Summary
Children of women treated with antiepileptic drugs (AEDs) are at increased risk for adverse outcomes detectable in the neonatal period, which may be associated with the amount of AED in the fetal circulation. Placental passage of AEDs can be measured by calculating the ratio of umbilical cord to maternal AED concentrations collected at delivery. The aims of this study were to determine the umbilical cord concentrations and umbilical to maternal ratios for AEDs, and to determine whether higher cord concentrations are associated with increased risk of neonatal complications. AED cord and maternal blood concentrations from 70 mother-newborn dyads and neonatal complications were recorded. Logistic regressions were performed to determine the association between AED concentrations and complications. Mean umbilical to maternal ratios for total concentrations ranged from 0.79 for carbamazepine to 1.20 for valproic acid, and mean umbilical to maternal ratios for free concentrations ranged from 0.86 for valproic acid to 1.42 for carbamazepine, indicating complete placental passage. Neither umbilical cord concentrations nor umbilical to maternal ratios were associated with adverse neonatal outcomes. Additional investigations are warranted to delineate the relationship between quantified fetal AED exposure and neonatal complications.
Keywords: epilepsy, pregnancy, placental passage, antiepileptic drugs, umbilical cord concentrations
Introduction
Children of women treated with antiepileptic drugs (AEDs) may be at increased risk for adverse outcomes detectable in the neonatal period, including premature delivery, small for gestational age birthweight, low Apgar scores, and major congenital malformations.1 A recent study of hospitalization records during delivery reported increased odds ratios for prematurity, poor fetal growth, fetal distress, fetal abnormalities, and stillbirth in infants born to women with epilepsy.2 Both seizures and their treatment likely contribute to these risks, and it is unknown if the amount of AED exposure at term increases the risk for neonatal complications.
The clearance of most AEDs increases in pregnancy and may require maternal dose adjustment to maintain therapeutic benefit. Several pregnancy registries have provided data showing that higher AED doses at conception confer a higher risk for major congenital malformations.1,3 However, pregnancy registries most often do not report AED doses after the first trimester, systematic measurements of AED blood concentrations, or neonatal complications other than major congenital malformations.
Factors other than maternal dose may also correlate with fetal exposure. Drug properties including molecular weight and dissociation constant affect placental passage, with drugs weighing greater than 500 daltons and those that are predominantly dissociated at physiologic pH passing incompletely.4 Protein binding does not directly influence placental passage,4 but may affect fetal exposure due to maternal drug-drug interactions. Maternal variation in placental transporter proteins also influences placental passage.5 Fetal AED concentrations cannot be directly measured during pregnancy. Umbilical cord concentrations at delivery and calculation of umbilical cord to maternal concentration ratios can serve as a proxy for fetal exposure and a means to compare individual medication exposure. The purpose of this study was to determine the umbilical cord concentrations and umbilical to maternal ratios for several commonly prescribed AEDs, and to determine whether higher AED umbilical cord concentrations or umbilical to maternal ratios are associated with an increased risk of neonatal complications.
Methods
Pregnant women who were treated with AEDs for epilepsy or bipolar disorder at a single academic center were followed prospectively in an observational study examining the pharmacokinetic properties of AEDs during pregnancy and delivery (Emory Women’s Epilepsy and Mental Health Programs) from December 2002 until March 2006. The Institutional Review Board of Emory University School of Medicine approved the study. Women were informed of all available treatment options, and written informed consent was obtained. Only women who chose to continue AEDs during pregnancy and whose infants had umbilical cord AED levels drawn at the time of delivery were included in this cohort analysis.
Umbilical cord and maternal venous blood were collected at the time of delivery. At each collection, blood was centrifuged at 2,750 rpm at 3°C for 10 minutes, and plasma or serum was transferred in 600 μL aliquots to polypropylene tubes, and stored at −80 degrees Celsius until assay. All assays were completed blind to maternal dose. Total drug levels were measured for patients taking carbamazepine, phenytoin, valproic acid, lamotrigine, levetiracetam, oxcarbazepine, and topiramate by liquid chromatography/mass spectrometry. Free (unbound) drug levels were determined by first warming the sample to 37°C then placing an aliquot of the sample into a Millipore Centrifree cartridge (1000 MW cutoff) and centrifuging the sample at 2500×G for 20 mins. Concentrations of unbound drug were determined in the filtrate by liquid chromatography/mass spectrometry as above. Free drug was assessed for patients taking carbamazepine, phenytoin, valproic acid, and lamotrigine.
In order to compare umbilical cord concentrations across different AEDs, the percentage of the upper limit of the therapeutic range was calculated using the following upper limits: 12 mcg/mL for total carbamazepine, 20 mcg/mL for total phenytoin, 120 mcg/mL for total valproic acid, 20 mcg/mL for total lamotrigine, 46 mcg/mL for total levetiracetam, 35 mcg/mL for total oxcarbazepine, 20 mcg/mL for total topiramate, 3 mcg/mL for free carbamazepine, 2 mcg/mL for free phenytoin, and 23 mcg/mL for free valproic acid. The therapeutic range of free lamotrigine is not established.
Demographic information was collected at the time of enrollment. Medical records were obtained for review from the treating obstetrician, the delivery hospital, and the treating pediatrician. Neonatal complications evaluated included prematurity (delivery prior to 37 weeks gestational age), small for gestational age birthweight, neonatal intensive care unit or special care nursery admission, major congenital malformations, and Apgar scores less than 7 at 1 and 5 minutes after delivery.
Logistic regressions were performed to ascertain the effect of umbilical cord AED levels and umbilical to maternal AED ratios on neonatal complications. Statistical analyses were performed using IBM SPSS Statistics Version 22.
Results
Umbilical cord samples were collected at delivery for 70 pregnancies. Two mother-newborn dyads were included in the study twice for two different pregnancies; each pregnancy was counted separately. Sixty-six mothers were taking one AED at the time of delivery. AEDs included carbamazepine (n=8), lamotrigine (n=36), levetiracetam (n=7), oxcarbazepine (n=4), phenytoin (n=3), topiramate (n=2), and valproic acid (n=6). Four mothers were taking two AEDs. Polytherapy combinations included carbamazepine and phenytoin (n=1), carbamazepine and levetiracetam (n=1), and lamotrigine and levetiracetam (n=2). There were no significant differences between AED groups with regard to age, number of prior pregnancies, diagnosis (epilepsy, bipolar disorder, or both), race, marital status, or education level.
All women received prenatal care for the majority of pregnancy. All were singleton pregnancies. For the four mothers taking two AEDs, umbilical cord samples were assayed for both medications, resulting in 74 umbilical cord AED concentration measurements. Nine of the mother-child pairs had only umbilical cord levels tested and thus 65 maternal samples were available for analysis of umbilical cord to maternal AED ratios. Maternal venous samples were collected a mean of 24.1 ± 56.4 minutes after the umbilical cord samples.
Mean umbilical cord AED concentrations by medication are shown in Table 1. Mean umbilical to maternal ratios by medication are shown in Figure 1. There were no significant differences in umbilical to maternal ratios between medications for either total (p = 0.31) or free (p = 0.42) AED levels.
Table 1.
| a. Umbilical cord concentration by medication | ||||||||
|---|---|---|---|---|---|---|---|---|
| CBZ | LEV | LTG | OXC | PHT | TPM | VPA | Total | |
| Sample Size (n) | 10 | 10 | 38 | 4 | 4 | 2 | 6 | 74 |
| Total AED Concentration | ||||||||
| [Umbilical Cord] Mean (mcg/mL) | 3.71 ± 1.00 | 22.75 ± 21.81 | 5.79 ± 3.58 | 1.65 ± 0.84 | 10.20 ± 5.24 | 9.95 ± 1.63 | 48.60 ± 17.12 | |
| Range (mcg/mL) | 1.70 – 4.90 | 4.00 – 76.00 | 1.10 – 16.30 | 0.50 – 2.50 | 6.30 – 17.50 | 8.80 – 11.10 | 26.00 – 71.00 | |
| Free (unbound) AED Concentration | ||||||||
| [Umbilical Cord] Mean (mcg/mL) | 1.50 ± 0.31 | N/A | 2.29 ± 1.58* | N/A | 1.50 ± 0.57 | N/A | 3.87 ± 4.22 | |
| Range (mcg/mL) | 0.90 – 2.00 | N/A | 0.30 – 8.00* | N/A | 0.80 – 2.20 | N/A | 0.30 – 10.00 | |
| b. Maternal dose at delivery and placental passage by medication | ||||||||
|---|---|---|---|---|---|---|---|---|
| CBZ | LEV | LTG | OXC | PHT | TPM | VPA | Total | |
| Sample Size (n) | 9 | 10 | 33 | 3 | 3 | 2 | 5 | 65 |
| Maternal AED Dose | ||||||||
| Dose at Delivery Mean (mg/d) | 1216.67 ± 312.25 | 2666.67 ± 1419.73** | 626.52 ± 254.64 | 1650.00 ± 1050.00 | 466.67 ± 76.38 | 700.00 ± 100.00 | 1093.75 ± 373.26*** | |
| Range (mg/d) | 800 – 1750 | 750 – 5000** | 125 – 1200 | 450 – 2400 | 400 – 550 | 600 – 800 | 625 – 1500*** | |
| Total AED Placental Passage | ||||||||
| Placental Passage ([cord]/[maternal]) | 0.79 ± 0.19 | 1.12 ± 0.46 | 1.17 ± 0.48 | 1.11 ± 0.10 | 1.10 ± 0.32 | 0.85 ± 0.02 | 1.20 ± 0.23 | |
| Range | 0.51 – 1.19 | 0.57 – 2.17 | 0.66 – 3.06 | 1.00 – 1.19 | 0.72 – 1.33 | 0.83 – 0.87 | 1.00 – 1.56 | |
| Free (Unbound) AED Placental Passage | ||||||||
| Placental Passage ([cord]/[maternal]) | 1.42 ± 1.22 | N/A | 1.15 ± 0.36**** | N/A | 1.10 ± 0.19 | N/A | 0.86 ± 0.31 | |
| Range | 0.90 – 4.67 | N/A | 0.63 – 2.31**** | N/A | 0.88 – 1.25 | N/A | 0.30 – 1.01 | |
| Factors Associated with Placental Passage | ||||||||
| Molecular Weight6 | 236.27 | 170.21 | 259.09 | 252.27 | 252.26 | 339.37 | 144.21 | |
| Protein Binding6 | 75% | 0% | 55% | 60% (active mtabolite 40%) |
90% | 15% | 90% | |
| Dissociation Constant | 13.97 | −2 (active metabolite 3.67)8 | 5.77 | − 0.657 | 8.337 | 8.539 | 4.87 | |
| c. Neonatal outcomes by medication | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Neonatal outcomes | CBZ | LEV | LTG | OXC | PHT | TPM | VPA | Poly-therapy | Total |
| Sample Size (n) | 8 | 7 | 36 | 4 | 3 | 2 | 6 | 4 | 70 |
| Premature (<37 weeks gestation) | 2 (25%) | 0 (0%) | 1 (2.8%) | 0 (0%) | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0) | 4 (5.7%) |
| Small for Gestational Age (<10th percentile) | 1 (12.5%) | 1 (14.3%) | 3 (8.3%) | 1 (25%) | 0 (0%) | 1 (50%) | 2 (33.3%) | 1 (25%) | 10 (14.3%) |
| Apgar < 7 at 1 minute | 1 (12.5%) | 1 (14.3%) | 2 (5.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (75%) | 7 (10%) |
| Apgar < 7 at 5 minutes | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 |
| Admission to NICU or Special Care Nursery | 2 (25%) | 1 (14.3%) | 6 (16.7%) | 0 (0%) | 0 (0%) | 1 (50%) | 0 (0%) | 2 (50%) | 12 (17.1%) |
| Major Malformation | 1 (12.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (25%) | 2 (2.9%) |
CBZ = carbamazepine. LEV = levetiracetam. LTG = lamotrigine. OXC = oxcarbazepine. PHT = phenytoin. TPM = topiramate. VPA = valproic acid.
n=36
n=9
n=4
n=31
NICU = neonatal intensive care unit.
Figure 1.
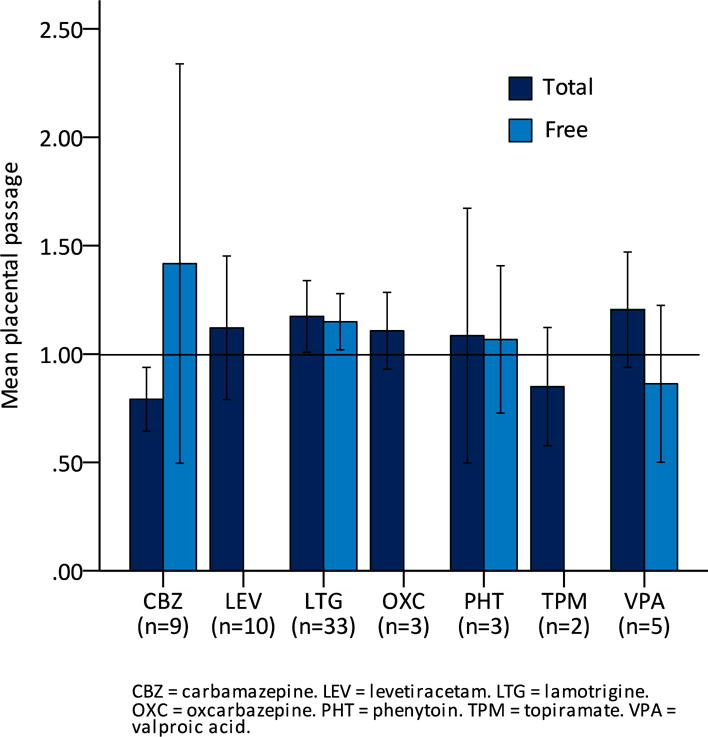
Mean placental passage (ratio of umbilical cord concentration to maternal concentration) of total and free antiepileptic drugs by medication.
Four infants (5.7%) were premature, ten (14.3%) had small for gestational age birthweight, twelve (17.1%) were admitted to the neonatal intensive care unit or special care nursery, two (2.9%) had major congenital malformations, seven (10%) had an Apgar score less than 7 at 1 minute after delivery, and none (0%) had an Apgar score less than 7 at 5 minutes after delivery (Table 1).
Higher umbilical cord concentrations of the total drug as a percentage of the upper limit of the therapeutic range were not associated with increased likelihood of prematurity, small for gestational age birthweight, neonatal intensive care unit or special care nursery admission, major congenital malformation, or Apgar score lower than 7 at 1 minute after delivery, nor were higher umbilical cord concentrations of the free drug as a percentage of the upper limit of the therapeutic range for carbamazepine, phenytoin, and valproic acid.
Higher umbilical to maternal total ratios were not associated with increased likelihood of prematurity, small for gestational age birthweight, neonatal intensive care unit or special care nursery admission, major congenital malformations, or Apgar scores lower than 7 at 1 minute after delivery, nor were higher umbilical to maternal free ratios for carbamazepine, phenytoin, and valproic acid.
Discussion
Maternal AED concentrations during pregnancy can be used to estimate fetal AED exposure. Umbilical cord AED concentrations at the time of delivery, especially when compared to maternal concentrations, provide valuable information about fetal AED exposure in utero.
Previously reported umbilical to maternal ratios for total drug concentrations include 0.73 for carbamazepine,10 0.91 for phenytoin,10 1.59 for valproic acid,10 0.89 for lamotrigine,11 and 1.14 for levetiracetam,12 while previously reported free ratios include 1.42 for carbamazepine,10 1.10 for phenytoin,10 and 0.50 for valproic acid.10 Umbilical to maternal ratios for oxcarbazepine13 and topiramate14 have been reported at close to unity. These studies were largely limited by small sample sizes of 5–35 patients, with the exception of Kacirova et al.,11 which included 63 patients on lamotrigine.
The umbilical to maternal ratios observed in this study are comparable to those reported in prior studies. Mean umbilical to maternal ratios for total concentrations ranged from 0.79 for carbamazepine to 1.20 for valproic acid, indicating extensive placental passage of all AEDs and a high degree of fetal exposure. Despite this exposure, however, few infants had neonatal complications. Reassuringly, higher umbilical cord AED concentrations and higher umbilical to maternal ratios were not associated with an increased likelihood of adverse neonatal outcomes.
Carbamazepine had the highest placental passage of free drug. This may be explained by its high dissociation constant, which causes it to be minimally ionized at physiologic pH and thus transfer completely or near-completely across the placenta. Conversely, valproate had the lowest placental passage of free drug, perhaps due to its low dissociation constant and incomplete transfer across the placenta. Despite this, in utero valproate exposure is associated with significantly lower IQ scores at 3, 4.5, and 6 years in a dose-dependent manner compared to carbamazepine, phenytoin, or lamotrigine.15 Thus, the particular adverse neurodevelopmental effects of a particular medication likely outweigh the amount of drug exposure.
Our study is limited by the small sample size with relatively few adverse neonatal outcomes overall. We also comment only on AED concentrations at delivery. Additionally, the umbilical and maternal samples in this study were collected in the early to mid-2000s; current fetal AED exposure may differ as AED selection and dosing practices have changed over time. A larger prospective study could help clarify the relationship between the amount of prenatal AED exposure at different gestational ages with a variety of child outcomes including long-term cognitive and behavioral outcomes.
Conclusions
Umbilical cord concentrations and umbilical to maternal ratios vary among AEDs, but with most ratios approximating unity. Our findings confirm prior reports demonstrating complete placental passage of several commonly prescribed AEDs. In this small sample, higher umbilical cord concentrations and umbilical to maternal ratios were not associated with adverse neonatal outcomes. A larger study is warranted to determine if individual fetal exposure is a major determinant of adverse outcomes, and further if maternal daily dose is a viable proxy for fetal exposure. Such data could have a significant impact on balancing the clinical response to changes in AED clearance in pregnancy with the risks of fetal exposure.
Acknowledgments
This work was supported by funds received from the National Institutes of Health, National Institute of Mental Health, award number P50 MH 68036 (Stowe, Newport, Pennell) and the National Institute of Neurological Disorders and Stroke and Eunice Kennedy Shriver National Institute of Child Health and Human Development, award number U01-NS038455 (Stowe, Pennell). The authors would like to thank Robert Glynn, PhD of Harvard Catalyst for his assistance with statistical analysis.
Footnotes
Conflict of Interest Statement:
Dr. Anna M. Bank has no conflicts to disclose. Dr. Zachary N. Stowe has participated in the speaker’s bureaus for GlaxoSmithKline, Pfizer, and Wyeth; served on the advisory boards for GlaxoSmithKline and Bristol Myers Squibb; has received research grant support from Pfizer, GlaxoSmithKline, Wyeth, and the National Institutes of Health, and clinical trials sponsored by Sage Therapeutics and Janssen Pharmaceuticals. Dr. Newport has received research support from Eli Lilly, Glaxo SmithKline (GSK), Janssen, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the National Institutes of Health (NIH), Takeda Pharmaceuticals, and Wyeth. He has served on speakers’ bureaus and/or received honoraria from Astra-Zeneca, Eli Lilly, GSK, Pfizer and Wyeth. He has served on advisory boards for GSK. He has never served as a consultant to any biomedical or pharmaceutical corporations. Neither he nor family members have ever held equity positions in biomedical or pharmaceutical corporations. Dr. James C. Ritchie has nothing to disclose. Dr. Page B. Pennell has participated in the speaker’s bureau and advisory boards for GlaxoSmithKline and UCB Pharma and received research support from GSK, UCB Pharma, Marinus Pharmaceuticals, and the NIH at the time this data was gathered, and she continues to receive support from the NIH.
Ethical Publication Statement:
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Pennell PB. Use of Antiepileptic Drugs During Pregnancy: Evolving Concepts. Neurotherapeutics. 2016;13:811–820. doi: 10.1007/s13311-016-0464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDonald SC, Bateman BT, McElrath TF, et al. Mortality and Morbidity During Delivery Hospitalization Among Pregnant Women with Epilepsy in the United States. JAMA Neurol. 2015;72:981–988. doi: 10.1001/jamaneurol.2015.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 4.Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28:235–269. doi: 10.2165/00003088-199528030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wang JS, Newport DJ, Stowe ZN, et al. The emerging importance of transporter proteins in the psychopharmacological treatment of the pregnant patient. Drug Metab Rev. 2007;39:723–746. doi: 10.1080/03602530701690390. [DOI] [PubMed] [Google Scholar]
- 6.Patsalos PN, Bourgeois BFD. The Epilepsy Prescriber’s Guide to Antiepileptic Drugs. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 7.National Center for Biotechnology Information. PubChem Compound Database; CID 2554, 3878, 34312, 1775, 3121. Available at: https://pubchem.ncbi.nlm.nih.gov. Accessed January 27, 2017.
- 8.Center for Drug Evaluation and Research. Review of Environmental Assessment for Keppra Oral Solution. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021505_s000_Keppra_EAFONSI.pdf. Accessed January 27, 2017.
- 9.Center for Drug Evaluation and Research. Environmental Assessment and Finding of No Significant Impact for Topamax (topiramate) Tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020505_000_environmental_rvw.pdf. Accessed January 27, 2017.
- 10.Takeda A, Okada H, Tanaka H, et al. Protein binding of four antiepileptic drugs in maternal and umbilical cord serum. Epilepsy Res. 1992;13:147–151. doi: 10.1016/0920-1211(92)90070-a. [DOI] [PubMed] [Google Scholar]
- 11.Kacirova I, Grundmann M, Brozmanova H. Serum levels of lamotrigine during delivery in mothers and their infants. Epilepsy Res. 2010;91:161–165. doi: 10.1016/j.eplepsyres.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Johannessen SI, Helde G, Brodtkorb E. Levetiracetam Concentrations in Serum and in Breast Milk at Birth and During Lactation. Epilepsia. 2005;46:775–777. doi: 10.1111/j.1528-1167.2005.54804.x. [DOI] [PubMed] [Google Scholar]
- 13.Myllynen P, Pienimaki P, Jouppila P, et al. Transplacental Passage of Oxcarbazepine and Its Metabolites in Vivo. Epilepsia. 2001;42:1482–1485. doi: 10.1046/j.1528-1157.2001.14301.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohman I, Vitols S, Luef G, et al. Topiramate Kinetics during Delivery, Lactation, and in the Neonate: Preliminary Observations. Epilepsia. 2002;43:1157–1160. doi: 10.1046/j.1528-1157.2002.12502.x. [DOI] [PubMed] [Google Scholar]
- 15.Meador KJ, Baker GA, Browning N, et al. NEAD Study Group Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


