Abstract
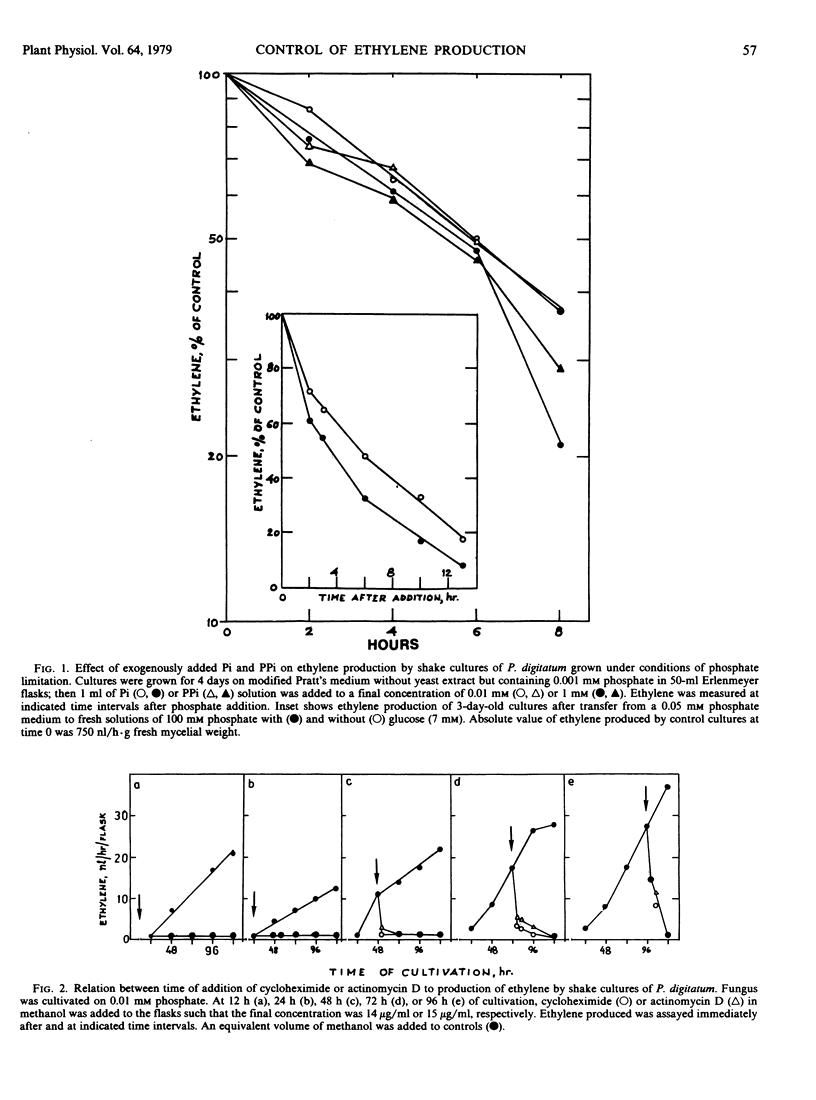
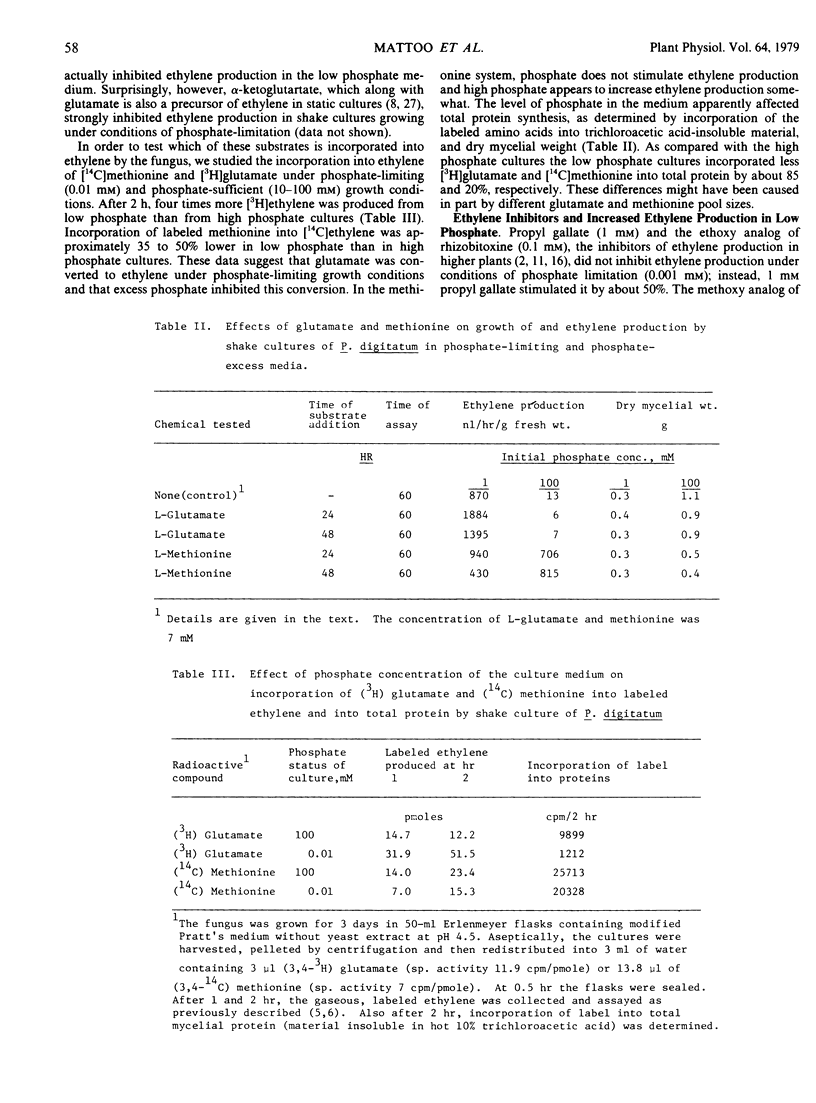
Characterization of the phosphate effect on ethylene production by Penicillium digitatum is reported. A low level of phosphate (0.001 millimolar) was about 200 to 500 times as effective as a high phosphate level (100 millimolar) in stimulating ethylene production and the stimulation was readily reversed by addition of phosphate. This phosphate effect did not operate in static cultures. The precursor of ethylene in the stimulated low phosphate system was glutamate but not α-ketoglutarate, which is a precursor in static systems. Actinomycin D and cycloheximide effectively inhibited the low phosphate/high ethylene-producing system. Alkaline phosphatase and protein kinase activities were higher in low than in high phosphate systems. We suggest that phosphate level regulates ethylene production by P. digitatum and that the regulation involves a phosphorylation or dephosphorylation reaction of some enzyme system associated with ethylene production. Phosphate-mediated control of ethylene production may also involve the transcriptional and translational machinery of the fungal cell. P. digitatum apparently can produce widely different levels of ethylene by different pathways, depending on culture conditions under which it is grown.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D. Adenylate metabolism of embryonic axes from deteriorated soybean seeds. Plant Physiol. 1977 Apr;59(4):610–614. doi: 10.1104/pp.59.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Lieberman M., Anderson J. D. Inhibition of ethylene production in fruit slices by a rhizobitoxine analog and free radical scavengers. Plant Physiol. 1978 Jun;61(6):886–888. doi: 10.1104/pp.61.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chalutz E., Lieberman M. Inhibition of Ethylene Production in Penicillium digitatum. Plant Physiol. 1978 Jan;61(1):111–114. doi: 10.1104/pp.61.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalutz E., Lieberman M. Methionine-induced Ethylene Production by Penicillium digitatum. Plant Physiol. 1977 Sep;60(3):402–406. doi: 10.1104/pp.60.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. W., Yang S. F. The biogenesis of ethylene in Penicillium digitatum. Arch Biochem Biophys. 1973 Jul;157(1):73–82. doi: 10.1016/0003-9861(73)90391-3. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control by phosphate of candicidin production. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1103–1109. doi: 10.1016/0006-291x(76)90767-1. [DOI] [PubMed] [Google Scholar]
- Martín J. F., Liras P., Demain A. L. ATP and adenylate energy charge during phosphate-mediated control of antibiotic synthesis. Biochem Biophys Res Commun. 1978 Aug 14;83(3):822–828. doi: 10.1016/0006-291x(78)91468-7. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Lieberman M. Localization of the Ethylene-synthesizing System in Apple Tissue. Plant Physiol. 1977 Nov;60(5):794–799. doi: 10.1104/pp.60.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Shah Z. M. Nucleotide degrading enzymes in Neurospora crassa. Z Allg Mikrobiol. 1974;14(7):581–591. doi: 10.1002/jobm.3630140705. [DOI] [PubMed] [Google Scholar]
- Primrose S. B. Formation of ethylene by Escherichia coli. J Gen Microbiol. 1976 Jul;95(1):159–165. doi: 10.1099/00221287-95-1-159. [DOI] [PubMed] [Google Scholar]
- Rossomando E. F., Hesla M. A. Time-dependent changes in Dictyostelium discoideum adenylate cyclase activity upon incubation with ATP. J Biol Chem. 1976 Nov 10;251(21):6568–6573. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Spalding D. H., Lieberman M. Factors affecting the production of ethylene by Penicillium digitatum. Plant Physiol. 1965 Jul;40(4):645–648. doi: 10.1104/pp.40.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. C., Spencer M. L-methionine as an ethylene precursor in Saccharomyces cerevisiae. Can J Microbiol. 1977 Dec;23(12):1669–1674. doi: 10.1139/m77-240. [DOI] [PubMed] [Google Scholar]


