Abstract
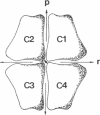
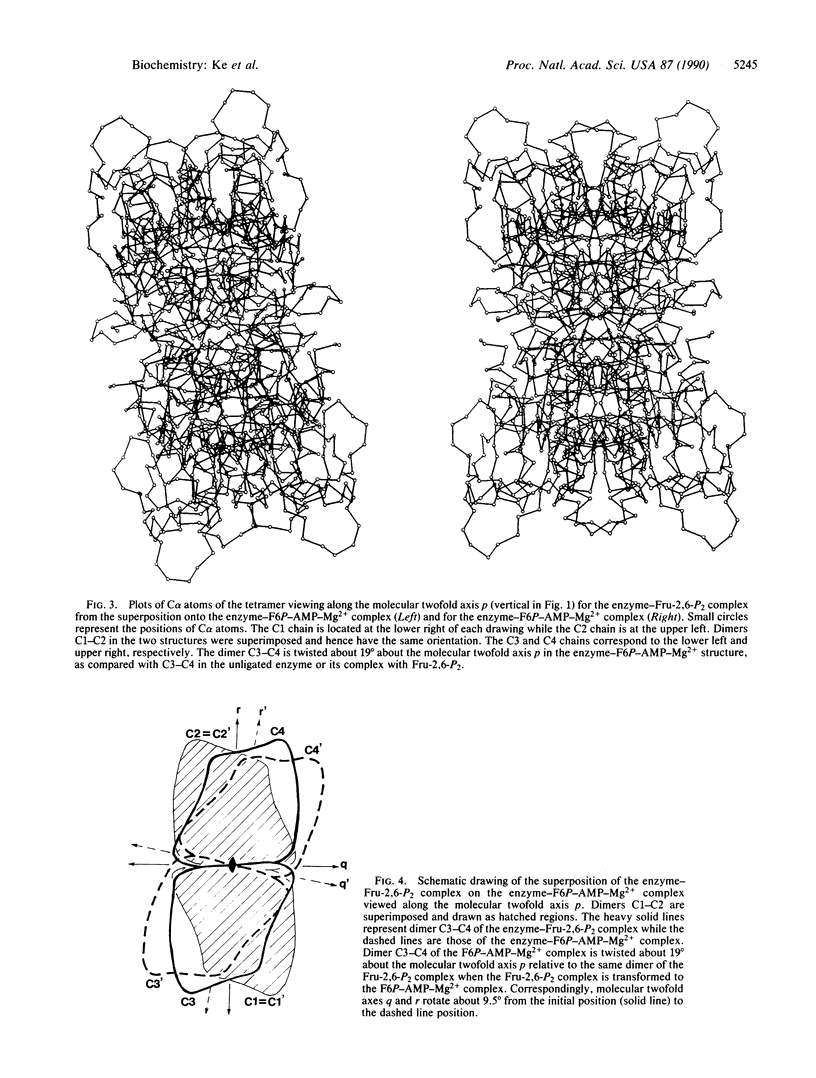
The crystal structure of fructose-1,6-bisphosphatase (EC 3.1.3.11) complexed with fructose 6-phosphate, AMP, and Mg2+ has been solved by the molecular replacement method and refined at 2.5-A resolution to a R factor of 0.215, with root-mean-square deviations of 0.013 A and 3.5 degrees for bond lengths and bond angles, respectively. No solvent molecules have been included in the refinement. This structure shows large quaternary and tertiary conformational changes from the structures of the unligated enzyme or its fructose 2,6-bisphosphate complex, but the secondary structures remain essentially the same. Dimer C3-C4 of the enzyme-fructose 6-phosphate-AMP-Mg2+ complex twists about 19 degrees relative to the same dimer of the enzyme-fructose 2,6-bisphosphate complex if their C1-C2 dimers are superimposed on one another. Nevertheless, many interfacial interactions between dimers of C1-C2 and C3-C4 are conserved after quaternary structure changes occur. Residues of the AMP domain (residues 6-200) show large migrations of C alpha atoms relative to barely significant positional changes of the FBP domain (residues 201-335).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arneson R. M., Geller A. M., Byrne W. L. Binding of adenosine 5'-monophosphate to bovine liver fructose 1,6-bisphosphatase. Enzyme. 1979;24(2):132–136. doi: 10.1159/000458641. [DOI] [PubMed] [Google Scholar]
- Benkovic P. A., Frey W. A., Benkovic S. J. The binding of products, metal ion, and a substrate analog to rabbit liver fructose bisphosphatase. Arch Biochem Biophys. 1978 Dec;191(2):719–726. doi: 10.1016/0003-9861(78)90412-5. [DOI] [PubMed] [Google Scholar]
- Benkovic S. J., deMaine M. M. Mechanism of action of fructose 1,6-bisphosphatase. Adv Enzymol Relat Areas Mol Biol. 1982;53:45–82. doi: 10.1002/9780470122983.ch2. [DOI] [PubMed] [Google Scholar]
- Black W. J., Van Tol A., Fernando J., Horecker B. L. Isolation of ahighly active fructose diphosphatase from rabit muscle: its subunit structure and activation by monovalent cations. Arch Biochem Biophys. 1972 Aug;151(2):576–590. doi: 10.1016/0003-9861(72)90535-8. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Chatterjee T., Reardon I., Heinrikson R. L., Marcus F. Des-1-25-fructose-1,6-bisphosphatase, a nonallosteric derivative produced by trypsin treatment of the native protein. J Biol Chem. 1985 Nov 5;260(25):13553–13559. [PubMed] [Google Scholar]
- Cruz Z. M., Tanizaki M. M., El-Dorry H. A., Bacila M. On the nucleotide binding domain of fructose-1,6-bisphosphatase. Arch Biochem Biophys. 1979 Dec;198(2):424–433. doi: 10.1016/0003-9861(79)90516-2. [DOI] [PubMed] [Google Scholar]
- El-Dorry H. A., Chu D. K., Dzugaj A., Botelho L. H., Pontremoli S., Horecker B. L. Primary structure of the S-peptide formed by digestion of rabbit liver fructose 1,6-biphosphatase with subtilisin. Arch Biochem Biophys. 1977 Aug;182(2):763–773. doi: 10.1016/0003-9861(77)90558-6. [DOI] [PubMed] [Google Scholar]
- Fisher W. K., Thompson E. O. Amino acid sequence studies on sheep liver fructose-bisphosphatase. II. The complete sequence. Aust J Biol Sci. 1983;36(3):235–250. doi: 10.1071/bi9830235. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., Harrison D. A., Dyer T. A. Sequence of the Escherichia coli fructose-1,6-bisphosphatase gene. Nucleic Acids Res. 1988 Sep 12;16(17):8707–8707. doi: 10.1093/nar/16.17.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H. M., Thorpe C. M., Seaton B. a., Lipscomb W. N., Marcus F. Structure refinement of fructose-1,6-bisphosphatase and its fructose 2,6-bisphosphate complex at 2.8 A resolution. J Mol Biol. 1990 Apr 5;212(3):513–539. doi: 10.1016/0022-2836(90)90329-k. [DOI] [PubMed] [Google Scholar]
- Ke H., Thorpe C. M., Seaton B. A., Marcus F., Lipscomb W. N. Molecular structure of fructose-1,6-bisphosphatase at 2.8-A resolution. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1475–1479. doi: 10.1073/pnas.86.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratowich N., Mendicino J. Role of enzyme interactions in the regulation of gluconeogenesis. Modification of the binding properties of fructose 1,6-diphosphatase by adenosine monophosphate, adenosine triphosphate, and fructose 1,6-diphosphate. J Biol Chem. 1974 Sep 10;249(17):5485–5494. [PubMed] [Google Scholar]
- MacGregor J. S., Hannappel E., Xu G. J., Pontremoli S., Horecker B. L. Conservation of primary structure at the proteinase-sensitive site of fructose 1,6-bisphosphatases. Arch Biochem Biophys. 1982 Sep;217(2):652–664. doi: 10.1016/0003-9861(82)90547-1. [DOI] [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Reardon I., Heinrikson R. L. Complete amino acid sequence of pig kidney fructose-1,6-bisphosphatase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7161–7165. doi: 10.1073/pnas.79.23.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Saidel L. J., Keim P. S., Heinrikson R. L. The covalent structure of pig kidney fructose 1,6-bisphosphatase: sequence of the 60-residue NH2-terminal peptide produced by digestion with subtilisin. Arch Biochem Biophys. 1981 Jul;209(2):687–696. doi: 10.1016/0003-9861(81)90330-1. [DOI] [PubMed] [Google Scholar]
- Marcus F., Haley B. E. Inhibition of fructose-1,6-biphosphatase by the photoaffinity AMP analog, 8-azidoadenosine 5'-monophosphate. J Biol Chem. 1979 Jan 25;254(2):259–261. [PubMed] [Google Scholar]
- Marcus F., Harrsch P. B. Amino acid sequence of spinach chloroplast fructose-1,6-bisphosphatase. Arch Biochem Biophys. 1990 May 15;279(1):151–157. doi: 10.1016/0003-9861(90)90475-e. [DOI] [PubMed] [Google Scholar]
- Marcus F., Harrsch P. B., Moberly L., Edelstein I., Latshaw S. P. Spinach chloroplast fructose-1,6-bisphosphatase: identification of the subtilisin-sensitive region and of conserved histidines. Biochemistry. 1987 Nov 3;26(22):7029–7035. doi: 10.1021/bi00396a026. [DOI] [PubMed] [Google Scholar]
- Marcus F., Rittenhouse J., Gontero B., Harrsch P. B. Function, structure and evolution of fructose-1,6-bisphosphatase. Arch Biol Med Exp (Santiago) 1987;20(3-4):371–378. [PubMed] [Google Scholar]
- Marcus F., Rittenhouse J., Moberly L., Edelstein I., Hiller E., Rogers D. T. Yeast (Saccharomyces cerevisiae) fructose-1,6-bisphosphatase. Properties of phospho and dephospho forms and of two mutants in which serine 11 has been changed by site-directed mutagenesis. J Biol Chem. 1988 May 5;263(13):6058–6062. [PubMed] [Google Scholar]
- McGrane M. M., El-Maghrabi M. R., Pilkis S. J. The interaction of fructose 2,6-bisphosphate and AMP with rat hepatic fructose 1,6-bisphosphatase. J Biol Chem. 1983 Sep 10;258(17):10445–10454. [PubMed] [Google Scholar]
- Meek D. W., Nimmo H. G. The interaction of fructose 2,6-bisphosphate with an allosteric site of rat liver fructose 1,6-bisphosphatase. FEBS Lett. 1983 Aug 22;160(1-2):105–109. doi: 10.1016/0014-5793(83)80946-6. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Higgins S. J., Thornton S. D., Start C. The activities of fructose diphosphatase in flight muscles from the bumble-bee and the role of this enzyme in heat generation. Biochem J. 1972 Jun;128(1):89–97. doi: 10.1042/bj1280089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Tipton K. F. The allosteric properties of beef-liver fructose bisphosphatase. Eur J Biochem. 1975 Oct 15;58(2):575–585. doi: 10.1111/j.1432-1033.1975.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., McGrane M. M., Pilkis J., Claus T. H. The role of fructose 2,6-bisphosphate in regulation of fructose-1,6-bisphosphatase. J Biol Chem. 1981 Nov 25;256(22):11489–11495. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. J Biol Chem. 1981 Apr 25;256(8):3619–3622. [PubMed] [Google Scholar]
- Pontremoli S., Grazi E., Accorsi A. Fructose diphosphatase from rabbit liver. X. Isolation and kinetic properties of the enzyme--adenosine monophosphate complex. Biochemistry. 1968 Oct;7(10):3628–3633. doi: 10.1021/bi00850a041. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., De Flora A., Horecker B. L. Conversion of neutral to alkaline liver fructose 1,6-bisphosphatase: changes in molecular properties of the enzyme. Proc Natl Acad Sci U S A. 1973 Mar;70(3):661–664. doi: 10.1073/pnas.70.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Traniello S. Conversion of "neutral" to "alkaline" fructose 1,6-diphosphatase by controlled digestion with papain. Arch Biochem Biophys. 1971 Dec;147(2):762–766. doi: 10.1016/0003-9861(71)90436-x. [DOI] [PubMed] [Google Scholar]
- Preiss J., Biggs M. L., Greenberg E. The effect of magnesium ion concentration on the pH optimum of the spinach leaf alkaline fructose diphosphatase. J Biol Chem. 1967 May 10;242(9):2292–2294. [PubMed] [Google Scholar]
- Raines C. A., Lloyd J. C., Longstaff M., Bradley D., Dyer T. Chloroplast fructose-1,6-bisphosphatase: the product of a mosaic gene. Nucleic Acids Res. 1988 Aug 25;16(16):7931–7942. doi: 10.1093/nar/16.16.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. T., Hiller E., Mitsock L., Orr E. Characterization of the gene for fructose-1,6-bisphosphatase from Saccharomyces cerevisiae and Schizosaccharomyces pombe. Sequence, protein homology, and expression during growth on glucose. J Biol Chem. 1988 May 5;263(13):6051–6057. [PubMed] [Google Scholar]
- Sarngadharan M. G., Watanabe A., Pogell B. M. Binding of adenosine 5'-monophosphate and substrate by rabbit liver fructose 1,6-diphosphatase. Biochemistry. 1969 Apr;8(4):1411–1419. doi: 10.1021/bi00832a016. [DOI] [PubMed] [Google Scholar]
- Suda H., Xu G. J., Kutny R. M., Natalini P., Pontremoli S., Horecker B. L. Location of lysyl residues at the allosteric site of fructose 1,6-bisphosphatase. Arch Biochem Biophys. 1982 Aug;217(1):10–14. doi: 10.1016/0003-9861(82)90473-8. [DOI] [PubMed] [Google Scholar]
- TAKETA K., POGELL B. M. ALLOSTERIC INHIBITION OF RAT LIVER FRUCTOSE 1,6-DIPHOSPHATASE BY ADENOSINE 5'-MONOPHOSPHATE. J Biol Chem. 1965 Feb;240:651–662. [PubMed] [Google Scholar]
- Tejwani G. A., Pedrosa F. O., Pontremoli S., Horecker B. L. The purification of properties of rat liver fructose 1,6-bisphosphatase. Arch Biochem Biophys. 1976 Nov;177(1):253–264. doi: 10.1016/0003-9861(76)90435-5. [DOI] [PubMed] [Google Scholar]
- Tejwani G. A. Regulation of fructose-bisphosphatase activity. Adv Enzymol Relat Areas Mol Biol. 1983;54:121–194. doi: 10.1002/9780470122990.ch3. [DOI] [PubMed] [Google Scholar]
- Traniello S., Melloni E., Pontremoli S., Sia C. L., Horecker R. L. Rabbit liver fructose 1,6-diphosphatase. Properties of the native enzyme and their modification by subtilisin. Arch Biochem Biophys. 1972 Mar;149(1):222–231. doi: 10.1016/0003-9861(72)90317-7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981 May;78(5):2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalitis J. Modification of native sheep liver fructose-1,6-bisphosphatase by subtilisin. Biochem Biophys Res Commun. 1976 May 17;70(2):323–330. doi: 10.1016/0006-291x(76)91049-4. [DOI] [PubMed] [Google Scholar]
- el-Maghrabi M. R., Pilkis J., Marker A. J., Colosia A. D., D'Angelo G., Fraser B. A., Pilkis S. J. cDNA sequence of rat liver fructose-1,6-bisphosphatase and evidence for down-regulation of its mRNA by insulin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8430–8434. doi: 10.1073/pnas.85.22.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]