Abstract
Many breast cancer survivors have to deal with a variety of psychological and physiological sequelae including impaired immune responses. The primary purpose of this randomized controlled trial was to determine the efficacy of a mindfulness‐based stress reduction (MBSR) intervention for mood disorders in women with breast cancer. Secondary outcomes were symptom experience, health status, coping capacity, mindfulness, posttraumatic growth, and immune status. This RTC assigned 166 women with breast cancer to one of three groups: MBSR (8 weekly group sessions of MBSR), active controls (self‐instructing MBSR) and non‐MBSR. The primary outcome measure was the Hospital Anxiety and Depression Scale. Secondary outcome measures were: Memorial Symptom Assessment Scale, SF‐36, Sense of Coherence, Five Facets of Mindfulness Questionnaire, and Posttraumatic Growth Index. Blood samples were analyzed using flow cytometry for NK‐cell activity (FANKIA) and lymphocyte phenotyping; concentrations of cytokines were determined in sera using commercial high sensitivity IL‐6 and IL‐8 ELISA (enzyme‐linked immunosorbent assay) kits. Results provide evidence for beneficial effects of MBSR on psychological and biological responses. Women in the MBSR group experienced significant improvements in depression scores, with a mean pre‐MBSR HAD‐score of 4.3 and post‐MBSR score of 3.3 (P = 0.001), and compared to non‐MBSR (P = 0.015). Significant improvements on scores for distress, symptom burden, and mental health were also observed. Furthermore, MBSR facilitated coping capacity as well as mindfulness and posttraumatic growth. Significant benefits in immune response within the MBSR group and between groups were observed. MBSR have potential for alleviating depression, symptom experience, and for enhancing coping capacity, mindfulness and posttraumatic growth, which may improve breast cancer survivorship. MBSR also led to beneficial effect on immune function; the clinical implications of this finding merit further research.
Keywords: Breast cancer, immune response, mindfulness‐based stress reduction, randomized clinical trial
Introduction
Although there have been enormous improvements in breast cancer diagnosis and advances in treatment, less attention has been paid to alleviating patients' breast cancer experience by preserving their physical, functional, and psychosocial well‐being 1. Women with breast cancer are challenged to cope over time with a high symptom burden and distress, which affects their well‐being and quality of life 2, 3, 4, 5, 6, 7. The prevalence of mood disorders is highest in the first year after breast cancer diagnosis and then decreases gradually over time 8.
Still, individuals report persistent coexistent physical and psychological symptoms that contribute to interference with daily life after breast cancer treatment 9.
An increasing body of research has established an association between distress and changes in immune function 10, 11. Distress seems to have a significant negative effect on immune function, such as lowered natural killer cells (NK cells) and T lymphocytes (T cells) 11, 12. T cells have been linked to breast cancer recurrence and survival 13, 14, 15. Other important parameters are cytokines, such as interleukin‐6 (IL‐6) and interleukin‐8 (IL‐8), which are independently correlated with breast cancer disease stage and progression 16, 17, 18, 19.
Thus, previous research indicates a significant need for interventions to improve well‐being, alleviate distress and symptom burden, and to reinforce immunity in women during breast cancer diagnosis, treatment, and recovery.
Originating from ancient Buddhist and yoga traditions, mindfulness‐based interventions have become increasingly popular in the Western world. Mindfulness is described as a “way of being” and defined as the capacity for awareness in each moment, by “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally”20. The use of mindfulness‐based interventions in oncology has proliferated over the past decade and research in the field has rapidly expanded 21, 22 While there appears to be evidence to support the use of mindfulness‐based interventions with cancer patients, the overall quality of existing trials varies considerably 23.
Mindfulness‐based stress reduction (MBSR) is an 8‐week, standardized program combining mindfulness meditation, yoga and other techniques designed to reduce stress and improve well‐being and quality of life in patients with a wide range of chronic pain and stress disorders 20, 24, 25. MBSR and has also been shown to improve mood disorders 21, 26and reduce stress in cancer patients 27, 28. Furthermore, MBSR reduces fear of recurrence and improves physical functioning which in turn leads to reduced stress and anxiety in women with breast cancer 29. Evidence from nonrandomized, uncontrolled studies suggests that MBSR improves quality of life and coping, decreases stress and alters cortisol and immune patterns 30, 31, 32, 33.
These results raise important questions as to whether MBSR is related to positive outcomes in mood disorders, symptom burden and health status, as well as strengthened immune system functioning in breast cancer survivors. Despite growing evidence that MBSR influences immune function, there is a need for studies to determine how biomarkers relate to changes in mindfulness and psychosocial outcomes, including symptom reduction and well‐being 34. While noting the effectiveness of MBSR, authors of several reviews have pointed out the inherent methodological problems in the published studies 35, 36. There is also a need for randomized controlled studies with long‐term follow‐up 36.
The primary purpose of our study was to determine the efficacy of MBSR intervention for mood disorder symptom improvements in women with breast cancer. Secondary goals were to evaluate their symptom experience, distress, health status, coping capacity, mindfulness, posttraumatic growth, and immune status.
Patients and Methods
Study design
In this 3‐month follow‐up study, we present the first results of a 5‐year longitudinal, randomized, controlled trial (RTC). Details of this trial have been described elsewhere 37.
The trial was designed in accord with Consort recommendation 38, 39, 40. In an unblinded RTC, participants' expectations about the intervention may lead to a placebo effect in the intervention group and/or a negative response among controls. In order to minimize a potential placebo effect in the active intervention group and a “frustrebo response”41 in controls, a three‐armed design was chosen.
Patients diagnosed with breast cancer were consecutively recruited to participate after completion of adjuvant chemotherapy and/or radiation therapy, with or without endocrine therapy. Patients were excluded on the basis of having another advanced illness at diagnosis that might interfere with the ability to participate, ongoing major depression, ongoing Herceptin therapy, or who had previously, as well as during the intervention, used MBSR and other mind‐body programs (including yoga).
This trial was approved by the Regional Ethical Review Board, University of Gothenburg, and informed consent was obtained before enrolment.
Procedures
Eligible patients were contacted by research nurses at the first follow‐up appointment for patients receiving hormonal therapy or at the last treatment for patients undergoing chemotherapy. After oral and written information, interested patients provided written consent to participate in the study. Participants were first invited to a baseline health check‐up appointment, which included blood sample collection and questionnaire completion. Randomization was computerized and conducted in blocks of 9, 12, and 15, varied randomly. Assignment codes were kept in sequentially numbered, opaque, sealed envelopes, prepared by the research coordinator.
Intervention
Participants were randomized into one of three groups:
MBSR (8 weeks self‐instructing MBSR program + instructor and weekly group sessions), active controls (8 weeks self‐instructing MBSR program) or non‐MBSR (no intervention).
Participants in the MBSR group attended a standardized, group‐based, 8‐week course once a week for an average of 2 h each week with homework assignments consisting of 20 min sessions, 6 days/week. Participants were provided with information material, including a 20‐page introduction to mindfulness training, a compacted disk (CD) with meditation exercises, the training program and a diary in order to report the time allotted to mindfulness training including patients′ reflections about the meditation exercises. Led by a certified MBSR instructor, these weekly group sessions focused on the participants' experiences of mindfulness, and including gentle meditation and yoga training. Active controls received information material, a CD, 8 weeks of self‐instructing training program and a diary. The only difference between MBSR group and active controls was the weekly group sessions. Participants in both MBSR group and active controls were provided with written and oral instructions how to use information material, CD and diary. All participants received standard care for follow‐up for breast cancer according to the national and local guidelines 42.
Measures
Socio‐demographic data were collected through chart review and interviews. Clinical characteristics, patient self‐reported outcomes and biomarkers were collected at health checks both pre and postintervention. Follow‐ups for MBSR group and active controls were conducted 1 month after the intervention, and at similar time points. The same procedures, at similar time points of 3 months were conducted for those in the non‐MBSR group.
Primary outcome measures
Mood disorder
Mood disorder was measured using the Hospital Anxiety and Depression scale (HAD), which is one of most widely used instruments to screen for anxiety and depression in cancer patients 43, 44, 45. The HAD is a 14‐item questionnaire consisting of two subscales: anxiety and depression. Subscale scores range from 0 to 21; scores for each subscale are defined as: 0–7 (normal), 8–10 (possible cases), and 11–21 (cases of psychological morbidity) 46. The internal consistency of reliability for both subscales are satisfactory, with Cronbach's alpha 0.72–0.89, respectively, 0.78–0.93 45
Secondary outcomes measures
Symptom experience
Symptom experience was evaluated using the Memorial Symptom Assessment Scale (MSAS), a questionnaire consisting of 32 symptoms and symptom frequency, severity, and distress 47. The MSAS generates two subscales including physical and psychological symptoms, and two global indicators: Total Symptom Burden Scale (TMSAS) and the Global Symptom Distress Index (GDI). The MSAS is a reliable and valid multidimensional measure of symptom experience in cancer populations 47, 48 including the Swedish version of the MSAS 49.
Health status
Health status was measured using the 36‐item Short Form Health Survey (SF‐36), which consists of eight scaled scores: vitality, physical functioning, bodily pain, general health perceptions, physical, emotional and social role functioning, and mental health. The maximum score is 100 points. Reliability measurements of the SF‐36 are consistently good 50, 51, 52, 53.
Coping capacity
Coping capacity was evaluated using the Sense of Coherence scale (SOC) 54, 55, which consists of a 7‐point Likert scale evaluating perceived comprehensibility (5 items), manageability (4 items) and meaningfulness (4 items). Higher scores represent stronger sense of coherence. Reliability and validity of the SOC scale have been demonstrated, with Cronbach's α ranging from 0.74 to 0.93 56, 57, 58.
Mindfulness
Mindfulness was measured using the 29‐item short form Five Facets of Mindfulness Questionnaire (FFMQ–Swedish version), consisting of five key facets of mindfulness: observing, describing, acting with awareness, nonjudging, and nonreactivity to inner experience 59, 60.
Personal growth
Personal growth was evaluated using the Posttraumatic Growth Inventory (PTGI), which measures positive life changes after traumatic events. The PTGI yields a total score based on five dimensions: relating to others, new possibilities, personal strength, spiritual change, and appreciation of life 61. The PTGI has shown good reliability in previous research with a total score Cronbach's α of 0.96 62.
Lymphocyte distribution in peripheral blood
Lymphocyte distribution in peripheral blood was analyzed by flow cytometry using a FACSCanto II flow cytometer and the FACSDiva software. The absolute number of blood lymphocytes was determined with Trucount reference beads using the method recommended by the manufacturer. The following subpopulations were reported CD3+, CD3+4+ and CD3+8+T cells, CD19+B cells, and CD3‐16+56+NK cells. The results for each subpopulation were expressed as the percentage of lymphocytes and as the number of cells × 109/L. Antibodies to the antigens above. Trucount beads, the FACSCanto II flow cytometer and the FACSDiva software were all from BD Biosciences, Mountain View, CA.
NK‐cell activity
NK‐cell activity was measured using a Flow‐cytometric Assay of Natural Killer cell Immune response in Activated whole blood (FANKIA) a modified version of a previously published method using flow cytometry and stained K562 cells as target cells 63. Whole blood was mixed with a defined number of target cells transfected with the gene for green fluorescent protein (GFP) 64. After incubation the same volume was collected from tubes with: blood and target cells, target cells and medium; and blood and medium and analyses were performed as described above. The lytic activity was defined as the reduction in the number of target cells after mixing with the blood, expressed in percentage of target cells.
Determination of cytokine concentrations
Determination of cytokine concentrations were determined in sera using commercial high sensitivity IL‐6 and IL‐8 ELISA kits (R&D Systems, Inc., Abingdon, UK) according to the instructions from the manufacturer.
Data analysis
Sample size calculation was based on the primary outcome: breast cancer patient's mood disorder symptoms. A one‐unit change on the HAD‐subscales from baseline to 3‐month follow‐up was regarded as clinically relevant. The detection of such a difference would require 50 participants per group (a total of 150 participants) to achieve a statistical power of 80%. Descriptive statistics were used to summarize socio‐demographic and clinical characteristics. Spearman's correlation coefficients were calculated to determine the strength of relationships between selected variables. As most of the variables we explored were of ordinal data type and most of the continuous variables deal with skewed distributions deviating from normal‐distribution, we used nonparametric tests (Wilcoxon's test for comparison within groups and Mann–Whitney's test for comparison between groups).
P < 0.05 was considered as statistically significant result.
Results
A total of 177 women consented participation and were randomly assigned to one of three groups. There were 11 drop‐outs after randomization, that is, two patients were excluded as they did not complete the intervention, two patients withdraw their participation due to rapid breast cancer disease progression, and seven patients did not visited first follow‐up (MBSR = 4; active controls = 5; non‐MBSR = 2).
The final groups were MBSR (n = 62), active controls (n = 52) and non‐MBSR (n = 52). Postintervention data were missing for one active control participant. A participation flowchart is depicted in Figure 1.
Figure 1.
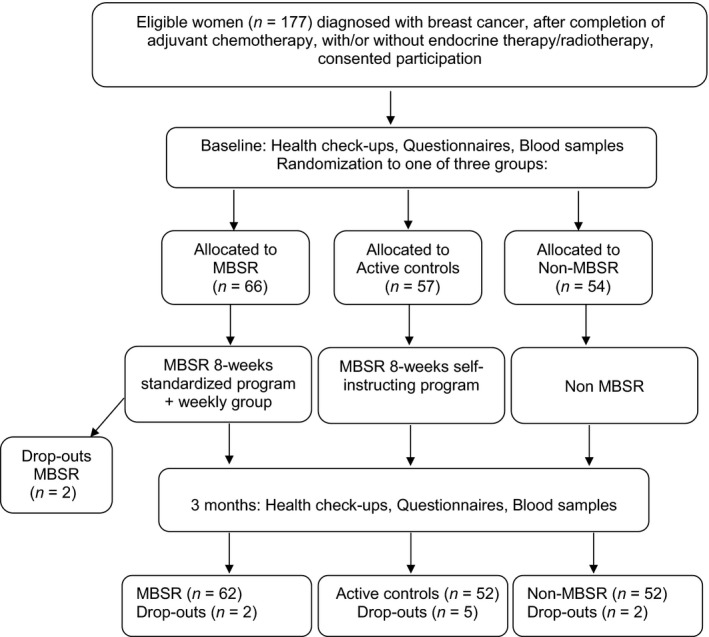
Flowchart of study design and randomization.
Participants' ranged from 34 to 80 years (mean = 57.2, SD = 10.2). No statistical differences were found between groups on demographic or clinical characteristics. Participant descriptions are listed in Table 1.
Table 1.
Baseline demographic data and clinical characteristics
| Characteristic | MBSR group (n = 62) | Active controls (n = 52) | Non‐MBSR (n = 52) | Total (n = 166) | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Marital status | ||||||||
| Married/cohabitation | 46 | 74.2 | 35 | 67.3 | 42 | 80.8 | 123 | 74.1 |
| Widowed | 3 | 4.8 | 3 | 5.8 | 4 | 7.7 | 10 | 6.0 |
| Divorced | 6 | 9.7 | 8 | 15.4 | 1 | 1.9 | 15 | 9.0 |
| Single | 5 | 8.1 | 4 | 7.7 | 4 | 7.7 | 13 | 7.8 |
| Partner without l.t.a | 2 | 3.2 | 2 | 3.8 | 1 | 1.9 | 5 | 3.0 |
| Living with | ||||||||
| Partner | 44 | 71.0 | 35 | 67.3 | 41 | 78.8 | 120 | 72.3 |
| Other | 1 | 1.6 | 0 | 0 | 1 | 1.9 | 2 | 1.2 |
| Children | 7 | 11.3 | 2 | 3.8 | 2 | 3.8 | 11 | 6.6 |
| Living alone | 10 | 16.1 | 15 | 28.8 | 8 | 15.4 | 33 | 19.9 |
| Education | ||||||||
| Primary school | 5 | 8.1 | 1 | 1.9 | 1 | 1.9 | 7 | 4.2 |
| Secondary school | 14 | 22.6 | 10 | 19.2 | 11 | 21.2 | 35 | 21.1 |
| Add education–lowerb | 6 | 9.7 | 6 | 11.5 | 10 | 19.2 | 22 | 13.3 |
| Add education– higherc | 7 | 11.3 | 11 | 21.2 | 5 | 9.6 | 23 | 13.9 |
| University | 30 | 48.4 | 24 | 46.2 | 25 | 48.1 | 79 | 47.6 |
| Children | ||||||||
| Yes | 57 | 91.9 | 49 | 94.2 | 45 | 86.5 | 151 | 91.0 |
| No | 5 | 8.1 | 3 | 5.8 | 7 | 13.5 | 15 | 9.0 |
| Employment status | ||||||||
| Working | 38 | 63.3 | 38 | 74.5 | 35 | 68.6 | 111 | 68.5 |
| Unemployed | 1 | 1.7 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Disability pensioner | 2 | 3.3 | 3 | 5.9 | 1 | 2.0 | 6 | 3.7 |
| Retired | 18 | 30.0 | 10 | 19.6 | 15 | 29.4 | 43 | 26.5 |
| Other | 1 | 1.7 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Surgery | ||||||||
| Mastectomy | 30 | 48.4 | 26 | 50.0 | 21 | 40.4 | 77 | 46.4 |
| Lumpectomy | 33* | 53.2 | 26 | 50.0 | 30 | 57.7 | 89 | 53.6 |
| Other | 0 | 0 | 0 | 0 | 1 | 1.9 | 1 | 0.6 |
| Tumor size | ||||||||
| <2 cm | 28 | 45.9 | 25 | 49.0 | 32 | 64.0 | 85 | 52.5 |
| 2–5 cm | 25 | 41.0 | 22 | 43.1 | 8 | 16.0 | 55 | 34.0 |
| >5 cm | 8 | 13.1 | 4 | 7.8 | 10 | 20.0 | 22 | 13.6 |
| Type of cancer | ||||||||
| Ductal | 43 | 69.4 | 37 | 71.2 | 40 | 76.9 | 120 | 72.3 |
| Lobular | 13 | 21.0 | 9 | 17.3 | 6 | 11.5 | 28 | 16.9 |
| Other | 6 | 9.7 | 6 | 11.5 | 6 | 11.5 | 18 | 10.8 |
| Receptor | ||||||||
| ER+/PgR+ | 40 | 69.0 | 34 | 72.3 | 39 | 78.0 | 113 | 72.9 |
| ER+/PgR‐ | 8 | 13.8 | 6 | 12.8 | 4 | 8.0 | 17 | 11.6 |
| ER‐/PgR+ | 0 | 0.0 | 0 | 0.0 | 1 | 2.0 | 1 | 0.6 |
| ER‐/PgR‐ | 10 | 17.2 | 7 | 14.9 | 6 | 12.0 | 24 | 14.8 |
| BRE (mean) | 6.6 | 6.5 | 6.6 | |||||
| Treatment postop | ||||||||
| Chemotherapy (CT) | 2 | 3.2 | 3 | 5.8 | 3 | 5.8 | ||
| Radiotherapy (RT) | 3 | 4.8 | 5 | 9.6 | 4 | 7.7 | ||
| Hormonal therapy (HT) | 12 | 19.4 | 14 | 26.9 | 6 | 11.5 | ||
| CT+RT | 11 | 17.8 | 6 | 11.5 | 7 | 13.5 | ||
| CT+ HT | 4 | 6.5 | 0 | 0 | 1 | 1.9 | ||
| RT+ HT | 16 | 25.8 | 13 | 25.0 | 16 | 30.8 | ||
| CT+RT+HT | 13 | 20.9 | 10 | 19.2 | 14 | 26.9 | ||
| No treatment | 1 | 1.6 | 1 | 1.9 | 1 | 1.9 | ||
ER, estrogen receptor; PgR, progesterone receptor.
Partner, not living together with.
Lower additional education.
Higher additional education.
Psychological response
Study results revealed significant changes in psychological and biological responses to the MBSR intervention, summarized in Tables 2, 3, 4, 5, 6, 7, 8.
Table 2.
Primary outcome: pre and postintervention mood disorder symptoms
| Measures HADa | Group | Preintervention | Postintervention | P‐valueb | P‐valuec | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (min‐max) | Mean (SD) | Median (min‐max) | ||||
| Anxiety | MBSR | 6.5 (4.3) | 6 (0–19) | 6.0 (3.9) | 6 (0–16) | 0.069 | 0.080 |
| Active Controls | 5.6 (3.9) | 4.5 (0–14) | 5.1 (3.9) | 5 (0–13) | 0.236 | 0.109 | |
| Non‐MBSR | 4.8 (3.6) | 4.5 (0–12) | 5.5 (4.1) | 5 (0–15) | 0.355 | Ref. | |
| Depression | MBSR | 4.3 (3.7) | 4 (0–14) | 3.3 (3.3) | 2 (0–12) | 0.001 a | 0.015 a |
| Active Controls | 3.4 (3.4) | 2 (0–14) | 3.0 (2.9) | 2 (0–12) | 0.292 | 0.472 | |
| Non‐MBSR | 3.5 (3.5) | 2 (0–14) | 3.8 (3.8) | 2 (0–15) | 0.892 | Ref. | |
Significant changes are marked in bold.
The Hospital Anxiety and Depression Scale (HAD).
Change over time (P‐value) within groups (Wilcoxon signed test).
Change over time (P‐value) between groups (comparison of MBSR‐Active controls vs. Non‐MBSR (ref.) (Mann–Whitney test).
Table 3.
Secondary outcome: symptom experience
| Measure MSASa | Group | Preintervention | Postintervention | P‐valueb | P‐valuec | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (min‐max) | Mean (SD) | Median (min‐max) | ||||
| Physical symptoms | MBSR | 0.7 (0.5) | 0.61 (0–2.30) | 0.6 (0.4) | 0.52 (0–2.15) | 0.007 a | 0.245 |
| Active controls | 0.5 (0.4) | 0.49 (0–1.45) | 0.5 (0.4) | 0.41 (0–2.13) | 0.372 | 0.966 | |
| Non‐MBSR | 0.6 (0.5) | 0.46 (0–2.11) | 0.5 (0.5) | 0.45 (0–2.34) | 0.475 | Ref. | |
| Psychological symptoms | MBSR | 1.4 (0.8) | 1.34 (0–3.22) | 1.2 (0.9) | 0.98 (0–3.32) | 0.008 a | 0.019 a |
| Active controls | 1.0 (0.9) | 0.77 (0–2.77) | 0.9 (0.8) | 0.69 (0–2.67) | 0.335 | 0.337 | |
| Non‐MBSR | 0.9 (0.8) | 0.76 (0–2.92) | 0.9 (0.8) | 0.83 (0–2.82) | 0.800 | Ref. | |
| Global distress | MBSR | 1.9 (0.6) | 1.93 (0–2.97) | 1.8 (0.6) | 1.80 (0–3.05) | 0.054 | 0.013 a |
| Active controls | 1.7 (0.8) | 1.80 (0–3.24) | 1.6 (0.8) | 1.60 (0–3.20) | 0.121 | 0.015 a | |
| Non‐MBSR | 1.6 (0.8) | 1.79 (0–4.0) | 1.7 (0.9) | 2.0 (0–3.2) | 0.103 | Ref. | |
| Total symptom burden | MBSR | 0.8 (0.5) | 0.71 (0–2.13) | 0.7 (0.5) | 0.55 (0–2.22) | 0.004 a | 0.097 |
| Active controls | 0.6 (0.4) | 0.56 (0–1.52) | 0.5 (0.3) | 0.49 (0–1.5) | 0.113 | 0.671 | |
| Non‐MBSR | 0.6 (0.4) | 0.54 (0–1.59) | 0.6 (0.4) | 0.56 (0.05–2.0) | 0.379 | Ref. | |
Significant changes are marked in bold.
MSAS, Memorial Symptom Assessment Scale.
Change over time (P‐value) within groups (Wilcoxon signed test).
Change over time (P‐value) between groups (comparison of MBSR‐Active controls vs. Non‐MBSR (ref.) (Mann–Whitney test).
Table 4.
Secondary outcome: health status
| Measure SF‐36a | Group | Preintervention | Postintervention | P‐valueb | P‐valuec | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (min‐max) | Mean (SD) | Median (min‐max) | ||||
| Vitality | MBSR | 49.5 (27.5) | 52.5 (29–75) | 60.9 (20.1) | 65 (45–76) | 0.000 a | 0.159 |
| Active Controls | 56.9 (24.4) | 60 (40–75) | 62.5 (23.7) | 65 (45–80) | 0.129 | 0.838 | |
| Non‐MBSR | 55.0 (25.8) | 55 (40–80) | 58.3 (24.4) | 60 (35–80) | 0.397 | Ref. | |
| Physical functioning | MBSR | 77.4 (16.7) | 80 (67.5–90) | 83.1 (15.4) | 90 (75–95) | 0.000 a | 0.822 |
| Active Controls | 83.7 (17.3) | 90 (75–95) | 84.8 (18.5) | 95 (80–95) | 0.412 | 0.129 | |
| Non‐MBSR | 78.5 (19.6) | 80 (70–95) | 82.8 (19.0) | 90 (71.0–95) | 0.007 a | Ref. | |
| Bodily pain | MBSR | 65.2 (26.4) | 67 (45–90) | 71.4 (23.5) | 77.5 (45–92) | 0.099 | 0.799 |
| Active Controls | 70.9 (20.7) | 77.5 (55–90) | 74.4 (25.2) | 79.5 (57–100) | 0.466 | 0.526 | |
| Non‐MBSR | 70 (23.1) | 67 (56–90) | 73.5 (27.1) | 85 (56–100) | 0.253 | Ref. | |
| General health perceptions | MBSR | 61.2 (21.6) | 60 (45–75) | 67.6 (19.1) | 70 (52–85) | 0.003 a | 0.087 |
| Active Controls | 67.2 (19.3) | 75 (55–80) | 70.6 (19.1) | 70 (60–85) | 0.111 | 0.465 | |
| Non‐MBSR | 69.3 (19.4) | 70 (60–85) | 69.6 (19.6) | 75 (55–80) | 0.832 | Ref. | |
| Physical role functioning | MBSR | 45.9 (42.9) | 50 (0–100) | 63.3 (39.7) | 75 (25–100) | 0.003 a | 0.822 |
| Active Controls | 42.2 (42.6) | 25 (0–100) | 67.6 (35.8) | 75 (50–100) | 0.001 a | 0.463 | |
| Non‐MBSR | 40.4 (41.5) | 25 (0–75) | 59.6 (42.3) | 75 (6–100) | 0.006 a | Ref. | |
| Emotional role functioning | MBSR | 61.7 (41.2) | 67 (33–100) | 72.1 (38.6) | 100 (33–100) | 0.053 | 0.900 |
| Active Controls | 69.9 (38.5) | 100 (33–100) | 77.1 (35.6) | 100 (67–100) | 0.168 | 0.652 | |
| Non‐MBSR | 60.9 (47.0) | 100 (0–100) | 72.4 (37.2) | 100 (33–100) | 0.093 | Ref. | |
| Social functioning | MBSR | 74.0 (23.0) | 75 (62–100) | 79.0 (20.7) | 87.5 (62–100) | 0.103 | 0.117 |
| Active Controls | 78.4 (25.9) | 87.5 (62–100) | 84.8 (22.8) | 100 (62–100) | 0.017 a | 0.105 | |
| Non‐MBSR | 72.1 (26.0) | 75 (50–100) | 84.1 (25.3) | 100 (75–100) | 0.001 a | Ref. | |
| Mental health | MBSR | 67.9 (19.0) | 72 (56–80) | 74.1 (17.1) | 76 (64–85) | 0.000 a | 0.001 a |
| Active Controls | 73.5 (22.7) | 80 (60–92) | 77.8 (17.4) | 80 (64–92) | 0.073 | 0.038 a | |
| Non‐MBSR | 76.2 (20.0) | 84 (64–88) | 74.4 (20.7) | 84 (60–92) | 0.299 | Ref. | |
Significant changes are marked in bold.
36‐items short form health survey (SF‐36).
Change over time (P‐value) within groups (Wilcoxon signed test).
Change over time (P‐value) between groups (comparison of MBSR‐Active controls vs. Non‐MBSR (ref.) (Mann–Whitney test).
Table 5.
Secondary outcome: coping capacity
| Measure SoCa | Group | Preintervention | Postintervention | P‐valueb | P‐valuec | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (min‐max) | Mean (SD) | Median (min‐max) | ||||
| Coping capacity | MBSR | 65.7 (13.7) | 67 (32–91) | 67.7 (12.0) | 66.5 (39–91) | 0.055 | 0.028 a |
| Active controls | 69.8 (13.7) | 71 (28–90) | 70.8 (12.5) | 75 (41–90) | 0.458 | 0.098 | |
| Non‐MBSR | 71.4 (11.1) | 72 (39–88) | 69.3 (11.5) | 69 (45–87) | 0.113 | Ref. | |
Significant changes are marked in bold.
SoC, Sense of Coherence.
Change over time (P‐value) within groups (Wilcoxon signed test).
Change over time (P‐value) between groups (comparison of MBSR‐Active controls vs. Non‐MBSR (ref.) (Mann–Whitney test).
Table 6.
Secondary outcome: facets of mindfulness
| Measure FFMQa | Group | Preintervention | Postintervention | P‐valueb | P‐valuec | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (min‐max) | Mean (SD) | Median (min‐max) | ||||
| Nonreactivity | MBSR | 2.9 (0.7) | 3.0 (1.3–4.5) | 3.3 (0.5) | 3.2 (2.0–4.7) | 0.000 a | 0.010 a |
| Active Controls | 3.2 (0.6) | 3.2 (1.8–4.3) | 3.3 (0.5) | 3.3 (1.8–4.3) | 0.318 | 0.759 | |
| Non‐MBSR | 3.1 (0.6) | 3.2 (1.0–4.3) | 3.2 (0.7) | 3.2 (1.0–4.3) | 0.552 | Ref. | |
| Observe | MBSR | 3.3 (0.7) | 3.3 (1.0–4.7) | 3.6 (0.5) | 3.7 (1.7–4.9) | 0.000 a | 0.006 a |
| Active Controls | 3.3 (0.7) | 3.3 (1.4–4.9) | 3.4 (0.7) | 3.4 (1.4–5.0) | 0.015 a | 0.376 | |
| Non‐MBSR | 3.1 (0.7) | 3.1 (1.6–4.1) | 3.2 (0.7) | 3.3 (1.0–4.6) | 0.190 | Ref. | |
| Awareness | MBSR | 3.3 (0.8) | 3.4 (1.8–5.0) | 3.3 (0.6) | 3.2 (2.0–5.0) | 0.751 | 0.783 |
| Active Controls | 3.5 (0.8) | 3.6 (1.8–5.0) | 3.4 (0.7) | 3.4 (2.0–5.0) | 0.280 | 0.224 | |
| Non‐MBSR | 3.4 (0.7) | 3.3 (1.8–4.8) | 3.4 (0.8) | 3.3 (2.0–5.0) | 0.984 | Ref. | |
| Describe | MBSR | 3.6 (0.7) | 3.7 (2.0–5.0) | 3.6 (0.6) | 3.8 (2.0–4.7) | 0.599 | 0.478 |
| Active Controls | 3.6 (0.6) | 3.7 (2.0–4.8) | 3.6 (0.7) | 3.7 (2.0–5.0) | 0.938 | 0.700 | |
| Non‐MBSR | 3.5 (0.7) | 3.7 (1.8–5.0) | 3.5 (0.8) | 3.6 (1.0–5.0) | 0.659 | Ref. | |
| Nonjudge | MBSR | 3.3 (0.8) | 3.4 (1.2–5.0) | 3.3 (0.8) | 3.2 (1.6–5.0) | 0.992 | 0.361 |
| Active Controls | 3.5 (0.8) | 3.4 (2.0–5.0) | 3.4 (0.7) | 3.4 (2.4–5.0) | 0.339 | 0.775 | |
| Non‐MBSR | 3.7 (0.8) | 3.6 (2.0–5.0) | 3.5 (0.9) | 3.6 (1.8–5.0) | 0.164 | Ref. | |
Significant changes are marked in bold.
FFMQ, Five Facets of Mindfulness Questionnaire.
Change over time (P‐value) within groups (Wilcoxon signed test).
Change over time (P‐value) between groups (comparison of MBSR‐Active controls vs. Non‐MBSR (ref.) (Mann–Whitney test).
Table 7.
Secondary outcome: Posttraumatic Growth
| Measure PTGIa | Group | Preintervention | Postintervention | P‐valueb | P‐valuec | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (min‐max) | Mean (SD) | Median (min‐max) | ||||
| Posttraumatic growth | MBSR | 59.78 (19.5) | 62.00 (17–103) | 64.65 (17.7) | 67.00 (6–100) | 0.005 | 0.111 |
| Active Controls | 55.92 (20.2) | 58.50 (0–92) | 57.13 (17.6) | 61.00 (17–89) | 0.498 | 0.049 | |
| Non‐MBSR | 52.58 (19.2) | 55.50 (13–85) | 51.57 (20.8) | 52.00 (0–94) | 0.933 | Ref. | |
Significant changes are marked in bold.
PTGI, Posttraumatic Growth Inventory.
Change over time (P‐value) within groups (Wilcoxon signed test).
Change over time (P‐value) between groups (comparison of MBSR‐Active controls vs. Non‐MBSR (ref.) (Mann–Whitney test).
Table 8.
Secondary outcome: immune response
| Measures | Group | Preintervention | Postintervention | P‐valuea | P‐valueb | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (quartiles) | Mean (SD) | Median (quartiles) | ||||
| Fankia% | MBSR | 19.1 (8.2) | 18 (14–25) | 22.0 (7.7) | 22 (17–27) | 0.015 | 0.142 |
| Active Controls | 22.0 (7.6) | 20 (14.5–26.5) | 18.8 (7.3) | 19 (16–23) | 0.668 | 0.602 | |
| Non‐MBSR | 19.2 (8.4) | 19 (13–24) | 19.6 (6.8) | 20 (15–25) | 0.731 | Ref | |
| Lymphocytesx10e9/I | MBSR | 1.5 (0.76) | 1.3 (0.98–1.7) | 1.4 (0.58) | 1.4 (0.98–1.7) | 0.997 | 0.559 |
| Active Controls | 1.6 (0.7) | 1.4 (1.2–1.9) | 1.5 (0.5) | 1.4 (1.1–1.7) | 0.415 | 0.280 | |
| Non‐MBSR | 1.4 (0.6) | 1.3 (0.96–1.7) | 1.4 (0.6) | 1.2 (0.97–1.8) | 0.518 | Ref | |
| CD3T% | MBSR | 72.8 (11.1) | 74 (66.7–82.0) | 71.4 (10.5) | 72 (67–80) | 0.027 | 0.222 |
| Active Controls | 72.9 (7.5) | 71 (68–78) | 71.5 (7.7) | 71 (68–77) | 0.289 | 0.061 | |
| Non‐MBSR | 76.3 (8.1) | 78 (71–82) | 73.4 (8.8) | 75 (67–79) | 0.001 | Ref | |
| CD3Tx10e9/I | MBSR | 1.1 (0.6) | 0.97 (0.67–1.3) | 1.0 (0.5) | 1.0 (0.63–1.3) | 0.671 | 0.800 |
| Active Controls | 1.2 (0.6) | 1.1 (0.8–1.4) | 1.1 (0.4) | 1.0 (0.7–1.2) | 0.263 | 0.424 | |
| Non‐MBSR | 1.0 (0.4) | 1 (0.8–1.3) | 1.0 (0.5) | 0.8 (0.7–1.3) | 0.937 | Ref | |
| CD3+4+Th% | MBSR | 41.7 (8.7) | 42 (37–48) | 41.1 (8.7) | 40.5 (35–47) | 0.274 | 0.253 |
| Active Controls | 44.9 (8.1) | 46 (39–50) | 44.5 (8.4) | 45 (41–50) | 0.987 | 0.080 | |
| Non‐MBSR | 46.2 (9.8) | 46 (38–55) | 44.3 (9.1) | 43.5 (39–50) | 0.021 | Ref | |
| CD3+4+x10e9/I | MBSR | 0.62 (0.31) | 0.54 (0.4–0.7) | 0.60 (0.29) | 0.53 (0.4–0.7) | 0.581 | 0.860 |
| Active Controls | 0.73 (0.4) | 0.69 (0.5–0.8) | 0.66 (0.3) | 0.65 (0.4–0.8) | 0.543 | 0.864 | |
| Non‐MBSR | 0.63 (0.28) | 0.62 (0.4–0.8) | 0.62 (0.31) | 0.56 (0.4–0.7) | 0.834 | Ref | |
| CD3+8+T cy/s% | MBSR | 29.6 (11.3) | 28 (20–37) | 28.4 (10.5) | 25.5 (20–38) | 0.035 | 1.00 |
| Active Controls | 27.9 (10.4) | 26 (21–35) | 26.7 (9.5) | 25 (20–34) | 0.041 | 0.819 | |
| Non‐MBSR | 28.6 (11.2) | 25 (21–38) | 27.8 (11.7) | 24.5 (19–36) | 0.132 | Ref | |
| CD3+8+x10e9/I | MBSR | 0.46 (0.33) | 0.37 (0.2–0.6) | 0.42 (0.26) | 0.33 (0.2–0.6) | 0.658 | 0.750 |
| Active Controls | 0.44 (0.26) | 0.38 (0.3–0.6) | 0.38 (0.18) | 0.34 (0.23–0.5) | 0.144 | 0.384 | |
| Non‐MBSR | 0.40 (0.27) | 0.32 (0.2–0.5) | 0.41 (0.30) | 0.28 (0.2–0.5) | 0.655 | Ref | |
| CD3‐16+56+NK% | MBSR | 16.7 (8.1) | 14.5 (11–21) | 16.1 (7.5) | 15 (10–21) | 0.329 | 0.077 |
| Active Controls | 15.9 (6.1) | 15 (12–20) | 15.2 (6.2) | 15 (11–19) | 0.057 | 0.025 | |
| Non‐MBSR | 14.8 (6.2) | 13.5 (10–19) | 15.7 (7.1) | 15.5 (10–18) | 0.127 | Ref | |
| CD3‐16+56+NKx10e9/I | MBSR | 0.24 (0.16) | 0.2 0.1–0.3) | 0.22 (0.10) | 0.2 (0.1–0.3) | 0.239 | 0.041 |
| Active Controls | 0.25 (0.14) | 0.24 (0.1–0.3) | 0.22 (0.13) | 0.19 (0.1–0.3) | 0.121 | 0.011 | |
| Non‐MBSR | 0.20 (0.11) | 0.17 (0.1–0.3) | 0.22 (0.13) | 0.18 (0.1–0.3) | 0.051 | Ref | |
| CD19B% | MBSR | 9.5 (8.4) | 7.5 (3.7–13) | 11.6 (7.6) | 10 (7–14) | 0.001 | 0.957 |
| Active Controls | 10.2 (4.8) | 10 (7–13) | 12.2 (5.5) | 12 (9–15) | 0.007 | 0.417 | |
| Non‐MBSR | 7.7 (5.4) | 8 (3–11) | 9.8 (4.7) | 9 (6–12) | 0.003 | Ref | |
| CD19Bx10e9/I | MBSR | 0.15 (0.16) | 0.1 (0.04–0.21) | 0.17 (0.15) | 0.1 (0.08–0.21) | 0.004 | 0.862 |
| Active Controls | 0.17 (0.12) | 0.14 (0.08–0.23) | 0.17 (0.09) | 0.17 (0.11–0.22) | 0.385 | 0.362 | |
| Non‐MBSR | 0.11 (0.08) | 0.1 (0.03–0.16) | 0.13 (0.07) | 0.13 (0.09–0.17) | 0.013 | Ref | |
| CD3+4+/CD3+8+quotient% | MBSR | 1.65 (0.73) | 1.6 1–2.2) | 1.72 (0.82) | 1.6 (1–2.2) | 0.220 | 0.313 |
| Active Controls | 1.92 (0.99) | 1.7 (1.2–2.3) | 1.99 (1.1) | 1.8 (1.2–2.3) | 0.272 | 0.336 | |
| Non‐MBSR | 1.98 (1.2) | 1.7 (1.1–2.6) | 2.06 (1.7) | 1.8 (1.1–2.5) | 0.812 | Ref | |
|
IL6‐HS (ELISA) |
MBSR | 1.0 (0.7–2.3) | 1.0 (0.6–1.7) | 0.750 | 0.847 | ||
| Active Controls | 1.2 (0.7–1.7) | 1.0 (0.7–2.1) | 0.983 | 0.528 | |||
| Non‐MBSR | 1.1 (0.5–2.5) | 1.2 (0.5–1.8) | 0.291 | Ref. | |||
|
IL8‐HS (ELISA) |
MBSR | 11.5 (9.3–5) | 12.0 (9.7–15.7) | 0.241 | 0.395 | ||
| Active Controls | 12.0 (9.0–4) | 12.0 (9.4–15.0) | 1.000 | 0.595 | |||
| Non‐MBSR | 12.0 (9.0–5) | 12.0 (9.3–16.0) | 0.894 | Ref. | |||
Significant changes are marked in bold.
Change over time (P‐value) within groups (Wilcoxon signed test).
Change over time (P‐value) between groups (comparison of MBSR‐Active controls vs. Non‐MBSR (ref.) (Mann–Whitney test).
On the primary outcome measures, MBSR participants reported significant improvements on depression symptoms both within group (mean = 4.3; SD = 3.7 to mean = 3.3; SD = 3.3; P = 0.001) as well as compared to non‐MBSR (P = 0.015), but not on anxiety symptoms (Table 2).
The number of reduced cases of depression were 7 (11%) in MBSR participants compared to 4 (8%) reduced cases of depression in active controls and non‐MBSR, respectively.
Pre and postintervention HAD scores for depression and anxiety are presented in Figure 2.
Figure 2.
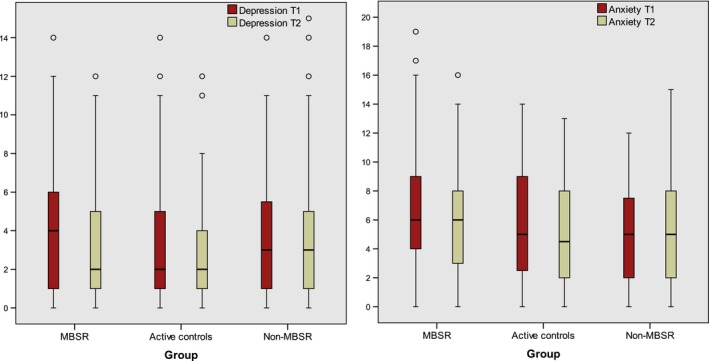
Box‐plot. Primary outcome: Pre and Postintervention Mood Disorder Symptoms, HAD scores for depression, respectively, anxiety subscales.
There were significant improvements in physical symptoms (mean = 0.7; SD = 0.5 to mean = 0.6; SD = 0.4; P = 0.007), psychological symptoms (mean = 1.4; SD = 0.8; to mean = 1.2; SD = 0.9; P = 0.008), and total symptom burden (mean = 0.8; SD = 0.5 to mean = 0.7; SD = 0.5; P = 0.004) within the MBSR. There were also significant improvements between MBSR versus non‐MBSR regarding psychological symptoms (P = 0.019), as well as global distress (P = 0.013) (Table 3).
Within the MBSR group, changes in health status were seen in improved vitality (mean = 49.5; SD = 27.5 to mean = 60.9; SD = 20.1; P < 0.001), physical functioning (mean = 77.4; SD = 16.7 to mean = 83.1; SD = 15.4; P < 0.001), general health perceptions (mean = 61.2; SD = 21.6 to mean = 67.6; SD = 19.1; P = 0.003), physical role functioning (mean = 45.9; SD = 42.9 to mean = 63.3; SD = 39.7; P = 0.003), and mental health (mean = 67.9; SD = 19.0 to mean = 74.1; SD = 17.1; P < 0.001). Changes were reported in physical functioning within the non‐MBSR group (mean = 78.5; SD = 19.6 to mean = 82.8; SD = 19.0; P = 0.007), physical role functioning with active controls (mean = 42.2; SD = 42.6 to mean = 67.6; SD = 35.8; P = 0.001), non‐MBSR (mean = 40.4; SD = 41.5 to mean = 59.6; SD = 42.3; P = 0.006), social functioning for active controls (mean = 78.4; SD = 25.9 to mean = 84.8; SD = 22.8; P = 0.017), non‐MBSR (mean = 72.1; SD = 26.0 to mean = 84.1; SD = 25.3; P = 0.001). A significant improvement between groups was reported in mental health for the MBSR group (P = 0.001) and active controls (P = 0.038) compared to non‐MBSR (Table 4).
Additional secondary outcome measures showed that MBSR participants reported improved coping capacity (P = 0.028) versus non‐MBSR (Table 5).
Enhanced elements of mindfulness were shown regarding Nonreactivity within MBSR (mean = 2.9; SD = 0.7 to mean = 3.3; SD = 0.5; P < 0.001), and between groups compared to non‐MBSR (P = 0.010), as well as on Observe both within MBSR group (mean = 3.3; SD = 0.7 to mean = 3.6; SD = 0.5; P < 0.001) and compared to non‐MBSR (P = 0.006) (Table 6). Enriched posttraumatic growth was reported within MBSR (mean = 59.78; SD = 19.5 to mean = 64.65; SD = 17.7; P = 0.005), and between groups for active controls versus non‐MBSR (P = 0.049) (Table 7).
Biological response
Mean baseline NK‐cell activity increased significantly (mean = 19.1; SD = 8.2 to mean = 22.0 SD = 7.7; P = 0.015) within the MBSR group. The absolute number of CD19+B‐lymphocytes increased (mean = 0.15; SD = 0.16 to mean = 0.17; SD = 0.15; P = 0.004). The proportion of CD3+T‐lymphocytes decreased (mean = 72.8; SD = 11.1 to mean = 71.4; SD = 10.5; P = 0.027) as did that of CD3+8+T‐lymphocytes (mean = 29.6; SD = 11.3 to mean = 28.4; SD = 10.5; P = 0.035), whereas the proportion of CD19+B‐lymphocytes increased (mean = 9.5; SD = 8.4 to mean = 11.6; SD = 7.6; P = 0.001). Analyses also demonstrated significant changes (P = 0.041) between MBSR participants and non‐MBSR regarding decrease in the absolute number of NK cells (CD3‐16+56+NKx10e9/l).
There were also some significant changes pre and postintervention in active controls and in the non‐MBSR group suggesting a decrease in the absolute number of NK cells (CD3‐16‐56+NKx10e9/I) for active controls (P = 0.011) compared to non‐MBSR. There were also significant changes for active controls regarding CD3‐16+56+NK% versus non‐MBSR (P = 0.025) (Table 8). There were no significant differences in serum concentrations of IL‐6 or IL‐8 between any of the study groups.
Discussion
Our trial provides evidence in support of the efficacy of MBSR for psychological and biological response among women with breast cancer. The primary purpose of this study was to determine the efficacy of MBSR intervention on mood disorder, that is, depression and anxiety. Our finding of improvements in depression is consistent with other RTCs that have evaluated MBSR and mood disorders in breast cancer patients 21, 26. Unlike those studies, our findings revealed no significant changes in anxiety. However, consistent with our trial, a meta‐analysis of mindfulness‐based interventions that included participants who met the diagnostic criteria for a current episode of anxiety or depressive disorder show that MBSR is effective for reducing symptoms of depression, but not anxiety 65.
MBSR participants reported significantly greater improvements in symptoms, especially psychological symptoms. In addition, their symptom burden and distress significantly decreased. MBSR participants also improved in functional status; in line with previous research showing significant intergroup improvements in mental health 66, our findings indicate significant improved mental health between groups.
A common assumption is that mindfulness increases the individual's ability to cope, but few RCTs have examined the effect of MBSR on coping capacity. The MBSR intervention appears to improve coping effectiveness in breast cancer patients 32, and behavioral and cognitive coping 67. Results from our trial show that women who participated in the MBSR experienced improved coping capacity, here measured as sense of coherence (SOC). Previous research has identified SOC as a significant predictor of distress, number and type of coping strategies in women with breast cancer 68, suggesting the lower the SOC, the higher the levels of symptom burden 3. While Antonovsky 54 believed that SOC is a relatively stable personality state, our findings show evidence that MBSR may influence SOC (i.e., to improve patients' ability to manage, comprehend, and finding meaning living with breast cancer).
Enhanced elements of mindfulness were shown for non‐reactivity and observing in the MBSR group. Future research is needed to explore the complexity and relations among the different dimensions of mindfulness, and to gain a deeper understanding about which factors facilitate the cultivation of mindfulness 69.
Our trial indicates that the benefits of MBSR may also extend to posttraumatic growth. Relatively little research has investigated the relationship between posttraumatic growth and immunity. A study of patients with hepatoma suggests that higher PTGI scores are associated with higher peripheral blood leukocytes and longer survival 70. Further research addressing the interrelationship of MBSR with posttraumatic growth and immune response is warranted.
In terms of biological response, changes in NK‐cell activity and numbers of both NK cells and B cells within the MBSR group as well as between groups were seen. Of note was the finding that there were no changes in numbers of IL‐6 or IL‐8. The clinical relevance of these discrete findings is difficult to estimate and more research is needed to fully explain the clinical meaning of these biological parameters. However, consistent with our findings, there is intriguing evidence suggesting that finding meaning and personal growth is associated with T‐cell levels 71 and NK‐cell activity 72. Furthermore, there have been prior reports that distress is associated with immune downregulation, including reduced NK‐cell activity 12, 32. In addition, participation in mindfulness training leads to a shift from proinflammatory response in cancer patients 33 and a pilot study has suggested that improvements in well‐being following MBSR was associated with increased NK activity and decreased CRP levels 73.
Given the beneficial efficacy of MBSR on both psychological and biological outcomes, future longitudinal studies may be needed to investigate the effect of these outcomes on disease progression and survival. There were several tendencies in favor for MBSR group, although not statistically significant, and for some outcome variables statistically significant changes could be detected only within MBSR group but not between the groups. A larger sample size might have resulted in more significant differences between groups. Several improvements were also seen in active controls (i.e., physical and social role functioning, and observing) as well as between groups (i.e., global distress, mental health, and posttraumatic growth). Future research is needed to explore who benefits from participating in an MBSR intervention with weekly group sessions versus using a self‐instructing training program.
This study is characterized by several strengths, including use of active and non‐MBSR controls, random assignment, inclusion of patient‐reported outcomes and immune response among a homogenous group of women diagnosed with breast cancer. To our knowledge, this is the first MBSR intervention study to include a comparison between standardized MBSR and both active controls using a self‐instructing program and passive controls. The notable study limitation was that all women who fulfilled the inclusion criteria were invited without undergoing screened for mood disorder before study invitation. Furthermore, despite randomization, there were differences in distribution regarding disease stage (tumor size and type of breast cancer) which might have affected study results.
In conclusion, results from this RCT suggest that MBSR is beneficial and leads to psychological and biological improvements. MBSR may hold potential for alleviating depression, distress and symptom experience, and to strengthen coping capacity, which may improve breast cancer survivorship. Since there were also positive changes in the active control group, it is important to provide the self‐training program to patients who prefer to practice themselves, without weekly group exercises. Finally, longitudinal studies are required to investigate whether these positive psychological and biological responses remain constant, increases or decreases over time.
Conflict of Interest
The author(s) indicated no potential conflicts of interest.
Supporting information
Figure S1. X. Typical result with dot plots showing CD3+, CD3+4+, CD3+8+, 19‐,and CD3‐16+56+ cells. Absolute numbers of cells were calculated using Trucount reference beads. Lymphocytes and Trucount beads were defined in a CD45 versus side scatter area (SSC‐A) and a CD19 versus SSC‐A plot, respectively. CD3+ and CD3‐ cells were then defined in a CD3 versus SSC‐A plot. Then CD3+4+ and CD3+8+ were defined in a plot of CD8 versus CD4. Finally CD19 and CD3‐16+56+ cells were defined in a plot of CD16+56+ versus CD19.
Figure S2. Y Typical result with dot plots showing regulatory T cells. Lymphocytes were defined in a forward scatter area (FSC‐A) versus side scatter area (SSC‐A) plot. CD3+4+ cells were then defined in a CD3 versus CD4 plot. Then CD3+4+25+ were defined in a plot of CD4 versus CD25. Finally CD3+4+25+FOXP3++ (regulatory T cells) cells were defined in a plot of CD25 versus FOXP3.
Acknowledgments
We thank all the women who participated in this study. We are grateful to research coordinator Anna‐Lena Emanuelsson Loft and biostatistician Salmir Nasic for their excellent assistance with data management and statistical analysis. We especially acknowledge the help and supervision provided by senior MBSR instructor Ola Schenström, specialist in community medicine. We also acknowledge MD Stig B Holmberg for medical support during the i.
ClinicalTrials.gov: This trial is registered at ClinicalTrials.gov:NCT01591915.
Cancer Medicine 2017; 6(5):1108–1122
References
- 1. Fallowfield, L. , and Jenkins V.. 2015. Psychosocial/survivorship issues in breast cancer: are we doing better? J. Natl Cancer Inst. 107:335. [DOI] [PubMed] [Google Scholar]
- 2. Given, C. W. , Sikorskii A., Tamkus D., Given B., You M., McCorkle R., et al. 2008. Managing symptoms among patients with breast cancer during chemotherapy: results of a two‐arm behavioral trial. J. Clin. Oncol. 26:5855–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenne Sarenmalm, E. , Browall M., and Gaston‐Johansson F.. 2014. Symptom burden clusters: a challenge for targeted symptom management. A longitudinal study examining symptom burden clusters in breast cancer. J. Pain Symptom Manage. 47:731–741. [DOI] [PubMed] [Google Scholar]
- 4. Hjerl, K. , Andersen E. W., Keiding N., Mortensen P. B., and Jorgensen T. 2002. Increased incidence of affective disorders, anxiety disorders, and non‐natural mortality in women after breast cancer diagnosis: a nation‐wide cohort study in Denmark. Acta Psychiatr. Scand. 105:258–264. [DOI] [PubMed] [Google Scholar]
- 5. Zabora, J. , BrintzenhofeSzoc K., Curbow B., Hooker C., and Piantadosi S. 2001. The prevalence of psychological distress by cancer site. Psychooncology 10:19–28. [DOI] [PubMed] [Google Scholar]
- 6. Mehnert, A. , and Koch U.. 2007. Prevalence of acute and post‐traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: a prospective study. Psychooncology 16:181–188. [DOI] [PubMed] [Google Scholar]
- 7. Mehnert, A. , and Koch U.. 2008. Psychological comorbidity and health‐related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register‐based sample of long‐term breast cancer survivors. J. Psychosom. Res. 64:383–391. [DOI] [PubMed] [Google Scholar]
- 8. Burgess, C. , Cornelius V., Love S., Graham J., Richards M., and Ramirez A.. 2005. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ 330:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger, A. M. , Visovsky C., Hertzog M., Holtz S., and F. R. Loberiza Jr, 2012. Usual and worst symptom severity and interference with function in breast cancer survivors. J. Support. Oncol. 10:112–118. [DOI] [PubMed] [Google Scholar]
- 10. Segerstrom, S. C. , and Miller G. E.. 2004. Psychological stress and the human immune system: a meta‐analytic study of 30 years of inquiry. Psychol. Bull. 130:601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thornton, L. M. , Andersen B. L., Crespin T. R., and Carson W. E. 2007. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav. Immun. 21:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersen, B. L. , Farrar W. B., Golden‐Kreutz D., Kutz L. A., MacCallum R., Courtney M. E., et al. 1998. Stress and immune responses after surgical treatment for regional breast cancer. J. Natl Cancer Inst. 90:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiltschke, C. , Krainer M., Budinsky A. C., Berger A., Muller C., Zeillinger R., et al. 1995. Reduced mitogenic stimulation of peripheral blood mononuclear cells as a prognostic parameter for the course of breast cancer: a prospective longitudinal study. Br. J. Cancer 71:1292–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamasaki, S. , Kan N., Harada T., Ichinose Y., Moriguchi Y., Li L., et al. 1993. Relationship between immunological parameters and survival of patients with liver metastases from breast cancer given immuno‐chemotherapy. Breast Cancer Res. Treat. 26:55–65. [DOI] [PubMed] [Google Scholar]
- 15. Standish, L. J. , Sweet E. S., Novack J., Wenner C. A., Bridge C., Nelson A., et al. 2008. Breast cancer and the immune system. J. Soc. Integr. Oncol. 6:158–168. [PMC free article] [PubMed] [Google Scholar]
- 16. Rao, V. S. , Dyer C. E., Jameel J. K., Drew P. J., and Greenman J., 2006. Potential prognostic and therapeutic roles for cytokines in breast cancer (Review). Oncol. Rep. 15:179–185. [DOI] [PubMed] [Google Scholar]
- 17. Yokoe, T. , Iino Y., and Morishita Y.. 2000. Trends of IL‐6 and IL‐8 levels in patients with recurrent breast cancer: preliminary report. Breast Cancer 7:187–190. [DOI] [PubMed] [Google Scholar]
- 18. Bachelot, T. , Ray‐Coquard I., Menetrier‐Caux C., Rastkha M., Duc A., and Blay J. Y. 2003. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone‐refractory metastatic breast cancer patients. Br. J. Cancer 88:1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salgado, R. , Junius S., Benoy I., Van Dam P., Vermeulen P., Van Marck E., et al. 2003. Circulating interleukin‐6 predicts survival in patients with metastatic breast cancer. Int. J. Cancer 103:642–646. [DOI] [PubMed] [Google Scholar]
- 20. Kabat‐Zinn, J. , Massion A. O., Kristeller J., Peterson L. G., Fletcher K. E., Pbert L., et al. 1992. Effectiveness of a meditation‐based stress reduction program in the treatment of anxiety disorders. Am. J. Psychiatry 149:936–943. [DOI] [PubMed] [Google Scholar]
- 21. Hoffman, C. J. , Ersser S. J., Hopkinson J. B., Nicholls P. G., Harrington J. E., Thomas P. W.. 2012. Effectiveness of mindfulness‐based stress reduction in mood, breast‐ and endocrine‐related quality of life, and well‐being in stage 0 to III breast cancer: a randomized, controlled trial. J. Clin. Oncol. 30:1335–1342. [DOI] [PubMed] [Google Scholar]
- 22. Gotink, R. A. , Chu P., Busschbach J. J., Benson H., Fricchione G. L., and Hunink M. G. 2015. Standardised mindfulness‐based interventions in healthcare: an overview of systematic reviews and meta‐analyses of RCTs. PLoS ONE 10:e0124344. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Piet, J. , Wurtzen H., and Zachariae R.. 2012. The effect of mindfulness‐based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta‐analysis. J. Consult. Clin. Psychol. 80:1007–1020. [DOI] [PubMed] [Google Scholar]
- 24. Kabat‐Zinn, J. 1994. Wherever you go, there you are: Mindfulness meditation for everyday life. Piatkus, London. [Google Scholar]
- 25. Marcus, M. T. , Liehr P. R., Schmitz J., Moeller F. G., Swank P., Fine M., et al. 2007. Behavioral therapies trials: a case example. Nurs. Res. 56:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wurtzen, H. , Dalton S. O., Elsass P., Sumbundu A. D., Steding‐Jensen M., Karlsen R. V., et al. 2013. Mindfulness significantly reduces self‐reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I‐III breast cancer. Eur. J. Cancer 49:1365–1373. [DOI] [PubMed] [Google Scholar]
- 27. Speca, M. , Carlson L. E., Goodey E., and Angen M.. 2000. A randomized, wait‐list controlled clinical trial: the effect of a mindfulness meditation‐based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom. Med. 62:613–622. [DOI] [PubMed] [Google Scholar]
- 28. Branstrom, R. , Kvillemo P., Brandberg Y., and Moskowitz J. T.. 2010. Self‐report mindfulness as a mediator of psychological well‐being in a stress reduction intervention for cancer patients–a randomized study. Ann. Behav. Med. 39:151–161. [DOI] [PubMed] [Google Scholar]
- 29. Lengacher, C. A. , Shelton M. M., Reich R. R., Barta M. K., Johnson‐Mallard V., Moscoso M. S., et al. 2014. Mindfulness based stress reduction (MBSR(BC)) in breast cancer: evaluating fear of recurrence (FOR) as a mediator of psychological and physical symptoms in a randomized control trial (RCT). J. Behav. Med. 37:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Branstrom, R. , Kvillemo P., and Akerstedt T.. 2013. Effects of mindfulness training on levels of cortisol in cancer patients. Psychosomatics 54:158–164. [DOI] [PubMed] [Google Scholar]
- 31. Carlson, L. E. , Speca M., Faris P., and Patel K. D.. 2007. One year pre‐post intervention follow‐up of psychological, immune, endocrine and blood pressure outcomes of mindfulness‐based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav. Immun. 21:1038–1049. [DOI] [PubMed] [Google Scholar]
- 32. Witek‐Janusek, L. , Albuquerque K., Chroniak K. R., Durazo‐Arvizu R., and Mathews H. L.. 2008. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav. Immun. 22:969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carlson, L. E. , Speca M., Patel K. D., and Goodey E. 2003. Mindfulness‐based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom. Med. 65:571–581. [DOI] [PubMed] [Google Scholar]
- 34. Rouleau, C. R. , Garland S. N., and Carlson L. E.. 2015. The impact of mindfulness‐based interventions on symptom burden, positive psychological outcomes, and biomarkers in cancer patients. Cancer Manag. Res. 7:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baer, R. A. 2003. Mindulness training as a clinical intervention: a conceptual review. Clin. Psychol. Sci. Pract. 10:125–143. [Google Scholar]
- 36. Grossman, P. , Niemann L., Schmidt S., and Walach H. 2004. Mindfulness‐based stress reduction and health benefits. A meta‐analysis. J. Psychosom. Res. 57:35–43. [DOI] [PubMed] [Google Scholar]
- 37. Kenne Sarenmalm, E. , Martensson L. B., Holmberg S. B., Andersson B. A., Oden A., and Bergh I.. 2013. Mindfulness based stress reduction study design of a longitudinal randomized controlled complementary intervention in women with breast cancer. BMC Complement Altern. Med. 13:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Altman, D. G. , Schulz K. F., Moher D., Egger M., Davidoff F., Elbourne D., et al. 2001. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann. Intern. Med. 134:663–694. [DOI] [PubMed] [Google Scholar]
- 39. Zwarenstein, M. , Treweek S., Gagnier J. J., Altman D. G., Tunis S., Haynes B., et al. 2008. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boutron, I. , Moher D., Altman D. G., Schulz K. F., Ravaud P. and C. Group . 2008. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Ann. Intern. Med. 148:W60–W66. [DOI] [PubMed] [Google Scholar]
- 41. Power, M. , and Hopayian K.. 2011. Exposing the evidence gap for complementary and alternative medicine to be integrated into science‐based medicine. J. R. Soc. Med. 104:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Group TSBC : National Guidelines, 2008. [Google Scholar]
- 43. Zigmond, A. S. , and Snaith R. P.. 1983. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67:361–370. [DOI] [PubMed] [Google Scholar]
- 44. Mykletun, A. , Stordal E., and Dahl A. A.. 2001. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br. J. Psychiatry 179:540–544. [DOI] [PubMed] [Google Scholar]
- 45. Herrmann, C. 1997. International experiences with the Hospital Anxiety and Depression Scale–a review of validation data and clinical results. J. Psychosom. Res. 42:17–41. [DOI] [PubMed] [Google Scholar]
- 46. Bjelland, I. , Dahl A. A., Haug T. T., and Neckelmann D. 2002. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 52:69–77. [DOI] [PubMed] [Google Scholar]
- 47. Portenoy, R. K. , Thaler H. T., Kornblith A. B., Lepore J. M., Friedlander‐Klar H., Kiyasu E., et al. 1994. The memorial symptom assessment scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur. J. Cancer 30A:1326–1336. [DOI] [PubMed] [Google Scholar]
- 48. Tranmer, J. E. , Heyland D., Dudgeon D., Groll D., Squires‐Graham M., and Coulson K.. 2003. Measuring the symptom experience of seriously ill cancer and noncancer hospitalized patients near the end of life with the memorial symptom assessment scale. J. Pain Symptom Manage. 25:420–429. [DOI] [PubMed] [Google Scholar]
- 49. Browall, M. , Kenne Sarenmalm E., Nasic S., Wengstrom Y., and Gaston‐Johansson F.. 2013. Validity and reliability of the Swedish version of the Memorial Symptom Assessment Scale (MSAS): an instrument for the evaluation of symptom prevalence, characteristics, and distress. J. Pain Symptom Manage. 46:131–141. [DOI] [PubMed] [Google Scholar]
- 50. McHorney, C. A. , Ware J. E. Jr, Lu J. F., and Sherbourne C. D.. 1994. The MOS 36‐item Short‐Form Health Survey (SF‐36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 32:40–66. [DOI] [PubMed] [Google Scholar]
- 51. Sullivan, M. , Karlsson J., and Ware J. E. Jr. 1995. The swedish SF‐36 health survey–I. evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc. Sci. Med. 41:1349–1358. [DOI] [PubMed] [Google Scholar]
- 52. Persson, L. O. , Karlsson J., Bengtsson C., Steen B., and Sullivan M. 1998. The Swedish SF‐36 Health Survey II. Evaluation of clinical validity: results from population studies of elderly and women in Gothenborg. J. Clin. Epidemiol. 51:1095–1103. [DOI] [PubMed] [Google Scholar]
- 53. Sullivan, M. , and Karlsson J.. 1998. The Swedish SF‐36 Health Survey III. Evaluation of criterion‐based validity: results from normative population. J. Clin. Epidemiol. 51:1105–1113. [DOI] [PubMed] [Google Scholar]
- 54. Antonovsky, A. 1987. Unraveling the mysteries of health: how people manage stress and stay well. Jossey‐Bass, San Francisco. [Google Scholar]
- 55. Antonovsky, A. 1993. The structure and properties of the sense of coherence scale. Soc. Sci. Med. 36:725–733. [DOI] [PubMed] [Google Scholar]
- 56. Langius, A. , Bjorvell H., and Antonovsky A.. 1992. The sense of coherence concept and its relation to personality traits in Swedish samples. Scand. J. Caring Sci. 6:165–171. [DOI] [PubMed] [Google Scholar]
- 57. Gilbar, O. 1998. Coping with threat. Implications for women with a family history of breast cancer. Psychosomatics 39:329–339. [DOI] [PubMed] [Google Scholar]
- 58. Thome, B. , and Hallberg I. R.. 2004. Quality of life in older people with cancer – a gender perspective. Eur. J. Cancer Care (Engl) 13:454–463. [DOI] [PubMed] [Google Scholar]
- 59. Baer, R. A. , Smith G. T., Hopkins J., Krietemeyer J., and Toney L.. 2006. Using self‐report assessment methods to explore facets of mindfulness. Assessment 13:27–45. [DOI] [PubMed] [Google Scholar]
- 60. Lilja, J. L. , Frodi‐Lundgren A., Hanse J. J., Josefsson T., Lundh L. G., Skold C., et al. 2011. Five Facets Mindfulness Questionnaire–reliability and factor structure: a Swedish version. Cogn. Behav. Ther. 40:291–303. [DOI] [PubMed] [Google Scholar]
- 61. Tedeschi, R. G. , and Calhoun L. G.. 1996. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J. Trauma. Stress 9:455–471. [DOI] [PubMed] [Google Scholar]
- 62. Smith, S. G. , and Cook S. L.. 2004. Are reports of posttraumatic growth positively biased? J. Trauma. Stress 17:353–358. [DOI] [PubMed] [Google Scholar]
- 63. Godoy‐Ramirez, K. , Franck K., and Gaines H.. 2000. A novel method for the simultaneous assessment of natural killer cell conjugate formation and cytotoxicity at the single‐cell level by multi‐parameter flow cytometry. J. Immunol. Methods 239:35–44. [DOI] [PubMed] [Google Scholar]
- 64. Chung, H. J. , Park C. J., Lim J. H., Jang S., Chi H. S., Im H. J., et al. 2010. Establishment of a reference interval for natural killer cell activity through flow cytometry and its clinical application in the diagnosis of hemophagocytic lymphohistiocytosis. Int. J. Lab. Hematol. 32:239–247. [DOI] [PubMed] [Google Scholar]
- 65. Strauss, C. , Cavanagh K., Oliver A., and Pettman D. 2014. Mindfulness‐based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta‐analysis of randomised controlled trials. PLoS ONE 9:e96110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ledesma, D. , and Kumano H.. 2009. Mindfulness‐based stress reduction and cancer: a meta‐analysis. Psychooncology 18:571–579. [DOI] [PubMed] [Google Scholar]
- 67. Henderson, V. P. , Clemow L., Massion A. O., Hurley T. G., Druker S., Hebert J. R.. 2012. The effects of mindfulness‐based stress reduction on psychosocial outcomes and quality of life in early‐stage breast cancer patients: a randomized trial. Breast Cancer Res. Treat. 131:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kenne Sarenmalm, E. , Browall M., Persson L. O., Fall‐Dickson J., and Gaston‐Johansson F. 2013. Relationship of sense of coherence to stressful events, coping strategies, health status, and quality of life in women with breast cancer. Psychooncology 22:20–27. [DOI] [PubMed] [Google Scholar]
- 69. Grossman, P. 2008. On measuring mindfulness in psychosomatic and psychological research. J. Psychosom. Res. 64:405–408. [DOI] [PubMed] [Google Scholar]
- 70. Dunigan, J. T. , Carr B. I., and Steel J. L.. 2007. Posttraumatic growth, immunity and survival in patients with hepatoma. Dig. Dis. Sci. 52:2452–2459. [DOI] [PubMed] [Google Scholar]
- 71. Bower, J. E. , Kemeny M. E., Taylor S. E., and Fahey J. L. 1998. Cognitive processing, discovery of meaning, CD4 decline, and AIDS‐related mortality among bereaved HIV‐seropositive men. J. Consult. Clin. Psychol. 66:979–986. [DOI] [PubMed] [Google Scholar]
- 72. Bower, J. E. , Kemeny M. E., Taylor S. E., and Fahey J. L.. 2003. Finding positive meaning and its association with natural killer cell cytotoxicity among participants in a bereavement‐related disclosure intervention. Ann. Behav. Med. 25:146–155. [DOI] [PubMed] [Google Scholar]
- 73. Fang, C. Y. , Reibel D. K., Longacre M. L., Rosenzweig S., Campbell D. E., and Douglas S. D.. 2010. Enhanced psychosocial well‐being following participation in a mindfulness‐based stress reduction program is associated with increased natural killer cell activity. J. Altern. Complement. Med. 16:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. X. Typical result with dot plots showing CD3+, CD3+4+, CD3+8+, 19‐,and CD3‐16+56+ cells. Absolute numbers of cells were calculated using Trucount reference beads. Lymphocytes and Trucount beads were defined in a CD45 versus side scatter area (SSC‐A) and a CD19 versus SSC‐A plot, respectively. CD3+ and CD3‐ cells were then defined in a CD3 versus SSC‐A plot. Then CD3+4+ and CD3+8+ were defined in a plot of CD8 versus CD4. Finally CD19 and CD3‐16+56+ cells were defined in a plot of CD16+56+ versus CD19.
Figure S2. Y Typical result with dot plots showing regulatory T cells. Lymphocytes were defined in a forward scatter area (FSC‐A) versus side scatter area (SSC‐A) plot. CD3+4+ cells were then defined in a CD3 versus CD4 plot. Then CD3+4+25+ were defined in a plot of CD4 versus CD25. Finally CD3+4+25+FOXP3++ (regulatory T cells) cells were defined in a plot of CD25 versus FOXP3.


