggesting that histone modifications may play a role in sex-specific epigenetic programming of the cortex and hippocampus, and that at the very least histone modifications are hormone sensitive. Surprisingly, no difference in global histone acetylation was observed in the POA, an area that is sexually differentiated (Tsai et al., 2009). However, when a region- and gene-specific approach was taken, sex differences were identified. Investigation of total acetylated H4, a mark associated with active transcription, in the mPOA at the promoters of 2 genes necessary for sexual differentiation of the brain, Esr1 and aromatase (Cyp19a1), showed that each promoter was differentially acetylated in males and female rats. Sex differences in acetylated H4 were observed for Exon 1b of Esr1 on E21 (males > females) and Esr1 and Cyp19a1 on P3 (males < females). Similar results were reported for total acetylated H3. More specifically, on E21, acetylated H3 at the Cyp19a1 promoter was greater in males than in females, but greater in females than males on P3, illustrating the dynamic changes in histone acetylation over the span of 5 days (Matsuda et al., 2011). This result suggests that transcription is more active in the male brain during the prenatal testosterone surge (~E17 to E19), and that this falls off postnatally. Interestingly, if males are treated with the general HDAC inhibitor, trichostatin A by infusion into the mPOA on P1, adult male reproductive behavior was altered as indicated by decreased intromission ratio (number of intromissions/sum of intromissions and mounts). This suggests that a developmental decrease in acetylation in the mPOA is important for the organization of adult reproductive behaviors in males [N.B., females were not studied; (Matsuda et al., 2011)]. HDAC activity is necessary for the organization of the BNST. A pharmacological inhibitor of HDAC, valproic acid (VPA), was administered to mice on P1 and P2, and volume and cell numbers of the BNST were analyzed at ~P21 (Murray et al., 2009). VPA treatment eliminated the sex difference in BNST volume and cell number and blocked androgen-dependent masculinization of the male BNST, suggesting that histone acetylation is associated with the androgen-induced masculinization of the brain. VPA had no effect in the suprachiasmatic nucleus, a hypothalamic region that is not structurally sexually dimorphic (Murray et al., 2009).
Endogenous HDACs comprise a class of enzymes made up of a number of unique molecules. Of these, HDAC1 through HDAC11 and sirtuins (Sirts) show tissue-specific expression. In order to test the specific role of HDACs 2 and 4 in sexual differentiation of the brain, Matsuda and colleagues knocked down their expression in the mPOA of rats using antisense oligonucleotides on P0 and P1 (Matsuda et al., 2011). The premise for this work was the finding from the same study that HDAC2 and HDAC4 associated with Esr1 Exon 1b and Cyp19a1 promoters to a greater degree in males than in females (Matsuda et al., 2011), suggestive of the possibility that they play a role in developmental deacetylation of the promoters. Results showed that knocking down HDAC2 and HDAC4 resulted in a reduced intromission ratio in adult males, suggested that these HDACs were involved in sexual differentiation of the mPOA (Matsuda et al., 2011).
While histone acetylation is associated with transcriptional activation, histone methylation is associated with activation or repression of gene expression (Fig. 3) depending on which residues are methylated and the number of methyl groups added. Therefore, translating the role of histone methylation in sexual differentiation of the brain is much more difficult (Jenuwein and Allis, 2001). Limited evidence suggests that histone methylation may play a role in sexual differentiation of the brain. Sex differences in histone methyltransferases, which catalyze the addition of a methyl group onto the histone tail, have been reported in a few studies. Levels of MLL1/KMT2a, a histone methyltransferase complex, were higher in the PFC of females compared to males (Huang et al., 2007). Additionally, KDM5c/SMCX/JARID1c, a H3K4 specific demethylase, is X-linked, resulting in its sex-specific expression in brain (Xu et al., 2008).
To our knowledge, only two studies have directly linked histone methylation to sexual differentiation of the brain, with results supporting sex differences that are observed in a region-specific manner. Tsai et al. (Tsai et al., 2009) reported that H3K9me3, a repressive mark, was greater in males than females on P0 and P6 but not at E18 in the cortex and hippocampus of mice, suggesting a shift from activating to repressive marks in males during early development. However, testosterone propionate during gestation had no effect on H3K9me3 in females or males (Tsai et al., 2009). Genome-wide changes in H3K4me3, a mark associated with transcriptionally active genes, were investigated by ChIP-seq (chromatin immunoprecipitation followed by sequencing) in the BNST and POA. Sex differences were observed at 248 loci, with enrichment in females greater than males in 176 and enrichment greater in males at 72 loci. Those loci with significant enrichment in females also displayed significant increases in the mRNA expressed from those loci (Shen et al., 2015). While studies linking histone modifications to sexual differentiation of the brain are scarce, we are beginning to identify potential histone marks involved in these processes. Further research is necessary to understand the developmental timeline of histone changes as well as the characterization of sex differences and developmental profiles for the numerous histone “writers” and “erasers.”
3.3. Histone modifications as targets for EDCs
3.3.1. 3a BPA and phthalates
To our knowledge, no studies have been published on the role of chromatin modifications in models of endocrine disruption in the brain. However, such studies have been conducted in other reproductive tissues and reproductive cancers [Reviewed in (Seachrist et al., 2016)], with results suggesting that exposures to BPA or phthalates (Fig. 1), a class of EDCs in plasticizers, personal care products, intravenous tubing, and used in other applications, caused changes in chromatin modification.
In primary cortical neuronal cultures from rat, mouse, and humans, BPA exposure delayed the perinatal chloride shift caused by a decrease in potassium chloride cotransporter (Kcc2) mRNA expression (Yeo et al., 2013). BPA increased MeCP2 binding and decreased H3K9ac at the Kcc2 promoter and transcription start site respectively. HDAC inhibition with decitabine and trichostatin A, as well as knockdown of HDAC1 and combined knockdown of HDAC1 and 2, rescued the mRNA expression effects of BPA. Finally, these effects were greater in females than in males, indicative of sex differences in the histone modification response to BPA in this cell culture system (Yeo et al., 2013).
In human neuroblastoma lines, DEHP caused dose-dependent cell death via activation of caspase 3. These effects were prevented by the class II HDAC inhibitor MC-1588 (HDAC 4–7 and 9–10). Phthalates increased expression of HDAC4 and reduced HDAC5 but not HDAC 6, 7 or 10 suggesting that the toxic effects of phthalates occur through increased expression of HDAC4 and deacetylation of the cell survival protein S3 (Guida et al., 2014).
While these studies were performed in cell culture, they provide intriguing molecular evidence that BPA and phthalates can directly alter the machinery regulating histone modifications.
4. Noncoding RNAs as targets for endocrine disruptors
4.1. Introduction to noncoding RNAs
Complete sequencing of the transcriptome in numerous mammalian species has revealed evidence for a large and diverse numbers of RNA transcripts that are not translated into protein (O’Carroll and Schaefer, 2013). Since the time of this recent discovery, these noncoding RNAs have been found to have important roles in cell function, particularly in the regulation of gene expression. While the functions of some noncoding RNAs have been known for decades, e.g. tRNA and those involved in splicing, the recent identification of microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and piwi-interacting RNAs (piRNAs), among others, have revealed a role of these noncoding RNAs in regulating gene expression through myriad mechanisms. MiRNAs are short sequences of RNA (21–24 bp) that repress translation by binding target sequences of specific mRNAs, leading to mRNA degradation or inhibition of translation of that mRNA. In animals, a single miRNA can inhibit numerous mRNA targets and in some cases, miRNAs can influence histone modification or DNA methylation to repress transcription as well (Hawkins and Morris, 2010; Tan et al., 2015). Long noncoding RNAs (lncRNAs) are longer RNA sequences (>200 bps) that regulate gene expression by forming RNA-protein complexes and modulating transcription factors or chromatin-regulatory proteins. Additionally, recent evidence suggests that lncRNAs can influence histone conformation through direct interactions with the chromatin to change the conformation, thus altering the availability of certain DNA sequences to transcriptional machinery and inducing an active or repressive transcriptional state [Reviewed in (Kim and Shiekhattar, 2016)].
4.2. Noncoding RNAs and sexual differentiation of the brain
Few studies have investigated the role of non-coding RNAs in sexual differentiation of the brain and those that have focused on miRNAs. For example, Morgan and Bale (Morgan and Bale, 2011) used a qPCR array to not only show that a number of miRNAs are sexually dimorphic in the whole brain of mice on P1, but that aromatase inhibition on P1 demasculinized miRNA expression on P2, suggesting that miRNAs are sensitive to alterations in gonadal hormones during the critical period of sexual differentiation and may be playing a role in sex-specific brain development. Similar sex differences and hormonal responses were observed in halibut (Bizuayehu et al., 2012). Additionally, these effects may be region- and age- specific as our lab has identified developmental sex differences (P15–P90) in miRNAs in the MPN but not VMH of rats, which are sensitive to perinatal estrogen exposure (Topper et al., 2015). Finally, there is evidence that miRNAs, specifically let-7 and lin-28, are necessary for the initiation of puberty in rats and monkeys and are altered by neonatal hormonal manipulation in a sex-specific manner (Sangiao-Alvarellos et al., 2013).
While the sexually dimorphic lncRNA, xist, is one of the most extensively studied lncRNAs for its role in X-inactivation (Galupa and Heard, 2015), few studies have investigated the role lncRNAs may play a role in sexual differentiation of the brain. However, one study identified MHM, a lncRNA in chickens, as playing a role in female-specific gonadogenesis (Roeszler et al., 2012). The dearth of research on noncoding RNAs highlights the need for more research in the field of epigenetics and sexual differentiation of the brain.
4.3. Noncoding RNAs and PCBs
There are a few studies investigating how EDCs alter miRNAs in peripheral reproductive tissues (Zhang and Pan, 2009; Choi et al., 2011; Kovanecz et al., 2014; Lesiak et al., 2014), but little on central reproductive neuroendocrine systems. Our lab directly tested the hypothesis that miRNA expression in the hypothalamus is altered by prenatal exposures to PCBs. Using the model described earlier for effects of A1221 (1 mg/kg), estradiol benzoate (EB, 50 μg/kg), or vehicle (all administered on E16 and E18) on hypothalamic gene and protein expression, and on reproductive behaviors, developmental expression profiles of eight miRNAs (mir-132, mir-219, mir-7, mir-9, mir-145, mir-124a, let-7a, and let-7b) were profiled. Measurements were made in the MPN and VMN of male and female exposed offspring across postnatal development and puberty, on P15, 30 (peripubertal), 45 (late puberty) and P90 (adulthood) (Topper et al., 2015). In the MPN, the most common expression pattern of miRNAs was a developmental increase, seen in both sexes for 7 of the 8 miRNAs. Interestingly, expression of 6 of the 8 miRNAs was increased on P30 specifically in the females, an effect found in both the A1221 and EB groups compared to the vehicle-exposed females (Topper et al., 2015). In the males, a completely different response pattern was seen, with expression of 6 of 8 miRNAs decreased by A1221, but not EB, at P90. These results are very interesting because they show sex-dependence in the effects of prenatal PCBs on miRNA expression that differ by age (P30 in females, P90 in males), directionality (up-regulated miRNA expression in the PCB females, down-regulated in the PCB males), as well as similarities/differences to the EB treated groups (A1221 and EB had similar effects in females, but different in males). Considering that our experiment only measured 8 miRNAs, it is important to study effects of EDCs on a broader spectrum of these and other noncoding RNAs.
In that same study (Topper et al., 2015) we also measured the same 8 miRNAs in the VMN. While all of the measured miRNAs underwent developmental increases from P15 to P90, there were relatively few effects of treatment. Thus, there is also region-specificity in EDC actions on developing miRNA expression. Furthermore, this study also measured expression of several mRNAs predicted by bioinformatics programs to be regulated by the selected miRNAs. Interestingly, expression changes in miRNA expression were not associated with mRNA expression change in their predicted targets. It is possible that this lack of the predicted correlation was due to the relatively small number of genes investigated, both for miRNAs and mRNAs. The relationships among these systems are complex and typically involve numerous players, most of which were not measured. Furthermore, considering that miRNAs inhibit translation of mRNA to protein, it is possible that the protein products would be affected. However, no proteins were measured in that study. These data suggest novel and potentially exciting evidence that miRNAs may be altered by perinatal exposure to PCBs, providing evidence of another epigenetic mechanism underlying altered behavior and physiology in the adult organism.
To our knowledge, only one study has investigated how EDCs alter lncRNAs, specifically X-inactivation in the brain of female mice (Kumamoto and Oshio, 2013). Exposure to a high (50 mg/kg), but not low (0.02 mg/kg) dose of BPA on E6 and E15 altered expression of two X-inactivation related mRNAs in the cerebrum of mice. Expression of the X-chromosome inactivating factor Tsix was increased on P2, ~P21 and ~P60 and Xist expression decreased on ~P21 and ~P60 (both doses). These expression changes were associated with a down-regulation of neurodevelopment genes (Kumamoto and Oshio, 2013). Although more research is necessary, these data provide compelling evidence that lncRNAs are altered by prenatal exposure to BPA and provide a possible link for how EDCs might alter chromatin modifications.
5. General conclusions and future directions
5.1. Epigenetic mechanisms for sexual differentiation of the brain
It is clear that epigenetic mechanisms including DNA methylation, histone modifications and non-coding RNAs, all contribute to sexual differentiation of the brain. Currently, DNA methylation is the most comprehensively studied in the field because of its relative stability in comparison to other epigenetic mechanisms. Our review of the data reveals several emergent properties with regard to epigenetic mechanisms underlying sexual differentiation of the brain: (1) activity of the methylation machinery (DNMT enzymatic activity) but not gene expression is sexually dimorphic in hypothalamic regions prior to P15. After P15, sex differences in gene expression begin to be observed. (2) Exposure to a masculinizing dose of estradiol or testosterone can reverse sex differences in activity and gene expression in the hypothalamus. (3) Postnatal sex differences in histone acetylation (males > females) are seemingly transient and can be reversed by exposure to testosterone in the neonatal hippocampus and cortex but not the POA. (4) In the hypothalamus, there are not genome-wide sex differences in histone modifications but rather, they are gene-specific. (5) Sex differences in miRNA expression are observed in the brain in a region-specific manner. While these results are promising, much more research is necessary to confirm these initial studies, to better understand the role that each of these epigenetic molecular mechanisms plays in sexual differentiation of the brain, and to determine how they work in concert to maintain long-term alterations in gene and protein expression throughout the life cycle. It is imperative to understand the baseline mechanisms of sexual differentiation of the brain so we can compare them to the effects of perinatal exposure to EDCs in the brain and develop a comprehensive understanding of how sex differences in the epigenome arise, develop, are maintained throughout the life cycle, and may be perturbed.
5.2. Epigenetic machinery targeted by EDCs
Compared to knowledge on brain sexual differentiation, evidence that EDCs alter epigenetic mechanisms in the brain is more limited. Much of what we know comes from the studies focused on chemicals and cancer, and work done in that field can serve as a model for future EDC studies. Given the role of the brain in regulating reproductive physiology, behavior, metabolism and stress, greater focus on the brain as a sensitive target to the epigenetic impacts of EDCs is much-needed. In fact, many of the long-term effects of EDCs and epigenetic mechanisms reported for reproductive cancers may be the result of altered reproductive physiology earlier in life, and could include contributions from the three levels of the HPG axis through feed-forward and feedback mechanisms. Future research must integrate how EDCs might alter a combination of epigenetic mechanisms to affect the “epigenetic conversation,” resulting in altered behavior, metabolism, physiology and stress responses in the organism as a whole.
By considering the data in an age- and sex-specific context we show that exposure to EDCs early in life not only alter sexual differentiation of the brain, but in many cases alter the developmental trajectory of specific brain regions. For example, expression of Dnmt1 and 3a undergo striking sex- and region specific developmental changes that are disrupted by EDCs. Additionally, the alterations reviewed herein affect females more often than males, suggesting that the female brain may be more sensitive to disruption by perinatal EDC exposure and could explain the long-term alterations in reproductive physiology and behavior previously reported. This contributes to our understanding of the molecular mechanisms underlying sex-specific development and may aid in identifying sex-specific windows for intervention after early life exposures to EDCs.
Table 4.
Perinatal hormone effects on sex steroid hormone receptor expression and methylation in the brain.
| Esr1 - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | References (all Rat studies) |
|---|---|---|---|---|---|---|---|
| DNA methylation - POA | F + E2 = M (P1) | No Δ (P20) | F + E2 = M (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - POA | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | ||||
| mRNA/protein expression - AVPV | No Δ (P1) | M + E2 = F (P21) | Rat (Cao et al., 2013; Rebuli et al., 2014) | ||||
| F + E2 = M (P60) | Rat (Dickerson et al., 2011b) | ||||||
| mRNA/protein expression - mPOA | No Δ (P1) | No Δ (P10) | Rat (Takagi et al., 2005; Cao et al., 2013) | ||||
|
F + E2 > F M + E2 > M (P1) |
Rat (Dickerson et al., 2011a) | ||||||
| DNA methylation - MBH | No Δ (P1) | No Δ (P20) | No Δ (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - ARC | No Δ (P1) | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P60) | Rat (Cao et al., 2013; Walker et al., 2014) | |
| mRNA/protein expression - VMH | No Δ (P1) | Rat (Cao et al., 2013) | |||||
|
| |||||||
| Esr2 - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | |
|
| |||||||
| DNA methylation - POA | No Δ (P1) | F + E2 > M (P20) | No Δ (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - POA | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | ||||
| mRNA/protein expression - AVPV | No Δ (P1) | No Δ (P15) | F + E2 > F (P30) | F + E2 > F (P45) |
F + E2 > F (P90) No Δ for M (P90) |
Rat (Cao et al., 2013; Rebuli et al., 2014; Walker et al., 2014) | |
| mRNA/protein expression - mPOA | No Δ (P1) | F + E2 > F (P10) | M + E2 = F (P90) | Rat (Takagi et al., 2005; Cao et al., 2013; Rebuli et al., 2014) | |||
| DNA methylation - MBH | No Δ (P1) | F + E2 = M (P20) | F + E2 = M (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - ARC | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Rat (Walker et al., 2014) | ||
| mRNA/protein expression - VMH | No Δ (P1) | Rat (Cao et al., 2013) | |||||
|
| |||||||
| Ar - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | |
|
| |||||||
| mRNA/protein expression - POA | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | ||||
| DNA methylation - AVPV | No Δ (P15) | No Δ (P30) | M + E2 < M (P45) | No Δ (P90) | Rat (Walker et al., 2014) | ||
| mRNA/protein expression - AVPV | F + E2 = M (P15) | No Δ (P30) | No Δ (P45) | F + E2 = M (P90) | |||
| mRNA/protein expression - ARC | No Δ (P15) | No Δ (P15) | M + E2 = F (P45) | No Δ (P90) | Rat (Walker et al., 2014) | ||
|
| |||||||
| Pgr - Brain region | Embryonic E0–E22 | Neonatal P0–P7 | Infant P8–P20 | Juvenile P21–P35 | Pubertal P36–P59 | Adulthood > P60 | |
|
| |||||||
| DNA methylation - POA | F + E2 > M & F (P1) | No Δ (P20) | No Δ (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - POA | No Δ (P1) | No Δ (P60) | Rat (Dickerson et al., 2011a, 2011b) | ||||
| mRNA/protein expression - AVPV | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Rat (Walker et al., 2014) | ||
|
F + T > F M + T = F (E22) |
Rat (Quadros et al., 2002b) | ||||||
| mRNA/protein expression - mPOA | F + E2 = M (P10) | Rat (Takagi et al., 2005) | |||||
|
F + T > F M + T < M (E22) |
Mouse (Quadros et al., 2002b) | ||||||
| DNA methylation - MBH | F + E2 > M, F (P1) |
F + E2 = M (3 sites) F + E2 > M, F (P20) |
F + E2 > M, F (P60) | Rat (Schwarz et al., 2010) | |||
| mRNA/protein expression - ARC | No Δ (P15) | No Δ (P30) | No Δ (P45) | No Δ (P90) | Rat (Walker et al., 2014) | ||
Effects of perinatal estradiol (E2) or testosterone on sex differences in expression and DNA methylation of sex steroid hormone receptors. Significant effects of E2 and T are shown (bolded) across brain regions and across developmental timepoints, from embryos through adults. When mRNA and protein levels are available, protein changes are underlined. For information regarding differences in dose and timing/route of exposure please see text. No change is indicated as No Δ. Abbreviations: E2 = estradiol; T = testosterone; F = females; M = males.
Acknowledgments
The authors would like to thanks Drs. Rosemary Bagot and Hannah Cates for their thoughtful review of drafts and helpful commentary.
Footnotes
Grant support: NIH T32 DA007135-31 (DMW), NIH RO1 ES020662 (ACG), NIH RO1 ES023254 (ACG).
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Annamalai J, Namasivayam V. Endocrine disrupting chemicals in the atmosphere: their effects on humans and wildlife. Environ Int. 2015;76:78–97. doi: 10.1016/j.envint.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Charlier TD, Cornil CA, Dickens MJ, Harada N, Konkle AT, Voigt C, Ball GF. Sex differences in brain aromatase activity: genomic and non-genomic controls. Front Endocrinol (Lausanne) 2011;2:34. doi: 10.3389/fendo.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58:114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Auyeung B, Norgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Cohen AS, Chakrabarti B, Ruta L, Lombardo MV. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20:369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough CA, Gorski RA. Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology. 1961;68:68–79. doi: 10.1210/endo-68-1-68. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Woutersen PJ, Slob AK. Sex difference in whole-body androgen content in rats on fetal days 18 and 19 without evidence that androgen passes from males to females. Biol Reprod. 1991;44:747–751. doi: 10.1095/biolreprod44.5.747. [DOI] [PubMed] [Google Scholar]
- Bell MR, Hart BG, Gore AC. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 2. Sex-specific neuromolecular effects in the brain. Mol Cell Endocrinol. 2016a;420:125–137. doi: 10.1016/j.mce.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Thompson LM, Rodriguez K, Gore AC. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm Behav. 2016b;78:168–177. doi: 10.1016/j.yhbeh.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizuayehu TT, Babiak J, Norberg B, Fernandes JM, Johansen SD, Babiak I. Sex-biased miRNA expression in Atlantic halibut (Hippoglossus hippoglossus) brain and gonads. Sex Dev. 2012;6:257–266. doi: 10.1159/000341378. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158:141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect. 2009;117:7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Koopman P. Precious cargo: regulation of sex-specific germ cell development in mice. Sex Dev. 2013;7:46–60. doi: 10.1159/000342072. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor alpha and androgen receptor is sex-, age- and region-dependent in mice. J Neuroendocrinol. 2015;27:264–276. doi: 10.1111/jne.12258. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Calderon-Gierszal EL, Prins GS. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose Bisphenol A exposure. PLoS ONE. 2015;10:e0133238. doi: 10.1371/journal.pone.0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and Kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, Patisaul HB. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133:157–173. doi: 10.1093/toxsci/kft035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B. The role of Sry in cellular events underlying mammalian sex determination. Curr Top Dev Biol. 1996;32:1–37. doi: 10.1016/s0070-2153(08)60423-8. [DOI] [PubMed] [Google Scholar]
- Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Res Bull. 1997;44:365–375. doi: 10.1016/s0361-9230(97)00216-5. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Chen F, Zhou L, Bai Y, Zhou R, Chen L. Sex differences in the adult HPA axis and affective behaviors are altered by perinatal exposure to a low dose of bisphenol A. Brain Res. 2014;1571:12–24. doi: 10.1016/j.brainres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Choi JS, Oh JH, Park HJ, Choi MS, Park SM, Kang SJ, Oh MJ, Kim SJ, Hwang SY, Yoon S. MiRNA regulation of cytotoxic effects in mouse sertoli cells exposed to nonylphenol. Reprod Biol Endocrinol. 2011;9:126. doi: 10.1186/1477-7827-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colciago A, Negri-Cesi P, Pravettoni A, Mornati O, Casati L, Celotti F. Prenatal Aroclor 1254 exposure and brain sexual differentiation: effect on the expression of testosterone metabolizing enzymes and androgen receptors in the hypothalamus of male and female rats. Reprod Toxicol. 2006;22:738–745. doi: 10.1016/j.reprotox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. Expression of the steroidogenic enzyme P450scc in the central and peripheral nervous systems during rodent embryogenesis. Endocrinology. 1995;136:2689–2696. doi: 10.1210/endo.136.6.7750493. [DOI] [PubMed] [Google Scholar]
- Consales C, Toft G, Leter G, Bonde JP, Uccelli R, Pacchierotti F, Eleuteri P, Jonsson BA, Giwercman A, Pedersen HS, Strucinski P, Goralczyk K, Zviezdai V, Spano M. Exposure to persistent organic pollutants and sperm DNA methylation changes in Arctic and European populations. Environ Mol Mutagen. 2016;57:200–209. doi: 10.1002/em.21994. [DOI] [PubMed] [Google Scholar]
- Cornejo MP, Hentges ST, Maliqueo M, Coirini H, Becu-Villalobos D, Elias CF. Neuroendocrine regulation of metabolism. J Neuroendocrinol. 2016 doi: 10.1111/jne.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC. Life imprints: living in a contaminated world. Environ Health Perspect. 2011;119:1208–1210. doi: 10.1289/ehp.1103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC. Epigenetic synthesis: a need for a new paradigm for evolution in a contaminated world. F1000 Biol Rep. 2012;4:18. doi: 10.3410/B4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. 2012;2012:713696. doi: 10.1155/2012/713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Forger NG. Sex differences in the brain: a whole body perspective. Biol Sex Differ. 2015;6:15. doi: 10.1186/s13293-015-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaulniers D, Xiao GH, Leingartner K, Chu I, Musicki B, Tsang BK. Comparisons of brain, uterus, and liver mRNA expression for cytochrome p450s, DNA methyltransferase-1, and catechol-o-methyltransferase in prepubertal female Sprague-Dawley rats exposed to a mixture of aryl hydrocarbon receptor agonists. Toxicol Sci. 2005;86:175–184. doi: 10.1093/toxsci/kfi178. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol. 2011a;252:36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011b;152:581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, Juraska JM. Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: a role for pubertal onset. Synapse. 2016 doi: 10.1002/syn.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol. 2007;17:675–683. doi: 10.1016/j.conb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzwilewski KL, Schantz SL. Prenatal chemical exposures and child language development. J Commun Disord. 2015;57:41–65. doi: 10.1016/j.jcomdis.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- Ehrhardt AA, Meyer-Bahlburg HF. Effects of prenatal sex hormones on gender-related behavior. Science. 1981;211:1312–1318. doi: 10.1126/science.7209510. [DOI] [PubMed] [Google Scholar]
- Faass O, Ceccatelli R, Schlumpf M, Lichtensteiger W. Developmental effects of perinatal exposure to PBDE and PCB on gene expression in sexually dimorphic rat brain regions and female sexual behavior. Gen Comp Endocrinol. 2013;188:232–241. doi: 10.1016/j.ygcen.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Falkner AL, Lin D. Recent advances in understanding the role of the hypothalamic circuit during aggression. Front Syst Neurosci. 2014;8:168. doi: 10.3389/fnsys.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, Auger CJ. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics. 2012;7:230–238. doi: 10.4161/epi.7.3.19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forger NG. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philos Trans R Soc Lond B: Biol Sci. 2016;371:20150114. doi: 10.1098/rstb.2015.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Strahan JA, Castillo-Ruiz A. Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol. 2016;40:67–86. doi: 10.1016/j.yfrne.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3beta-hydroxysteroid dehydrogenase in the rat brain. J Neurochem. 1998;71:2231–2238. doi: 10.1046/j.1471-4159.1998.71062231.x. [DOI] [PubMed] [Google Scholar]
- Galupa R, Heard E. X-chromosome inactivation: new insights into cis and trans regulation. Curr Opin Genet Dev. 2015;31:57–66. doi: 10.1016/j.gde.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George FW, Ojeda SR. Changes in aromatase activity in the rat brain during embryonic, neonatal, and infantile development. Endocrinology. 1982;111:522–529. doi: 10.1210/endo-111-2-522. [DOI] [PubMed] [Google Scholar]
- Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, de Vries GJ, Forger NG, Pellegrini M, Vilain E. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5:8. doi: 10.1186/2042-6410-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC. Prenatal programming and endocrinology. Endocrinology. 2015;156:3403–3404. doi: 10.1210/en.2015-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. Executive summary to EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:593–602. doi: 10.1210/er.2015-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev. 2014;35:961–991. doi: 10.1210/er.2013-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25:2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Graham MD, Pfaus JG. Infusions of ascorbic acid into the medial preoptic area facilitate appetitive sexual behavior in the female rat. Physiol Behav. 2013;122:140–146. doi: 10.1016/j.physbeh.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989a;12:92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Ferrell J, Sigmon R, Cooper R, Linder R, Rehnberg G, Goldman J, Laskey J. Correlation of sperm and endocrine measures with reproductive success in rodents. Prog Clin Biol Res. 1989b;302:193–206. discussion 206-199. [PubMed] [Google Scholar]
- Guida N, Laudati G, Galgani M, Santopaolo M, Montuori P, Triassi M, Di Renzo G, Canzoniero LM, Formisano L. Histone deacetylase 4 promotes ubiquitin-dependent proteasomal degradation of Sp3 in SH-SY5Y cells treated with di(2-ethylhexyl)phthalate (DEHP), determining neuronal death. Toxicol Appl Pharmacol. 2014;280:190–198. doi: 10.1016/j.taap.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Vertino PM, Cheng X. Molecular coupling of DNA methylation and histone methylation. Epigenomics. 2010;2:657–669. doi: 10.2217/epi.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20:633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins PG, Morris KV. Transcriptional regulation of Oct4 by a long non-coding RNA antisense to Oct4-pseudogene 5. Transcription. 2010;1:165–175. doi: 10.4161/trns.1.3.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Cory Slechta D, Thompson C, Hanson M. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985;5:40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisasue S, Seney ML, Immerman E, Forger NG. Control of cell number in the bed nucleus of the stria terminalis of mice: role of testosterone metabolites and estrogen receptor subtypes. J Sex Med. 2010;7:1401–1409. doi: 10.1111/j.1743-6109.2009.01669.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Holterhus PM. Molecular androgen memory in sex development. Pediatr Endocrinol Rev. 2011;9(Suppl 1):515–518. [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull E, Dominguez J. Male sexual behavior. In: Plant T, Zeleznik A, editors. Knobil and Neill’s Physiology of Reproduction. Elsevier; New York: 2015. pp. 2211–2285. [Google Scholar]
- Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev. 2011;32:317–355. doi: 10.1210/er.2010-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jin B, Li Y, Robertson KD. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2:607–617. doi: 10.1177/1947601910393957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Kito K, Kondo F. Factors influencing the migration of bisphenol A from cans. J Food Prot. 2003;66:1444–1447. doi: 10.4315/0362-028x-66.8.1444. [DOI] [PubMed] [Google Scholar]
- Kareta MS, Botello ZM, Ennis JJ, Chou C, Chedin F. Reconstitution and mechanism of the stimulation of de novo methylation by human DNMT3L. J Biol Chem. 2006;281:25893–25902. doi: 10.1074/jbc.M603140200. [DOI] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA, McElligott ZA. Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells. 2015;38:1–13. doi: 10.14348/molcells.2015.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimada K, Koopman P. Sry: the master switch in mammalian sex determination. Development. 2010;137:3921–3930. doi: 10.1242/dev.048983. [DOI] [PubMed] [Google Scholar]
- Keeley RJ, Bye C, Trow J, McDonald RJ. Strain and sex differences in brain and behaviour of adult rats: Learning and memory, anxiety and volumetric estimates. Behav Brain Res. 2015;288:118–131. doi: 10.1016/j.bbr.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141:4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, Jacobs DR, Steffes M, Lee DH. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect. 2010;118:370–374. doi: 10.1289/ehp.0901131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Shiekhattar R. Diverse regulatory interactions of long noncoding RNAs. Curr Opin Genet Dev. 2016;36:73–82. doi: 10.1016/j.gde.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR, Kurrasch DM. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci USA. 2015;112:1475–1480. doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM, Frohman LA. Nonirritative lesions of VMH: effects on plasma insulin, obesity, and hyperreactivity. Am J Physiol. 1985;248:E669–675. doi: 10.1152/ajpendo.1985.248.6.E669. [DOI] [PubMed] [Google Scholar]
- Kolodkin MH, Auger AP. Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. J Neuroendocrinol. 2011;23:577–583. doi: 10.1111/j.1365-2826.2011.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanecz I, Gelfand R, Masouminia M, Gharib S, Segura D, Vernet D, Rajfer J, Li DK, Kannan K, Gonzalez-Cadavid NF. Oral Bisphenol A (BPA) given to rats at moderate doses is associated with erectile dysfunction, cavernosal lipofibrosis and alterations of global gene transcription. Int J Impot Res. 2014;26:67–75. doi: 10.1038/ijir.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kumamoto T, Oshio S. Effect of fetal exposure to bisphenol A on brain mediated by X-chromosome inactivation. J Toxicol Sci. 2013;38:485–494. doi: 10.2131/jts.38.485. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25:1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Forbes-Lorman RM, Auger AP. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics. 2007;2:173–178. doi: 10.4161/epi.2.3.4841. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, Coleman P, Lemere CA, Hof PR, van den Hove DL, Rutten BP. The epigenetics of aging and neurodegeneration. Prog Neurobiol. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Pfaff DW. Sex difference in estradiol regulation of progestin receptor mRNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology. 1991;53:608–613. doi: 10.1159/000125781. [DOI] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, Wayman GA. The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci. 2014;34:717–725. doi: 10.1523/JNEUROSCI.2884-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Penell J, Luttropp K, Nordfors L, Syvanen AC, Axelsson T, Salihovic S, van Bavel B, Fall T, Ingelsson E, Lind PM. Global DNA hypermethylation is associated with high serum levels of persistent organic pollutants in an elderly population. Environ Int. 2013;59:456–461. doi: 10.1016/j.envint.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Quadros PS, Wagner CK. Effects of neonatal RU486 on adult sexual, parental, and fearful behaviors in rats. Behav Neurosci. 2001;115:58–70. doi: 10.1037/0735-7044.115.1.58. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Padmanabhan V. Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus. Toxicol Appl Pharmacol. 2010;247:98–104. doi: 10.1016/j.taap.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- Marieb E. Essentials of Human Anatomy and Physiology. 8. Pearson Benjamin Cummings; San Francisco, CA: 2006. The endocrine system; pp. 299–326. [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology. 2003;192:149–170. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- Matsuda KI. Epigenetic changes in the estrogen receptor alpha gene promoter: implications in sociosexual behaviors. Front Neurosci. 2014;8:344. doi: 10.3389/fnins.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Kawata M. Epigenetic mechanisms are involved in sexual differentiation of the brain. Rev Endocr Metab Disord. 2012;13:163–171. doi: 10.1007/s11154-012-9202-z. [DOI] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology. 2011;152:2760–2767. doi: 10.1210/en.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HB, Dedrick RL. Pharmacokinetics of PCBs. Annu Rev Pharmacol Toxicol. 1984;24:85–103. doi: 10.1146/annurev.pa.24.040184.000505. [DOI] [PubMed] [Google Scholar]
- Maze I, Noh KM, Soshnev AA, Allis CD. Every amino acid matters: essential contributions of histone variants to mammalian development and disease. Nat Rev Genet. 2014;15:259–271. doi: 10.1038/nrg3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. A lumpers versus splitters approach to sexual differentiation of the brain. Front Neuroendocrinol. 2011;32:114–123. doi: 10.1016/j.yfrne.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Sexual differentiation of the brain. Nature. 1981;291:610. doi: 10.1038/291610a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Coirini H, Danielsson A, Frankfurt M, Gould E, Mendelson S, Schumacher M, Segarra A, Woolley C. Steroid and thyroid hormones modulate a changing brain. J Steroid Biochem Mol Biol. 1991;40:1–14. doi: 10.1016/0960-0760(91)90160-7. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Rubinow DR, Stuber GD. Maternally responsive neurons in the bed nucleus of the stria terminalis and medial preoptic area: putative circuits for regulating anxiety and reward. Front Neuroendocrinol. 2015;38:65–72. doi: 10.1016/j.yfrne.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Pfaff DW. Specificity and neural sites of action of anisomycin in the reduction or facilitation of female sexual behavior in rats. Horm Behav. 1985;19:237–251. doi: 10.1016/0018-506x(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5alpha-reductase in the central nervous system: expression and modes of control. J Steroid Biochem Mol Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Mikaelsson MA, Miller CA. DNA methylation: a transcriptional mechanism co-opted by the developed mammalian brain? Epigenetics. 2011;6:548–551. doi: 10.4161/epi.6.5.15679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor alpha transcripts with alternative 5′-untranslated regions in the female rat preoptic area. J Endocrinol. 2007;194:201–212. doi: 10.1677/JOE-07-0014. [DOI] [PubMed] [Google Scholar]
- Monje L, Varayoud J, Munoz-de-Toro M, Luque EH, Ramos JG. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor alpha expression in nuclei controlling estrous cyclicity. Reprod Toxicol. 2010;30:625–634. doi: 10.1016/j.reprotox.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naule L, Picot M, Martini M, Parmentier C, Hardin-Pouzet H, Keller M, Franceschini I, Mhaouty-Kodja S. Neuroendocrine and behavioral effects of maternal exposure to oral bisphenol A in female mice. J Endocrinol. 2014;220:375–388. doi: 10.1530/JOE-13-0607. [DOI] [PubMed] [Google Scholar]
- Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne) 2012;3:48. doi: 10.3389/fendo.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Horm Behav. 2011;59:338–344. doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- O’Carroll D, Schaefer A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology. 2013;38:39–54. doi: 10.1038/npp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen S, Darcan S, Bayindir P, Karasulu E, Simsek DG, Gurler T. Effects of pesticides used in agriculture on the development of precocious puberty. Environ Monit Assess. 2012;184:4223–4232. doi: 10.1007/s10661-011-2257-6. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Balthazart J, Frye CA, Garcia-Segura LM, Herbison AE, Mensah-Nyagan AG, McCarthy MM, Melcangi RC. Milestones on steroids and the nervous system: 10 years of basic and translational research. J Neuroendocrinol. 2012;24:1–15. doi: 10.1111/j.1365-2826.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pelayo S, Oliveira E, Thienpont B, Babin PJ, Raldua D, Andre M, Pina B. Triiodothyronine-induced changes in the zebrafish transcriptome during the eleutheroembryonic stage: implications for bisphenol A developmental toxicity. Aquat Toxicol. 2012;110–111:114–122. doi: 10.1016/j.aquatox.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Pena CJ, Champagne FA. Neonatal overexpression of estrogen receptor-alpha alters midbrain dopamine neuron development and reverses the effects of low maternal care in female offspring. Dev Neurobiol. 2015;75:1114–1124. doi: 10.1002/dneu.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989;484:279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- Petrusz P, Flerko B. On the mechanism of sexual differentiation of the hypothalamus. Acta Biol Acad Sci Hung. 1965;16:169–173. [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Picot M, Naule L, Marie-Luce C, Martini M, Raskin K, Grange-Messent V, Franceschini I, Keller M, Mhaouty-Kodja S. Vulnerability of the neural circuitry underlying sexual behavior to chronic adult exposure to oral bisphenol a in male mice. Endocrinology. 2014;155:502–512. doi: 10.1210/en.2013-1639. [DOI] [PubMed] [Google Scholar]
- Pinel JP, Treit D, Rovner LI. Temporal lobe aggression in rats. Science. 1977;197:1088–1089. doi: 10.1126/science.560719. [DOI] [PubMed] [Google Scholar]
- Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC. Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol Appl Pharmacol. 2002;179:185–194. doi: 10.1006/taap.2002.9371. [DOI] [PubMed] [Google Scholar]
- Prewitt AK, Wilson ME. Changes in estrogen receptor-alpha mRNA in the mouse cortex during development. Brain Res. 2007;1134:62–69. doi: 10.1016/j.brainres.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS. Endocrine disruptors and prostate cancer risk. Endocr Relat Cancer. 2008;15:649–656. doi: 10.1677/ERC-08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, Kajdacsy-Balla A, van Breemen RB. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155:805–817. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros PS, Lopez V, De Vries GJ, Chung WC, Wagner CK. Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobiol. 2002a;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002b;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Wagner CK. Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology. 2008;149:3054–3061. doi: 10.1210/en.2007-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JG, Varayoud J, Kass L, Rodriguez H, Costabel L, Munoz-De-Toro M, Luque EH. Bisphenol a induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology. 2003;144:3206–3215. doi: 10.1210/en.2002-0198. [DOI] [PubMed] [Google Scholar]
- Rebuli ME, Cao J, Sluzas E, Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Patisaul HB. Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol Sci. 2014;140:190–203. doi: 10.1093/toxsci/kfu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC. The effects of prenatal PCBs on adult social behavior in rats. Horm Behav. 2015;73:47–55. doi: 10.1016/j.yhbeh.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150:3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeszler KN, Itman C, Sinclair AH, Smith CA. The long non-coding RNA, MHM, plays a role in chicken embryonic development, including gonadogenesis. Dev Biol. 2012;366:317–326. doi: 10.1016/j.ydbio.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116:1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama J, Chakraborty TR, Ng L, Gore AC. Effects of polychlorinated biphenyls on estrogen receptor-beta expression in the anteroventral periventricular nucleus. Environ Health Perspect. 2003;111:1278–1282. doi: 10.1289/ehp.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S, Manfredi-Lozano M, Ruiz-Pino F, Navarro VM, Sanchez-Garrido MA, Leon S, Dieguez C, Cordido F, Matagne V, Dissen GA, Ojeda SR, Pinilla L, Tena-Sempere M. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology. 2013;154:942–955. doi: 10.1210/en.2012-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ Health Perspect. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod Toxicol. 2003;17:15–23. doi: 10.1016/s0890-6238(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol. 2016;59:167–182. doi: 10.1016/j.reprotox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EY, Ahern TH, Cheung I, Straubhaar J, Dincer A, Houston I, de Vries GJ, Akbarian S, Forger NG. Epigenetics and sex differences in the brain: a genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Exp Neurol. 2015;268:21–29. doi: 10.1016/j.expneurol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Kataoka Y, Yamashita K, Ueki S. An important role of the central amygdaloid nucleus and mammillary body in the mediation of conflict behavior in rats. Brain Res. 1986;372:159–162. doi: 10.1016/0006-8993(86)91470-8. [DOI] [PubMed] [Google Scholar]
- Sibug R, Kuppers E, Beyer C, Maxson SC, Pilgrim C, Reisert I. Genotype-dependent sex differentiation of dopaminergic neurons in primary cultures of embryonic mouse brain. Brain Res Dev Brain Res. 1996;93:136–142. doi: 10.1016/0165-3806(96)00024-7. [DOI] [PubMed] [Google Scholar]
- Singh S, Li SS. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci. 2012;13:10143–10153. doi: 10.3390/ijms130810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Crews D, Logan C, Soukup RW. Eugen Steinach: the first neuroendocrinologist. Endocrinology. 2014;155:688–695. doi: 10.1210/en.2013-1816. [DOI] [PubMed] [Google Scholar]
- Spiller CM, Bowles J, Koopman P. Regulation of germ cell meiosis in the fetal ovary. Int J Dev Biol. 2012;56:779–787. doi: 10.1387/ijdb.120142pk. [DOI] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77:810–824. doi: 10.1016/j.neuron.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res Mol Brain Res. 1995;34:75–88. doi: 10.1016/0169-328x(95)00140-n. [DOI] [PubMed] [Google Scholar]
- Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol. 2014;4:80. doi: 10.3389/fonc.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ishikawa K, Sugiyama K, Furuta H, Nishimura F. Content and release of bisphenol A from polycarbonate dental products. Dent Mater J. 2000;19:389–395. doi: 10.4012/dmj.19.389. [DOI] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, Vasanthakumar A, Godley LA, Chang Q, Cheng X, He C, Jin P. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Shibutani M, Lee KY, Masutomi N, Fujita H, Inoue K, Mitsumori K, Hirose M. Impact of maternal dietary exposure to endocrine-acting chemicals on progesterone receptor expression in microdissected hypothalamic medial preoptic areas of rat offspring. Toxicol Appl Pharmacol. 2005;208:127–136. doi: 10.1016/j.taap.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Tan J, Qiu K, Li M, Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589:3175–3181. doi: 10.1016/j.febslet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Thomassin H, Flavin M, Espinas ML, Grange T. Glucocorticoid-induced DNA demethylation and gene memory during development. EMBO J. 2001;20:1974–1983. doi: 10.1093/emboj/20.8.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998;19:517–525. [PubMed] [Google Scholar]
- Topper VY, Walker DM, Gore AC. Sexually dimorphic effects of gestational endocrine-disrupting chemicals on microRNA expression in the developing rat hypothalamus. Mol Cell Endocrinol. 2015;414:42–52. doi: 10.1016/j.mce.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology. 1998;139:3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol Endocrinol. 2014;28:99–115. doi: 10.1210/me.2013-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Gore AC. Endocrine disrupting chemicals and the Brain. In: Gore AC, editor. Endocrine-Disrupting Chemicals: From Basic Research to Clinical Practice. Humana Press Inc; Totowa, NJ: 2007. pp. 63–109. [Google Scholar]
- Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154:2129–2143. doi: 10.1210/en.2012-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol Reprod. 2012;87:129. doi: 10.1095/biolreprod.112.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warita K, Mitsuhashi T, Ohta K, Suzuki S, Hoshi N, Miki T, Takeuchi Y. Gene expression of epigenetic regulatory factors related to primary silencing mechanism is less susceptible to lower doses of bisphenol A in embryonic hypothalamic cells. J Toxicol Sci. 2013;38:285–289. doi: 10.2131/jts.38.285. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: sex-specific deficits in associative ability and inhibitory control. Toxicol Appl Pharmacol. 2001;174:188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wizemann TM, Pardue ML, editors. Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academy Press; Washington, D.C: 2001. [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RL, Wang Q, Trevino LS, Bosland MC, Chen J, Medvedovic M, Prins GS, Kannan K, Ho SM, Walker CL. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10:127–134. doi: 10.1080/15592294.2015.1009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: The interplay between placenta and fetal brain. J Soc Gynecol Investig. 2005;12:67–76. doi: 10.1016/j.jsgi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS ONE. 2008;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376:563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Yeo M, Berglund K, Hanna M, Guo JU, Kittur J, Torres MD, Abramowitz J, Busciglio J, Gao Y, Birnbaumer L, Liedtke WB. Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Natl Acad Sci USA. 2013;110:4315–4320. doi: 10.1073/pnas.1300959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Chen QF, Liu ZP, Xu HF, Zhang XP, Xiang Q, Zhang WZ, Cui WM, Zhang X, Li N. Estrogen receptor alpha and beta expressions in hypothalamus-pituitary-ovary axis in rats exposed lactationally to soy isoflavones and bisphenol A. Biomed Environ Sci. 2010;23:357–362. doi: 10.1016/S0895-3988(10)60076-1. [DOI] [PubMed] [Google Scholar]
- Zhang B, Pan X. RDX induces aberrant expression of microRNAs in mouse brain and liver. Environ Health Perspect. 2009;117:231–240. doi: 10.1289/ehp.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Chen F, Chang F, Bai Y, Chen L. Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A. J Psychiatr Res. 2013;47:1535–1544. doi: 10.1016/j.jpsychires.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller TR, Dowling AL, Herzig CT, Iannacone EA, Gauger KJ, Bansal R. Thyroid hormone, brain development, and the environment. Environ Health Perspect. 2002;110(Suppl 3):355–361. doi: 10.1289/ehp.02110s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]