Abstract
We investigated the extent of cortical thinning in U.S. Veterans exposed to combat who varied in the severity of their posttraumatic stress disorder (PTSD) symptoms. In addition, we explored the neural correlates of PTSD symptom dimensions and the interactive effects of combat exposure and PTSD upon cortical thickness. Sixty-nine combat exposed Veterans completed high-resolution magnetic resonance imaging (MRI) scans to estimate cortical thickness. The Clinician Administered PTSD Scale (CAPS) and Combat Exposure Scale (CES) assessments were completed to measure current PTSD and historical combat severity, respectively. PTSD symptom dimensions (numbing, avoidance, reexperiencing, anxious arousal, and dysphoric arousal) were studied. Vertex-wise whole cerebrum analyses were conducted. We found widespread negative correlations between CAPS severity and cortical thickness, particularly within the prefrontal cortex. This prefrontal correlation remained significant after controlling for depression severity, medication status, and other potential confounds. PTSD dimensions, except anxious arousal, negatively correlated with cortical thickness in various unique brain regions. CES negatively correlated with cortical thickness in the left lateral prefrontal, regardless of PTSD diagnosis. A significant interaction between CES and PTSD diagnosis was found, such that CES negatively correlated with cortical thickness in the non-PTSD, but not in the PTSD, participants. The results underscore the severity of cortical thinning in U.S. Veterans suffering from high level of PTSD symptoms, as well as in Veterans with no PTSD diagnosis but severe combat exposure. The latter finding raises considerable concerns about a concealed injury potentially related to combat exposure in the post-9/11 era.
Keywords: PTSD, combat, Veteran, neuroimaging, cortical thickness
1. Introduction
Posttraumatic stress disorder (PTSD) is a disabling illness affecting approximately 7% of the adult U.S. population and up to 25% of combat exposed US Veterans, depending on cohort (Kessler et al., 2005). Yet, the understanding of the pathophysiology of PTSD is limited, with few effective pharmacological treatments. These under-addressed clinical needs are especially relevant to U.S. Veterans, with more than 2.6 million military personnel deployed to war zones in the post-9/11 era and an estimated 23% prevalence of PTSD among US Veterans (Fulton et al., 2015). Better insight into the neural correlates of combat exposure and PTSD symptoms may contribute to rational drug development of novel effective therapeutics. In this study of U.S. Veterans, we examined the relationship between PTSD symptoms, combat exposure, and cortical thickness, a measure of gray matter (GM) integrity. The models and design of the study focus on brain correlates of dimensional symptoms and trauma exposure while attempting to address some of the limitations of prior PTSD GM research, especially regarding the generalizability of results to the Veteran population.
Accumulating evidence implicates synaptic dysconnectivity in the pathophysiology of trauma and chronic stress (Pitman et al., 2012; Popoli et al., 2012; Reul and Nutt, 2008). It has been proposed that stressors initially activate glutamate circuits and trigger proinflammatory processes, which initiate a cascade of neural events that impair synaptic connectivity, contributing to the chronic effects of trauma and stress (Abdallah et al., 2015b; Averill et al., 2016). In this model, trauma and chronic stress impair glutamate neurotransmission and increase extrasynaptic glutamate levels, which precipitate excitotoxicity and affect synaptic integrity, as evidenced by a reduction of synaptic density and strength, as well as dendritic retraction and reduced arborization, in prefrontal and hippocampal circuits regulating emotion and stress responses (Abdallah et al., 2015a). In susceptible individuals subjected to major trauma, these stressor-induced synaptic deficits interact with predisposing vulnerabilities (e.g., amygdala reactivity, cortisol/NPY dysregulation) to impair fear and learning regulation and precipitate a constellation of PTSD symptoms, which further perpetuate the vicious effects of trauma and chronic stress (Abdallah et al., 2017; Averill et al., 2016). This synaptic model predicts that current PTSD symptoms and the severity of trauma history, as two major stressors, would negatively affect GM integrity. This stress-based model is most consistent with the “sustained threat” or “chronic stress” construct of the Negative Valence Systems in NIMH’s Research Domain Criteria (RDoC).
Structural Magnetic Resonance Imaging (MRI) can provide an indirect measure of GM integrity by estimating the volume or shape of a brain region. Preclinical evidence has demonstrated that stress induced changes in GM structure reflect underlying alterations in synaptic microstructure and density (Kassem et al., 2013). Extensive literature utilizing MRI methods over the past 2 decades has shown impaired GM integrity in patients diagnosed with PTSD. For example, meta-analyses in PTSD have reported reduced GM volume in the hippocampus, anterior cingulate cortex (ACC), and medial prefrontal cortex (PFC) (Kuhn and Gallinat, 2013; O’Doherty et al., 2015); structures that are broadly part of networks supporting emotion processing and the acquisition and extinction of fear. Several studies have reported reduced cortical thickness in various brain regions in PTSD patients (Geuze et al., 2008b; Hunter et al., 2011; Liu et al., 2012; Mueller et al., 2015; Qi et al., 2013; Woodward et al., 2009; Xie et al., 2013), but also see (Landre et al., 2010; Li et al., 2016). Some studies demonstrated a negative correlation between cortical thickness and PTSD severity (Corbo et al., 2014; Lindemer et al., 2013; Liu et al., 2012; Sadeh et al., 2016). Greater cortical thickness was previously associated with resilience and enhanced recovery from PTSD symptoms (Dickie et al., 2013; Lyoo et al., 2011; Nilsen et al., 2016), although not without inconsistencies (Helpman et al., 2016).
To date, MRI studies in PTSD have provided essential insight into putative pathophysiological pathways of the disorder (Bremner et al., 1995; Morey et al., 2012; Woodward et al., 2006; Woodward et al., 2009). However, additional state-of-the-art design and methods are still needed to complement prior evidence and begin to map GM abnormalities to the clinical characteristics of PTSD, as well as to the distinctive effects of trauma exposure. In particular, it is essential to investigate the effect of combat exposure on GM integrity in Veterans with and without PTSD. A recent report found a negative correlation between combat severity and cortical thickness in Veterans with chronic pain (Corbo et al., 2016). In this study, we focused on determining the association between combat severity and GM integrity, while attempting to address some of the gaps of prior evidence. For example, pioneering studies have primarily investigated GM integrity in case-control studies of those with a diagnosis of PTSD versus those without a diagnosis (Kuhn and Gallinat, 2013; O’Doherty et al., 2015). Though this approach has the strength of creating a large contrast, it also creates a potentially artificial dichotomization, especially if traumatic effects and related psychopathology are on a continuum of biological abnormalities and clinical severity. Therefore, we included combat exposed Veterans regardless of their PTSD diagnosis, aiming to capture a linear spread of PTSD symptom characteristics and severity, consistent with a dimensional approach such as RDoC. In addition, our study exclusion criteria were carefully selected to enhance the generalizability of the results to a clinical Veteran population.
In a cohort of 69 combat exposed US Veterans, we measured PTSD severity using the Clinician Administered PTSD Scale (CAPS) and confirmed that CAPS scores were continuously spread between low and high severity (Fig. S1). Our primary analysis examined the relationship between total CAPS scores and cortical thickness throughout the cerebrum, followed by region of interest (ROI) analysis of the prefrontal cortex (PFC) thickness. The PFC ROI analysis was used 1) based on the predictions of the synaptic model described above, 2) because the PFC plays a critical role in the pathophysiology of PTSD, and 3) because gray matter integrity within the PFC were previously correlated with PTSD pathology and recovery (Dickie et al., 2013; Lyoo et al., 2011; Woodward et al., 2006; Woodward et al., 2013). The PFC ROI analysis permitted the examination of several putative confounds, as well as reducing type I error due to multiple comparisons. Secondary analyses investigated the association between cortical thickness and five dimensions of PTSD symptoms (i.e., reexperiencing, anxious arousal, dysphoric arousal, numbing, and avoidance) (Pietrzak et al., 2012).
We also investigated whether combat exposure severity is directly related to cortical thickness or interacts with PTSD pathology. Consistent with the synaptic model of chronic stress, we predicted a negative correlation between PTSD severity and cortical thickness, in particular in the PFC. We hypothesized that combat exposure scale (CES) would negatively affect cortical thickness regardless of psychopathology and that CES would have an additive or synergistic effect in combination with PTSD pathology, such that the negative correlation between CES and thickness would be more robust in those with PTSD. This hypothesis was tested by first correlating CES with cortical thickness controlling for PTSD diagnosis (i.e., CES effects). Then, we studied the interaction between CES and PTSD.
2. Experimental Procedures
Participants
Sixty-nine combat exposed U.S. Veterans were recruited from the VA Connecticut Healthcare System (VACHS) and local Veteran communities (Abdallah et al., 2017). The study received IRB approval from the VACHS Human Subjects Subcommittee and Yale University Human Research Protection Program. All subjects completed written informed consent prior to participating in study procedures.
Participants ranged in age from 21 to 60 (mean ±SEM = 34.7 ±1.1) and had been deployed on at least one combat tour. Individuals were excluded from the study if they met any of the following criteria: a diagnosis of bipolar disorder or psychotic disorder, as assessed by the Structured Clinical Interview for the DSM-IV (SCID-IV) (First et al., 2002); current benzodiazepine use; a history of ADHD, learning disorder, moderate or severe traumatic brain injury (TBI), brain tumor, epilepsy, or a neurological disorder; current inpatient status; or an MRI contraindication. Given their high co-occurrence with PTSD in Veterans, stable antidepressants and comorbid mild TBI, depression, anxiety, and alcohol/substance disorders were not excluded to ensure the external validate of the study and the generalizability of the findings to the target population. These putative confounds were examined as covariates in post-hoc analyses.
Behavioral and Functional Assessments
PTSD diagnosis and symptom severity were assessed using the CAPS-IV (Blake et al., 1995; Weathers et al., 2001). A trained and certified clinical psychologist or master’s level clinician administered the CAPS-IV to assess PTSD symptoms. CAPS training included review of online training materials, observation of at least four CAPS sessions conducted by experienced clinicians, and rating of audio recordings for establishing inter-rater reliability. Kappa coefficients were between .80 and .90 for all interviewers. Psychiatric comorbidities were assessed using the SCID-IV (First et al., 2002). CES (Keane, 1989) was used to assess the extent of self-reported combat experiences. The Beck Depression Inventory (BDI), Second Edition (Beck, 1996) was used as a standardized measure of self-reported depressive symptomatology. The Beck Anxiety Inventory (BAI) was used as a self-report measure of anxiety symptoms (Beck, 1993). A neuropsychologist independently assessed TBI severity using American Congress of Rehabilitation Medicine criteria (Kay, 1993). The Wechsler Test of Adult Reading (WTAR) was administered as a measure of premorbid intellectual functioning (Holdnack, 2001).
Neuroimaging Methods
MRI scans were collected at 3.0 Tesla using a Siemens TIM Trio scanner with a 32-channel head coil. After localization, 2 × T1-weighted sagittal magnetization-prepared, rapid acquisition gradient-echo (MPRAGE; TR=2530ms; TE=2.71ms; TI=1200ms; Flip=7°) and 1 × T2-weighted (TR = 3200 ms; TE = 419 ms; Flip = 120°) scans were acquired with an isotropic voxel size of 1mm3. Cortical thickness estimates were computed using the fully automated Freesurfer package (http://surfer.nmr.mgh.harvard.edu; version 5.3; (Fischl, 2012)), as described in our previous studies (Abdallah et al., 2012). The standard Freesurfer methods, along with references, are provided in the Supplements. The structural scans were processed as follow: “recon-all –s $subject –i $first_T1_scan –i $second_T1_scan –T2 $T2_scan –all –T2pial -qcache”. Visual inspections for quality assurance were completed; no manual interventions were necessary. All image processing, parcellation, and quality control procedures were conducted while blinded to participants’ demographic and clinical characteristics.
Data Analysis
Demographic and psychiatric variables are presented in Table S1 & S2. Prior to conducting each analysis, the distribution of outcome measures was examined using probability plots and test statistics. Transformations and non-parametric tests were used as necessary. Whole-brain vertex-wise General Linear Modeling (GLM) was performed on the surface maps using Freesurfer’s QDEC package. Our primary GLM analysis examined the effects of CAPS total score on cortical thickness while controlling for age. Comparable GLMs were constructed to examine the effects of each of the 5 symptom dimensions on cortical thickness while controlling for age. To determine the effects of putative confounds, we first extracted the average cortical thickness of the anatomically defined PFC of each subject, as parcellated by Freesurfer. The PFC was selected for the post-hoc analyses because it was the primary brain region correlating with CAPS in the vertex-wise analysis. Then we conducted partial correlation analyses covarying for age and each of the following demographic and clinical measures: WTAR, BDI, BAI, and TBI and alcohol or substance use disorder status. To examine the effects of CES, we constructed a GLM with cortical thickness as outcome, diagnosis (PTSD) as a discrete variable, CES as a continuous variable, and age as a covariate. This GLM examined the main effect of CES and the interaction between PTSD diagnosis and CES. Average cortical thickness in each cluster that showed significant interaction was extracted per subject. To ascertain the direction of significant interactions, post-hoc analyses with FDR correction examined the slopes of the best-fit line relating CES and average thickness in each diagnostic group, controlling for age. Type I error correction for whole brain analyses was conducted based on cluster extent using Monte-Carlo simulation, as implemented in Freesurfer (Hagler et al., 2006), which provided a cluster corrected α < 0.01. Vertex thresholds used p values of 0.01 for the primary GLMs (i.e., CAPS and CES) and 0.001 for the secondary analyses (i.e., each of the 5 PTSD symptom dimensions).
3. Results
PTSD Symptoms
Following correction for multiple comparisons, we found significant negative correlations between CAPS scores and cortical thickness in 11 clusters (Fig. 1, Table 1). Participants with high symptom severity showed widespread reduced cortical thickness primarily in the left hemisphere and the PFC, including bilateral ventrolateral PFC, bilateral superior PFC, left dorsal ACC (dACC), left dorsolateral PFC, left anterior insula, right lateral occipital cortex, right fusiform, and right posterior cingulate. No positive correlations were found between CAPS and cortical thickness. The correlation between CAPS and PFC cortical thickness maintained significance (p < 0.05) after controlling for various demographic and clinical variables (see Methods).
Figure 1.
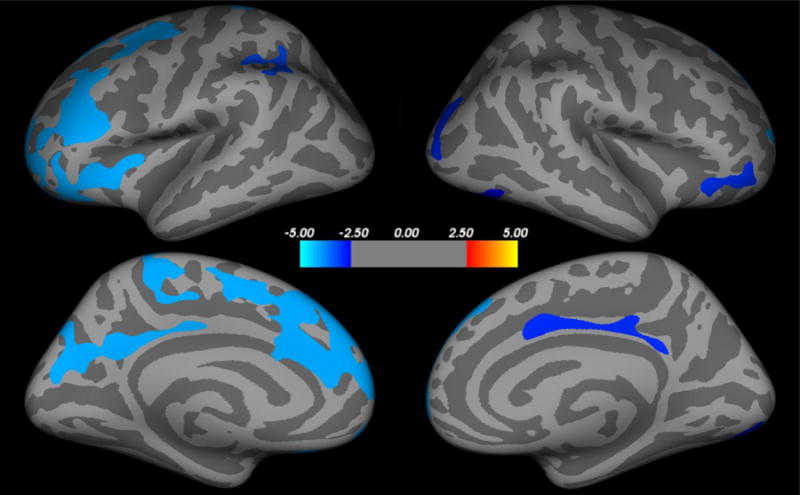
Correlation between cortical thickness and PTSD severity. PTSD symptom severity as measures by CAPS showed a widespread negative correlation with cortical thickness (blue clusters), but not positive correlation. CAPS = Clinician Administered PTSD Scale.
Table 1.
Clusters significantly correlating with cortical thickness
| Region | Side | Coordinates | Cluster Size (mm3) | Z values (peak) | Corrected p |
|---|---|---|---|---|---|
| CAPS Effects (negative)
| |||||
| Superior frontal | L | −7, 49, 34 | 6847 | − 6.5 | < 0.001 |
| Isthmus of cingulate | L | 1410 | − 5.9 | ||
| −9, −48, 28 | < 0.001 | ||||
| Insula | L | 1315 | − 3.5 | ||
| −30, 12, 6 | < 0.001 | ||||
| Superior frontal | R | 13, 46, 34 | 1183 | − 3.6 | |
| < 0.001 | |||||
| Postcentral | L | 867 | − 4.4 | ||
| −14, −31, 71 | < 0.001 | ||||
| Caudal middle frontal | L | 857 | − 4.0 | ||
| −25, −3, 41 | < 0.001 | ||||
| Superior Parietal | L | 652 | − 3.8 | ||
| −32, −47, 33 | 0.001 | ||||
| Lateral orbitofrontal | R | 42, 25, −12 | 626 | − 3.5 | |
| 0.002 | |||||
| Posterior cingulate | R | 5, 7, 35 | 603 | − 4.2 | |
| 0.002 | |||||
| Lateral occipital | R | 25, −83, 19 | 602 | − 4.0 | |
| 0.003 | |||||
| Lateral occipital | R | 20, −84, −1 | 511 | − 2.8 | |
| 0.009 | |||||
|
CES Effects (negative) | |||||
| Rostral middle frontal | L | 30, 36, 19 | 813 | − 3.9 | < 0.001 |
|
Diagnosis (PTSD vs. CC) * CES Interaction | |||||
| Supramarginal | L | −46, −31, 18 | 1200 | 4.0 | < 0.001 |
| Cuneus | L | 1147 | 4.9 | ||
| −11, −76, 29 | < 0.001 | ||||
| Superior frontal | L | 1080 | 4.4 | ||
| −8, 28, 51 | < 0.001 | ||||
| Cuneus | R | 5, −83, 21 | 865 | 3.6 | |
| < 0.001 | |||||
| Paracentral | R | 6, −10, 58 | 534 | 3.1 | |
| 0.007 | |||||
| Precentral | L | 523 | 2.8 | ||
| −40, −10, 44 | 0.008 | ||||
| Paracentral | L | 514 | 3.5 | ||
| −7, −27, 55 | 0.009 | ||||
Abbreviations – CC: combat control; PTSD: posttraumatic stress disorder; PFC: prefrontal cortex; L: left; R: right; CAPS-IV: Clinician Administered PTSD Scale for the DSM-IV; CES: Combat Exposure Scale.
Secondary analyses examining the effects of PTSD symptom dimensions found the following associations (Fig. 2): I. Dysphoric Arousal negatively correlated with cortical thickness clusters in left dorsolateral PFC, left superior PFC, right lateral occipital cortex, right posterior cingulate, left lingual gyrus and precuneus (Fig. 2A). II. Anxious Arousal did not correlate with cortical thickness. III. Reexperiencing negatively correlated with cortical thickness in the bilateral ventrolateral PFC and bilateral superior PFC (Fig. 2B). IV. Numbing negatively correlated with left dACC, left dorsolateral and ventrolateral PFC, and left inferior parietal cortex (Fig. 2C). 5. Avoidance negatively correlated with a cluster in the left lateral orbitofrontal cortex (Fig. 2D). No positive correlations were found between PTSD symptom dimensions and cortical thickness.
Figure 2.
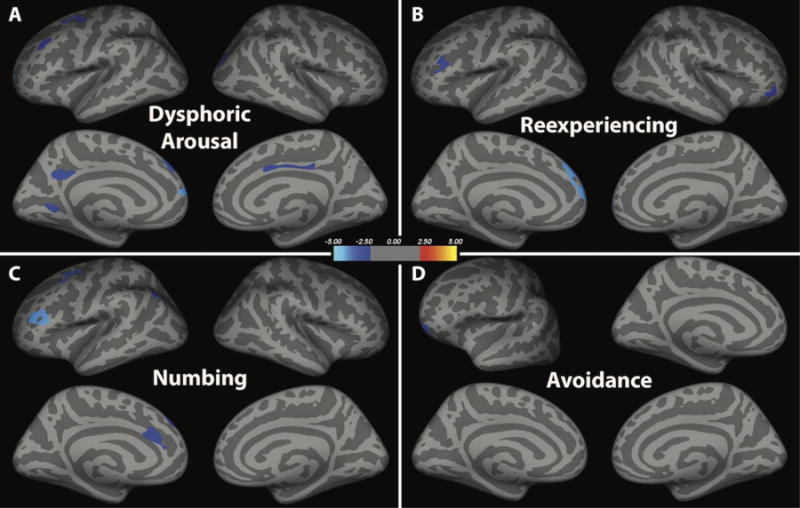
Correlation between cortical thickness and the 5 dimensions of PTSD symptoms. Four dimensions showed negative correlation with cortical thickness (blue clusters), but no positive correlations. The 5th dimension, anxious arousal, did not correlate with cortical thickness.
Combat Exposure
We found a main effect of CES, such that participants with more severe combat exposure – regardless of PTSD status – showed significant reductions in cortical thickness in a cluster in the lateral rostral middle frontal gyrus (Fig. 3, Table 1). The GLM also revealed an interaction between CES and diagnosis in 7 clusters (Fig. 4, Table 1). To delineate the pattern of interaction, we performed a linear fit between CES and cortical thickness, controlling for age, in each cluster and group (7 clusters × 2 groups). Following correction for multiple comparisons, 5 slopes remained significant, showing negative association between CES and cortical thickness in the non-PTSD participants in the left superior frontal cortex, bilateral paracentral gyri, and bilateral cuneus. In contrast, CES was not associated with cortical thickness in the PTSD participants (all corrected p values > 0.05). Conducting a multivariate analysis in which the 7 clusters were included as dependent measures with CES, CAPS, and CES-by-CAPS interaction as independent terms, showed comparable results of interaction between CES and CAPS effects [F(7,54) = 2.39, p = 0.03], such as Veterans with low PTSD symptoms showed more negative correlations between CES and cortical thickness.
Figure 3.
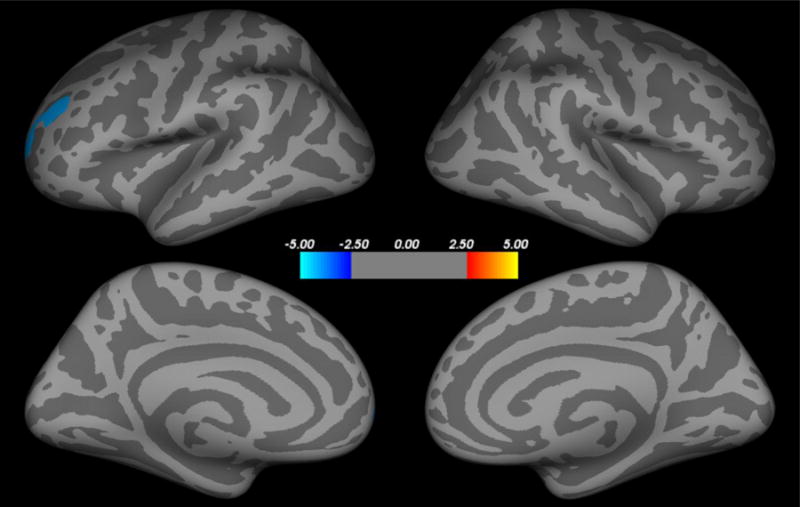
Correlation between cortical thickness and combat exposure severity. Historical self-report measure of combat exposure severity negatively correlated with cortical thickness in the left lateral prefrontal cortex (blue cluster), after controlling for age and PTSD status. There were no positive correlations between cortical thickness and combat exposure severity.
Figure 4.
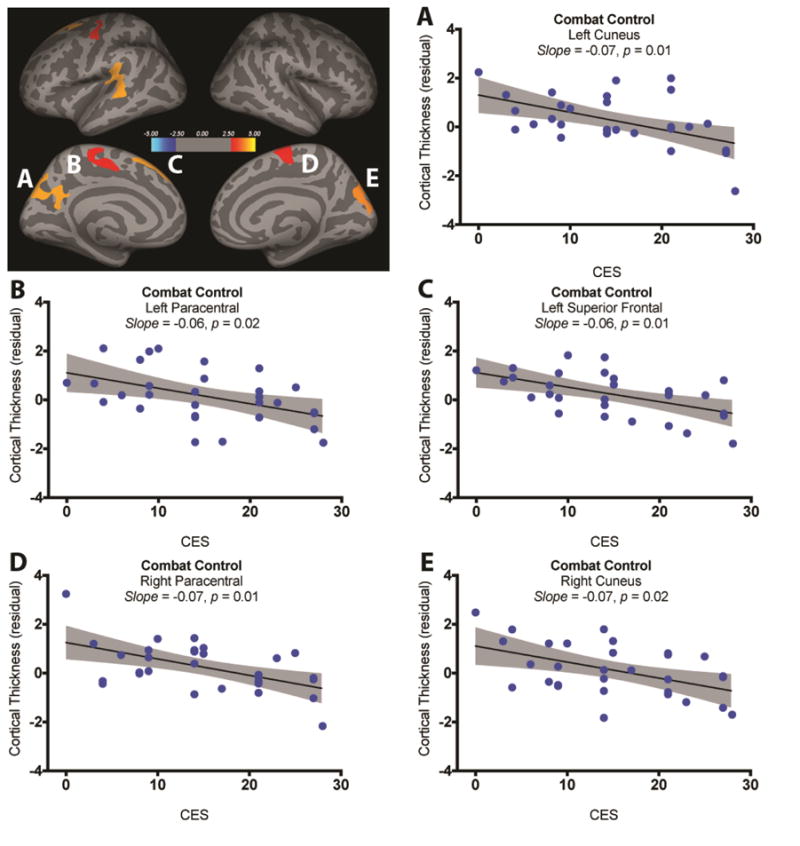
Interaction between combat exposure severity (CES) and PTSD diagnosis. Five clusters showed a significant interaction between CES and PTSD status (red-yellow clusters; A). Post-hoc analyses revealed significant negative association between CES and cortical thickness in the combat control group (B–E). There was no significant association between CES and cortical thickness in the PTSD group.
4. Discussion
Though prior studies have examined associations between PTSD and quantitative measurements of brain structure, few have examined the independent and interactive effects of trauma exposure and dimensional PTSD symptoms on cortical thickness, a robust measure of gray matter integrity. Consistent with our study hypothesis, we found that cortical thickness negatively correlated with total PTSD symptom severity in a number of brain regions relevant to emotional inhibition and emotion-cognition interactions. Secondary analyses of the relationship between cortical thickness and dimensional symptoms of PTSD also lent support to the hypothesis that different symptom dimensions would map onto different cortical regions. Moreover, we found that combat exposure was related to reductions in cortical thickness independent of age and PTSD diagnosis, partially supporting our third hypothesis. However, contrary to our prediction, we did not observe an additive effect of combat exposure and PTSD diagnosis in relationship to cortical thickness. In fact, the severity of combat exposure did not correlate with cortical thickness in the PTSD group, suggesting that the ongoing suffering from PTSD has confounded the brain effects of the historical stressor as measured by CES. Taken together, these results suggest that reduced cortical integrity within certain brain regions may be associated with severe trauma exposure and influence the expression of trauma-related symptoms. More importantly, the results highlight the brain sequelae of combat exposure, even in Veterans without PTSD.
Consistent with prior evidence, we found that ventrolateral and dorsolateral PFC, as well as left dorsal ACC, and right posterior cingulate, among other brain regions, showed significant reductions with increasing trauma-related symptom severity. Changes in cortical thickness may result from dendritic spine loss and/or glial dysfunction (Geuze et al., 2008a). To the extent that reduced cortical thickness may relate to reduced functioning, our findings may have relevance for models of brain functioning in PTSD. These brain regions overlap with networks that broadly support emotion processing, emotion regulation, and executive control, all of which are impacted in PTSD (Fitzgerald et al., 2016; Scott et al., 2016).
While highlighting the exploratory nature of the PTSD dimensional analysis, we note two observations that merit further assessment in future studies. First, there was relatively limited overlap between brain regions associated with each of the PTSD dimensions, putatively supporting the prospect of dimension-specific brain abnormalities. Second, the reduction in dACC thickness was associated with the numbing dimension (symptoms comparable to depression), but not arousal or reexperiencing (symptoms related to anxiety and fear expression). This observation is intriguing considering that activation of the dACC has been previously related to arousal and expression of conditioned fear (Fonzo et al., 2010; Mechias et al., 2010). Hyperactivation of the dACC was also proposed as predisposing vulnerability present in healthy twins of PTSD patients (Admon et al., 2013; Shin et al., 2011; Shin et al., 2009). Thus, the study results raise the question for future longitudinal investigations to determine whether the structural deficit in dACC contributes to a pathological behavioral shift from arousal and reexperiencing dimensions to depressive numbing –and perhaps dissociative– symptomatology.
The interaction effect observed in our combat exposure analysis is particularly interesting, as it shows that combat exposure is inversely related to cortical thickness in non-PTSD Veterans. Combat exposure requires sustained periods of heightened arousal and awareness, rapid processing of information, and assessment of threat, with high stakes. When not on combat missions or patrols, soldiers who live in a combat zone are at risk for potential harm, as attacks on bases can occur randomly and unpredictably. It has been consistently reported that chronic, unpredictable stress has adverse neurobiological effects (Arnsten, 2015). For example, animal models examining the impact of chronic stress show loss of dendrites and spines in pyramidal cells of the prefrontal cortex, which relate to working memory, attention, and cognitive flexibility. Similar findings have been reported in human research, whereby lower gray matter volume in the prefrontal cortex is observed in individuals with trauma exposure. In addition, alterations in prefrontal connectivity and dysregulation of the amygdala have been reported, which result in an emphasis on habitual or primitive responding versus flexible, goal-directed thinking (Ansell et al., 2012; Liston et al., 2009; Soares et al., 2013).
Our finding of reduced cortical thickness in combat exposed non-PTSD Veterans supports the notion that the effects of combat exposure may be associated with reductions in structural integrity outside of the context of PTSD. It also raises a critical question whether pre-deployment cortical thickness could serve as a biomarker of resilience to the impact of combat exposure. Consistent with this model, the cortical thickness of non-PTSD Veterans in the current study was affected negatively by the severity of combat exposure. However, these individuals were resilient to PTSD as well as retained a larger cortical thickness compared to Veterans with severe PTSD, as evident by the negative correlation between CAPS scores and cortical thickness. Initial evidence shows that individuals with higher prefrontal cortical thickness show enhanced emotional regulation and fear extinction compared than those with less cortical density (Bruehl et al., 2013; Hartley et al., 2011; Milad et al., 2005). A recent longitudinal study showed that individuals with greater gray matter volume in the ACC presented with fewer PTSD symptoms after experiencing a natural disaster (Sekiguchi et al., 2013). Similarly, greater cortical thickness was associated with reduced commission errors in a Go/No-GO task (Sadeh et al., 2015). Thus, future studies should examine whether pre-exposure cortical integrity confers resilience to trauma-related psychopathology.
There are several features of our study that make it notable. A somewhat unique feature of our study includes the examination of PTSD symptoms in relation to cortical thickness on a continuum (i.e., trauma-related stress symptom severity). Prior research has classified subjects into groups categorically, depending on whether subjects were diagnosed with PTSD. Categorical classification of PTSD creates logistical challenges, particularly when the comparison group has a relatively high degree of PTSD symptomology (e.g. subthreshold) despite not meeting DSM criteria and/or falling below a pre-determined symptom severity cut-score. Our approach also highlights the dimensional nature of stress exposure and PTSD symptoms, in line with the NIMH RDoC initiative (Cuthbert, 2014; Schutzwohl and Maercker, 1999).
In addition, to our knowledge, no studies have examined cortical thickness as it relates to symptom dimensions outlined in the five-factor model of PTSD. PTSD is a multi-faceted diagnosis that, like many mental disorders, manifests differently between individuals in the general population. For example, one individual may suffer primarily from hyperarousal symptoms early on in the illness course whereas another may experience numbing as a primary concern (Schell et al., 2004). Exploration of the relationship between different symptom presentations and potential neurobiological markers may assist in identifying pharmacological treatments that more effectively target dimensional PTSD symptoms.
Furthermore, much of the prior literature has focused primarily on neurobiological associations with PTSD, while limited studies have investigated independent contributions from combat exposure (Schnurr and Spiro, 1999; Taft et al., 1999). Early findings suggest an indirect relationship between combat exposure and physical health, and a stronger relationship between PTSD and physical health (Schnurr and Spiro, 1999; Taft et al., 1999). While the effects of PTSD and combat exposure are somewhat difficult to disentangle, the effects of combat exposure warrant further investigation given that some soldiers who experience combat do not develop PTSD. A high percentage of U.S. Veterans experience a variety of medical conditions and psychological distress (Agha et al., 2000; Kang et al., 2009). The mechanisms underlying the incidence of these conditions in the U.S. Veteran population could be investigated within a construct accounting for the detrimental neural effects of chronic combat stress.
Limitations of the study include a predominately male, moderately sized sample. Future studies would benefit from a larger and more representative sample of the U.S. Veteran population. Broadening the sample population would allow investigation of potential sex and racial differences in the neural consequences associated with combat exposure and stress-related psychopathology. Moreover, the use of a cross-sectional research design prevents conclusions about whether the group differences represent acquired neurobiological changes versus pre-existing vulnerabilities. For example, one twins’ study suggested that smaller hippocampal volume in PTSD could predate trauma and psychopathology (Gilbertson et al., 2002). However, a cortical twins’ study suggested that medial PFC GM deficit is putatively an acquired pathology in PTSD (Kasai et al., 2008). Although depression severity, medication and TBI status, alcohol/substance use comorbidities and other potential confounds did not affect the significant relationship between PTSD symptoms and GM integrity, many of the individuals within our sample had comorbidities, including mild TBI and substance use disorders, that limit our conclusions about etiology and vulnerability to neurobiological changes secondary to combat exposure. These comorbidities were not excluded because individuals with trauma and stress-related pathology often have multiple comorbidities, and excluding individuals with these diagnoses would have reduced the external validity and generalizability of our findings. Another limitation is that this dataset does not include information regarding early life stressors or number and duration of combat tours, which may contribute to the observed alterations in cortical thickness (Corbo et al., 2014; Yehuda et al., 2001). Finally, our sample was solely comprised of U.S. combat Veterans and may not generalize to other types of trauma exposure.
In conclusion, our results suggest that combat exposure is associated with reduced cortical thickness in multiple brain regions, even in individuals with low PTSD symptoms. To our knowledge, this is the first study to examine the interaction between combat exposure and PTSD effects on cortical thickness. Our study also explored the correlation between cortical thickness and PTSD symptoms dimensionally. Future research should include an exploration of the relationship between the neurobiological findings reported herein and functional outcome variables, such as cognition. In addition, future studies should investigate potential treatments for reversing or impeding the process of cortical thinning that is associated with combat exposure.
Supplementary Material
Acknowledgments
We would like to thank the Veterans who participated in this study for their invaluable contribution.
Role of Funding Source
This work was supported by the Department of Veterans Affairs (NCPTSD; IK2CX000772), NIMH (K23MH101498), and the Yale Center for Clinical Investigation (UL1RR024139). Portions of this research were presented at the 2015 Annual Meeting of the International Society for Traumatic Stress Studies (ISTSS). The funding source had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or other sponsoring institutions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors KMW, LAA, BS, JCS, IH, JHK, and CGA designed the study. Authors KMW, LAA, CK managed the literature searches. Authors KMW, BS, JCS, MT, AR, VW, CK, BM, and SMS acquired the data. CLA and CGA conducted the image processing. CGA undertook the statistical analysis. KMW and LAA wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Krystal has served as a scientific consultant to the following companies (the Individual Consultant Agreements listed are <$10,000 per year): Aisling Capital, Astellas Pharma Global Development, AstraZeneca Pharmaceuticals, Biocortech, Brintnall & Nicolini, Easton Associates, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Merz Pharmaceuticals, MK Medical Communications, Hoffmann–La Roche, SK Holdings, Sunovion Pharmaceuticals, Takeda Industries, and Teva Pharmaceutical Industries. He is on the Scientific Advisory Board for the following companies: Abbott Laboratories, Bristol-Myers-Squibb, Eisai, Eli Lilly, Forest Laboratories, Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Pfizer Pharmaceuticals, and Shire Pharmaceuticals. He holds <$150 in exercisable warrant options with Tetragenex Pharmaceuticals. He is on the Board of Directors of the Coalition for Translational Research in Alcohol and Substance Use Disorders. He was the principal investigator of a multicenter study in which Janssen Research Foundation provided drug and some support to the Department of Veterans Affairs. He is Editor of Biological Psychiatry. He has a patent on dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia (patent number 5447948) and is a coinventor on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1). He has a patent pending on intranasal administration of ketamine to treat depression. Dr. Abdallah has served as a consultant or on advisory boards for Genentech and Janssen. He also serves as editor for the journal Chronic Stress published by SAGE Publications, Inc. All other authors declare no conflicts of interest.
References
- Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci. 2015a;1344:66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Coplan JD, Jackowski A, Sato JR, Mao X, Shungu DC, Mathew SJ. Riluzole effect on occipital cortex: a structural and spectroscopy pilot study. Neurosci Lett. 2012;530:103–107. doi: 10.1016/j.neulet.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015b;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Wrocklage KM, Averill CL, Akiki T, Schweinsburg BC, Roy A, Martini B, Southwick S, Krystal JK, Scott JC. Anterior Hippocampal Dysconnectivity in Posttraumatic Stress Disorder: A Dimensional and Multimodal Approach. Translational psychiatry. 2017 doi: 10.1038/tp.2017.12. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Agha Z, Lofgren RP, Van Ruiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. Psychological Corporation; San Antonio, Texas: 1993. [Google Scholar]
- Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory - II. Psychological Corporation; San Antonio, Texas: 1996. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl H, Preissler S, Heuser I, Heekeren HR, Roepke S, Dziobek I. Increased prefrontal cortical thickness is associated with enhanced abilities to regulate emotions in PTSD-free women with borderline personality disorder. PLoS One. 2013;8:e65584. doi: 10.1371/journal.pone.0065584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo V, Salat DH, Amick MM, Leritz EC, Milberg WP, McGlinchey RE. Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Research: Neuroimaging. 2014;223:53–60. doi: 10.1016/j.pscychresns.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo V, Salat DH, Powell MA, Milberg WP, McGlinchey RE. Combat exposure is associated with cortical thickness in Veterans with a history of chronic pain. Psychiat Res-Neuroim. 2016;249:38–44. doi: 10.1016/j.pscychresns.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World psychiatry: official journal of the World Psychiatric Association (WPA) 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychological Medicine. 2013;43:645–653. doi: 10.1017/S0033291712001328. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P) Biometrics Research; New York: State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JM, MacNamara A, Kennedy AE, Rabinak CA, Rauch SA, Liberzon I, Phan KL. Individual differences in cognitive reappraisal use and emotion regulatory brain function in combat-exposed veterans with and without PTSD. Depress Anxiety. 2016 doi: 10.1002/da.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, Elbogen E, Beckham JC. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans: a meta-analysis. J Anxiety Disord. 2015;31:98–107. doi: 10.1016/j.janxdis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008a;41:675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Geuze E, Westenberg HGM, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008b;41:675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L, Papini S, Chhetry BT, Shvil E, Rubin M, Sullivan GM, Markowitz JC, Mann JJ, Neria Y. Ptsd Remission after Prolonged Exposure Treatment Is Associated with Anterior Cingulate Cortex Thinning and Volume Reduction. Depress Anxiety. 2016;33:384–391. doi: 10.1002/da.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdnack HA. Weschler Test of Adult Reading: WTAR. Psychological Corporation; San Antonio, Texas: 2001. [Google Scholar]
- Hunter M, Villarreal G, McHaffie GR, Jimenez B, Smith AK, Calais LA, Hanlon F, Thoma RJ, Canive JM. Lateralized abnormalities in auditory M50 sensory gating and cortical thickness of the superior temporal gyrus in post-traumatic stress disorder: preliminary results. Psychiatry Res. 2011;191:138–144. doi: 10.1016/j.pscychresns.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2009;51:401–410. doi: 10.1097/JOM.0b013e3181a2feeb. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, Hatton SN, Bennett MR. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Molecular neurobiology. 2013;47:645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Kay T, Harrington D, Adams R, Anderson T, Berrol S, Cicerone K, et al. Defination of mild traumatic brain injury. Journal of Head Trauma Rehabilitation. 1993;8:86–87. [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora C. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment. 1989;1:53–55. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Landre L, Destrieux C, Baudry M, Barantin L, Cottier JP, Martineau J, Hommet C, Isingrini M, Belzung C, Gaillard P, Camus V, El Hage W. Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiat Res-Neuroim. 2010;183:181–186. doi: 10.1016/j.pscychresns.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Li S, Huang X, Li L, Du F, Li J, Bi F, Lui S, Turner JA, Sweeney JA, Gong Q. Posttraumatic Stress Disorder: Structural Characterization with 3-T MR Imaging. Radiology. 2016:150477. doi: 10.1148/radiol.2016150477. [DOI] [PubMed] [Google Scholar]
- Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. NeuroImage Clinical. 2013;2:601–611. doi: 10.1016/j.nicl.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li YJ, Luo EP, Lu HB, Yin H. Cortical Thinning in Patients with Recent Onset Post-Traumatic Stress Disorder after a Single Prolonged Trauma Exposure. Plos One. 2012;7 doi: 10.1371/journal.pone.0039025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch Gen Psychiatry. 2011;68:701–713. doi: 10.1001/archgenpsychiatry.2011.70. [DOI] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, Nasser JD, Wagner HR, McCarthy G, Mid-Atlantic MW. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Ng P, Neylan T, Mackin S, Wolkowitz O, Mellon S, Yan XD, Flory J, Yehuda R, Marmar CR, Weiner MW. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiat Res-Neuroim. 2015;234:194–201. doi: 10.1016/j.pscychresns.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Nilsen AS, Hilland E, Kogstad N, Heir T, Hauff E, Lien L, Endestad T. Right temporal cortical hypertrophy in resilience to trauma: an MRI study. Eur J Psychotraumato. 2016;7 doi: 10.3402/ejpt.v7.31314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Tsai J, Harpaz-Rotem I, Whealin JM, Southwick SM. Support for a novel five-factor model of posttraumatic stress symptoms in three independent samples of Iraq/Afghanistan veterans: a confirmatory factor analytic study. J Psychiatr Res. 2012;46:317–322. doi: 10.1016/j.jpsychires.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Mu YF, Liu K, Zhang J, Huan Y, Tan QR, Shi M, Wang Q, Chen YC, Wang HH, Wang HN, Zhang NY, Zhang XL, Xiong LZ, Yin H. Cortical inhibition deficits in recent onset PTSD after a single prolonged trauma exposure. Neuroimage-Clin. 2013;3:226–233. doi: 10.1016/j.nicl.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, Nutt DJ. Glutamate and cortisol–a critical confluence in PTSD? J Psychopharmacol. 2008;22:469–472. doi: 10.1177/0269881108094617. [DOI] [PubMed] [Google Scholar]
- Sadeh N, Spielberg JM, Logue MW, Wolf EJ, Smith AK, Lusk J, Hayes JP, Sperbeck E, Milberg WP, McGlinchey RE, Salat DH, Carter WC, Stone A, Schichman SA, Humphries DE, Miller MW. SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Molecular Psychiatry. 2016;21:357–363. doi: 10.1038/mp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Spielberg JM, Miller MW, Milberg WP, Salat DH, Amick MM, Fortier CB, McGlinchey RE. Neurobiological indicators of disinhibition in posttraumatic stress disorder. Hum Brain Mapp. 2015;36:3076–3086. doi: 10.1002/hbm.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell TL, Marshall GN, Jaycox LH. All symptoms are not created equal: the prominent role of hyperarousal in the natural course of posttraumatic psychological distress. Journal of abnormal psychology. 2004;113:189–197. doi: 10.1037/0021-843X.113.2.189. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Spiro A., 3rd Combat exposure, posttraumatic stress disorder symptoms, and health behaviors as predictors of self-reported physical health in older veterans. J Nerv Ment Dis. 1999;187:353–359. doi: 10.1097/00005053-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Schutzwohl M, Maercker A. Effects of varying diagnostic criteria for posttraumatic stress disorder are endorsing the concept of partial PTSD. J Trauma Stress. 1999;12:155–165. doi: 10.1023/A:1024706702133. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Wrocklage KM, Schweinsburg BC, Southwick SM, Krystal JH. Prospective Memory in Posttraumatic Stress Disorder. Journal of the International Neuropsychological Society: JINS. 2016;22:724–734. doi: 10.1017/S1355617716000564. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, Araki T, Hanawa S, Nakagawa S, Miyauchi CM, Sakuma A, Kawashima R. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Mol Psychiatry. 2013;18:618–623. doi: 10.1038/mp.2012.51. [DOI] [PubMed] [Google Scholar]
- Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, Macklin ML, Gold AL, Karpf RD, Orr SP, Rauch SL, Pitman RK. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry. 2011;168:979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman AJ, Rauch SL, Pitman RK. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, Palha JA, Cerqueira JJ, Sousa N. Stress Impact on Resting State Brain Networks. PLoS One. 2013;8:e66500. doi: 10.1371/journal.pone.0066500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft CT, Stern AS, King LA, King DW. Modeling physical health and functional health status: the role of combat exposure, posttraumatic stress disorder, and personal resource attributes. J Trauma Stress. 1999;12:3–23. doi: 10.1023/A:1024786030358. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kuo JR, Schaer M, Kaloupek DG, Eliez S. Early adversity and combat exposure interact to influence anterior cingulate cortex volume in combat veterans. NeuroImage Clinical. 2013;2:670–674. doi: 10.1016/j.nicl.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1373–1382. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- Xie B, Qiu MG, Zhang Y, Zhang JN, Li M, Chen H, Zhang Y, Zhang JJ, Wang J, Chen W, Du HJ, Zhang SX. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Research. 2013;1490:225–232. doi: 10.1016/j.brainres.2012.10.048. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development and psychopathology. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


