Abstract
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that are involved in the degradation of various proteins in the extracellular matrix (ECM). Typically, MMPs have a propeptide sequence, a catalytic metalloproteinase domain with catalytic zinc, a hinge region or linker peptide, and a hemopexin domain. MMPs are commonly classified on the basis of their substrates and the organization of their structural domains into collagenases, gelatinases, stromelysins, matrilysins, membrane-type (MT)-MMPs, and other MMPs. MMPs are secreted by many cells including fibroblasts, vascular smooth muscle (VSM) and leukocytes. MMPs are regulated at the level of mRNA expression and by activation of their latent zymogen form. MMPs are often secreted as inactive proMMP form which is cleaved to the active form by various proteinases including other MMPs. MMPs cause degradation of ECM proteins such as collagen and elastin, but could influence endothelial cell function as well as VSM cell migration, proliferation, Ca2+ signaling and contraction. MMPs play a role in tissue remodeling during various physiological processes such as angiogenesis, embryogenesis, morphogenesis and wound repair, as well as in pathological conditions such as myocardial infarction, fibrotic disorders, osteoarthritis, and cancer. Increases in specific MMPs could play a role in arterial remodeling, aneurysm formation, venous dilation and lower extremity venous disorders. MMPs also play a major role in leukocyte infiltration and tissue inflammation. MMPs have been detected in cancer, and elevated MMP levels have been associated with tumor progression and invasiveness. MMPs can be regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs), and the MMP/TIMP ratio often determines the extent of ECM protein degradation and tissue remodeling. MMPs have been proposed as biomarkers for numerous pathological conditions and are being examined as potential therapeutic targets in various cardiovascular and musculoskeletal disorders as well as cancer.
Keywords: Cell Signaling, Collagen, Extracellular Matrix, Proteinases, Protein Degradation, Remodeling
1. INTRODUCTION
MMPs are a family of zinc-dependent endoproteases with multiple roles in tissue remodeling and degradation of various proteins in the extracellular matrix (ECM). MMPs promote cell proliferation, migration, and differentiation and could play a role in cell apoptosis, angiogenesis, tissue repair, and immune response. MMPs may also affect bioactive molecules on the cell surface and modulate various cellular and signaling pathways. Alterations in MMP expression and activity occur in normal biological processes e.g. during pregnancy and wound healing, but have also been observed in cardiovascular diseases such as atherosclerosis, aneurysms and varicose veins, musculoskeletal disorders such as osteoarthritis and bone resorption, and in various cancers. MMPs have also been implicated in tumor progression and invasiveness.
In this chapter, we will use data reported in PubMed and other scientific databases as well as data from our laboratory to provide a general overview of the biochemical and biological properties of MMPs with emphasis on MMP structure, tissue distribution, and protein substrates. We will then describe special properties of specific classes of MMPs and provide some examples of their role in cardiovascular diseases, inflammatory and musculoskeletal disorders, as well as cancer. We will then briefly discuss the regulation of MMP activity by endogenous tissue inhibitors of metalloproteinases (TIMPs). We will conclude the chapter by highlighting the potential benefits of MMPs as biomarkers and therapeutic targets in cardiovascular conditions, musculoskeletal disorders and cancer. Additional information regarding specific MMP functions can be found in other reports,1–4 and are elegantly reviewed in detail in the other chapters of this book.
2. MMP STRUCTURE
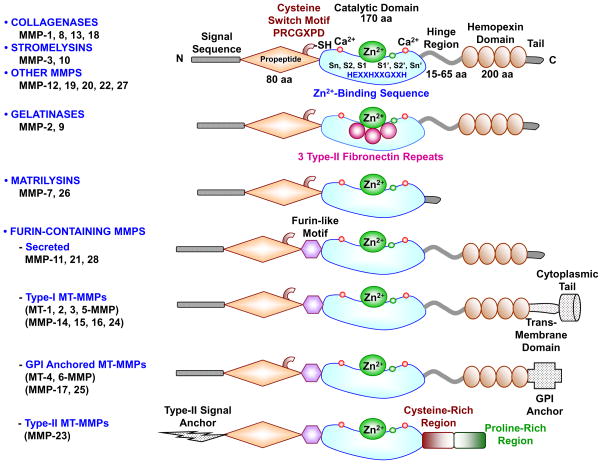
In the early 1960s, MMPs were first identified as a collagen proteolytic activity that causes ECM protein degradation during resorption of the tadpole tail.5 MMPs are now grown to a family of endopeptidases or matrixins that belong to the metzincins superfamily of proteases. MMPs are highly homologous, multidomain, zinc (Zn2+) containing metalloproteinases that degrade various protein components of ECM. The MMP family shares a common core structure. Typically MMPs consist of a propeptide of about 80 amino acids, a catalytic metalloproteinase domain of about 170 amino acids, a linker peptide (hinge region) of variable length, and a hemopexin domain of about 200 amino acids (Fig. 1).6–9
Fig. 1.
Major MMPs subtypes and their structure. A typical MMP consists of a propeptide, a catalytic metalloproteinase domain, a linker peptide (hinge region), and a hemopexin domain. The propeptide has a cysteine switch PRCGXPD whose cysteine sulfhydryl (–SH) group chelates the active site Zn2+, keeping the MMP in the latent proMMP zymogen form. The catalytic domain contains the Zn2+ binding motif HEXXHXXGXXH, two Zn2+ ions (one catalytic and one structural), specific S1, S2,…Sn and S1′, S2′,…Sn′ pockets, which confer specificity, and two or three Ca2+ ions for stabilization. Some MMPs show exceptions in their structures. Gelatinases have 3 type-II fibronectin repeats in the catalytic domain. Matrilysins have neither a hinge region nor a hemopexin domain. Furin-containing MMPs such as MMP-11, 21 and 28 have a furin-like pro-protein convertase recognition sequence in the propeptide C-terminus. MMP-28 has a slightly different cysteine switch motif PRCGVTD. Membrane-type MMPs (MT-MMPs) typically have a transmembrane domain and a cytosolic domain. MMP-17 and -25 have a glycosylphosphatidylinositol (GPI) anchor. MMP-23 lacks the consensus PRCGXPD motif, has a cysteine residue located in a different sequence ALCLLPA, may remain in the latent inactive proform through its type-II signal anchor, and has a cysteine-rich region and an immunoglobulin-like proline-rich region.
Most MMPs also share three important characteristics. First, MMPs show homology to collagenase-1 (MMP-1). MMP-7, -23 and -26 are exceptions as they lack the linker peptide and the hemopexin domain. MMP-23 has a unique C-terminal cysteine-rich domain and an immunoglobulin-like domain immediately after the C-terminus of the catalytic domain. Second, MMPs contain a cysteine switch motif PRCGXPD in which the cysteine sulfhydryl group chelates the active site Zn2+ thus keeping MMPs in their inactive proMMP zymogen form. Third, the catalytic domain of MMPs harbors a Zn2+-binding motif to which the Zn2+ ion is bound by three histidines from the conserved sequence HEXXHXXGXXH, with the assistance of a conserved glutamate, and a conserved methionine sequence XBMX (Met-turn) located 8-residues down from the Zn2+ binding motif that supports the structure surrounding the catalytic Zn2+ (Fig. 1).10–12
In vertebrates, the MMP family comprises 28 members, at least 23 are expressed in human tissues, and 14 of those MMPs are expressed in the vasculature (Table 1).10 MMPs are commonly classified on the basis of their substrates and the organization of their structural domains into collagenases, gelatinases, stromelysins, matrilysins, membrane-type (MT)-MMPs, and other MMPs. Additionally, different classes of MMPs have specific structural features that distinguish them from the prototypical MMP structure (Fig. 1).2,13,14 The topology of MMPs is well conserved, and a major difference between MMPs lies in the S1′ subsite, a well-defined hydrophobic pocket of variable depth that is critical for specific MMP-substrate interaction.15
Table 1.
Members of the MMP family, and their tissue distribution and substrates
| MMP (Other Name) Chromosome |
MW KDa Pro/Active | Distribution | Collagen Substrates | Non-Collagen ECM Substrates | Other Targets and Substrates |
|---|---|---|---|---|---|
|
Collagenases MMP-1 (Collagenase-1) 11q22.3 |
55/45 | Endothelium, intima, SMCs, fibroblasts, vascular adventitia, platelets, varicose veins (interstitial/fibroblast collagenase) | I, II, III, VII, VIII, X, gelatin | Aggrecan, nidogen, perlecan, proteoglycan link protein, serpins, tenascin-C, versican | Casein, α1-antichymotrypsin, α1-antitrypsin, α1-proteinase inhibitor, IGF-BP-3 and -5, IL-1β, L-selectin, ovostatin, pro-TNF-α, SDF-1 |
| MMP-8 (Collagenase-2) 11q22.3 |
75/55 | Macrophages, neutrophils (PMNL or neutrophil collagenase) | I, II, III, V, VII, VIII, X, gelatin | Aggrecan, elastin, fibronectin, laminin, nidogen | α2-antiplasmin, proMMP-8 |
| MMP-13 (Collagenase-3) 11q22.3 |
60/48 | SMCs, macrophages, varicose veins, preeclampsia, breast cancer | I, II, III, IV, gelatin | Aggrecan, fibronectin, laminin, perlecan, tenascin | Casein, plasminogen activator 2, proMMP-9 and -13, SDF-1 |
| MMP-18 (Collagenase-4) 12q14 |
70/53 | Xenopus (amphibian, Xenopus collagenase) heart, lung, colon | I, II, III, gelatin | α1-antitrypsin | |
|
Gelatinases MMP-2 (Gelatinase-A, Type IV Collagenase) 16q13-q21 |
72/63 | Endothelium, VSM, adventitia, platelets, leukocytes, aortic aneurysm, varicose veins | I, II, III, IV, V, VII, X, XI, gelatin | Aggrecan, elastin, fibronectin, laminin, nidogen, proteoglycan link protein, versican | Active MMP-9 and -13, FGF-R1, IGF-BP-3 and -5, IL-1β, pro-TNF-α, TGF-β |
| MMP-9 (Gelatinase-B, Type IV Collagenase) 20q11.2-q13.1 |
92/86 | Endothelium, VSM, adventitia, microvessels, macrophages, aortic aneurysm, varicose veins | IV, V, VII, X, XIV, gelatin | Aggrecan, elastin, fibronectin, laminin, nidogen, proteoglycan link protein, versican | CXCL5, IL-1β, IL2-R, plasminogen, pro-TNF-α, SDF-1, TGF-β |
|
Stromelysins MMP-3 (Stromelysin-1) 11q22.3 |
57/45 | Endothelium, intima, VSM, platelets, coronary artery disease, hypertension, varicose veins, synovial fibroblasts, tumor invasion | II, III, IV, IX, X, XI, gelatin | Aggrecan, decorin, elastin, fibronectin, laminin, nidogen, perlecan, proteoglycan, proteoglycan link protein, versican | Casein, α1-antichymotrypsin, α1-proteinase inhibitor, antithrombin III, E-cadherin, fibrinogen, IGF-BP-3, L-selectin, ovostatin, pro-HB-EGF, pro-IL-1β, proMMP-1, -8 and -9, pro-TNF-α, SDF-1 |
| MMP-10 (Stromelysin-2) 11q22.3 |
57/44 | Atherosclerosis, uterus, preeclampsia, arthritis, carcinoma cells | III, IV, V, gelatin | Aggrecan, elastin, fibronectin, laminin, nidogen | Casein, proMMP-1, -8 and -10 |
| MMP-11 (Stromelysin-3) 22q11.23 |
51/44 | Brain, uterus, angiogenesis | Does not cleave | Aggrecan, fibronectin, laminin | α1-antitrypsin, α1-proteinase inhibitor, IGF-BP-1 |
|
Matrilysins MMP-7 (Matrilysin-1) 11q21-q22 |
29/20 | Endothelium, intima, VSM, uterus, varicose veins (PUMP) | IV, X, gelatin | Aggrecan, elastin, enactin, fibronectin, laminin, proteoglycan link protein | Casein, β4 integrin, decorin, defensin, E-cadherin, Fas-ligand, plasminogen, proMMP-2, -7 and -8, pro-TNF-α, syndecan, transferrin |
| MMP-26 (Matrilysin-2, Endometase) 11p15 |
28/19 | Breast cancer, endometrial tumors | IV, gelatin | Fibrinogen, fibronectin, vitronectin | Casein, β1-proteinase inhibitor, fibrin, fibronectin, proMMP-2 |
|
Membrane-Type MMP-14 (MT1-MMP) 14q11-q12 |
66/56 | VSM, fibroblasts, platelets, brain, uterus, angiogenesis | I, II, III, gelatin | Aggrecan, elastin, fibrin, fibronectin, laminin, nidogen, perlecan, proteoglycan, tenascin, vitronectin | αvβ3 integrin, CD44, proMMP-2 and -13, pro-TNF-α, SDF-1, α1-proteinase inhibitor, tissue transglutaminase |
| MMP-15 (MT2-MMP) 16q13 |
72/50 | Fibroblasts, leukocytes, preeclampsia | I, gelatin | Aggrecan, fibronectin, laminin, nidogen, perlecan, tenascin, vitronectin | ProMMP-2 and -13, tissue transglutaminase |
| MMP-16 (MT3-MMP) 8q21.3 |
64/52 | Leukocytes, angiogenesis | I | Aggrecan, fibronectin, laminin, perlecan, vitronectin | Casein, proMMP-2 and -13 |
| MMP-17 (MT4-MMP) 12q24.3 |
57/53 | Brain, breast cancer | Gelatin | Fibrin | |
| MMP-24 (MT5-MMP) 20q11.2 |
57/53 | Leukocytes, lung, pancreas, kidney, brain, astrocytoma, glioblastoma | Gelatin | Chondroitin sulfate, dermatin sulfate, fibrin, fibronectin, N-cadherin | ProMMP-2 and -13 |
| MMP-25 (MT6-MMP) 16p13.3 |
34/28 | Leukocytes (Leukolysin), anaplastic astrocytomas, glioblastomas | IV, gelatin | Fibrin, fibronectin, proMMP-2, α1-proteinase inhibitor | |
|
Other MMPs MMP-12 (Metalloelastase) 11q22.3 |
54/45 - 22 | SMCs, fibroblasts, macrophages, great saphenous vein | IV, gelatin | Elastin, fibronectin, laminin | Casein, plasminogen |
| MMP-19 (RASI-1) 12q14 |
54/45 | Liver | I, IV, gelatin | Aggrecan, fibronectin, laminin, nidogen, tenascin | Casein |
| MMP-20 (Enamelysin) 11q22.3 |
54/22 | Tooth enamel | V | Aggrecan, cartilage oligomeric protein, amelogenin | |
| MMP-21 (Xenopus-MMP) 10q26.13 |
62/49 | Fibroblasts, macrophages, placenta | α1-antitrypsin | ||
| MMP-22 (Chicken-MMP) 1p36.3 |
51 | Chicken fibroblasts | Gelatin | ||
| MMP-23 (CA-MMP) 1p36.3 |
28/19 | Ovary, testis, prostate Other (type II) MT-MMP |
Gelatin | ||
| MMP-27 (Human MMP-22 homolog) 11q24 |
Heart, leukocytes, macrophages, kidney, endometrium, menstruation, bone, osteoarthritis, breast cancer | ||||
| MMP-28 (Epilysin) 17q21.1 |
56/45 | Skin, keratinocytes | Casein |
CA-MMP, cysteine array MMP; CXCL5, chemokine (C-X-C motif) ligand 5; FGF-R1, fibroblast growth factor receptor 1; IGF-BP, insulin-like growth factor binding protein; IL, interleukin; MW, molecular mass; PMNL, polymorphonuclear leukocytes; pro-HB-EGF, pro-heparin-binding epidermal growth factor-like growth factor; RASI-1, rheumatoid arthritis synovium inflamed-1; SDF-1, stromal cell-derived factor-1
3. SOURCES and TISSUE DISTRIBUTION of MMPS
MMPs are produced by multiple tissues and cells (Table 1). MMPs are secreted by connective tissue, pro-inflammatory, and uteroplacental cells including fibroblasts, osteoblasts, endothelial cells, vascular smooth muscle (VSM), macrophages, neutrophils, lymphocytes, and cytotrophoblasts.
Dermal fibroblasts and leukocytes are major sources of MMPs, especially MMP-2,16 and platelets are important sources of MMP-1, -2, -3, and -14.17 In general, MMPs are either secreted from the cells or anchored to the plasma membrane by proteoglycans such as heparan sulfate glycosaminoglycans.10 MT-MMPs and MMP-23 are anchored to the cell membrane by special trans-membrane domains.
Because MMPs play a major role in ECM remodeling, they are highly distributed in most connective tissues. MMPs have also been localized in many cell types, suggesting other biological roles for MMPs. For example, MMP-1, -2, -3, -7, -8, -9, -12, -13, and MT1-MMP and MT3-MMP, are expressed in various vascular tissues and cells.18 In the rat inferior vena cava (IVC), MMP-2 and -9 are localized in different layers of the venous wall including the intima, media and adventitia, suggesting interaction with signaling pathways in endothelial cells, VSM, and ECM, respectively.19 Other studies showed specific distribution of MMP-1, -2, -3, and -7 in endothelial cells and VSMCs, MMP-2 in the adventitia,20 MMP-9 in endothelial cells, medial VSMCs, and adventitial microvessels, and MMP-12 in VSMCs and fibroblasts of human great saphenous vein,21 Other studies showed intracellular localization of MMP-2 within cardiac myocytes, and colocalization of MMP-2 with troponin I within the cardiac myofilaments. MMP-2 activity has also been detected in nuclear extracts from both human heart and rat liver. Poly ADP-ribose polymerase is a nuclear matrix enzyme involved in DNA repair. Interestingly, poly ADP-ribose polymerase is susceptible to cleavage by MMP-2 in vitro, and its cleavage is blocked by MMP inhibitors. MMP-2 localization within the nucleus could play a role in degradation of poly ADP-ribose polymerase, and thereby affect DNA repair.22
4. MMP ACTIVATION
MMPs are regulated at multiple levels including mRNA expression, activation of the proenzyme to the active form, and the counteracting actions of endogenous TIMPs. MMPs are synthesized as pre-proMMPs, from which the signal peptide is removed during translation to generate proMMPs. In these zymogens or proMMPs, the cysteine from the PRCGXPD ‘cysteine switch’ motif coordinates with the catalytic Zn2+ to keep the proMMPs inactive.6 In order to process and activate these zymogens or proMMPs, the cysteine switch is cleaved and the prodomain is detached often by other proteolytic enzymes such as serine proteases, the endopeptidase furin, plasmin, or other MMPs to produce the active MMP forms.6 Furin-containing MMPs such as MMP-11, -21, and -28 and MT-MMPs have a furin-like pro-protein convertase recognition sequence at the C-terminus of the propeptide and are activated intracellularly by furin (Fig. 1).23 MT-MMPs first undergo intracellular activation by furin, then proceed to the cell surface where thay can cleave and activate other proMMPs.13 TIMPs are also essential for the formation of non-inhibitory proMMP/TIMP/MT-MMP complexs. Non-inhibitory complexes between progelatinases and TIMPs are restricted to proMMP-2 and TIMP-2, -3, or -4, and to MMP-9 and TIMP-1.24 For example, TIMP-2 first forms a complex with proMMP-2 by binding to its hemopexin domain, and the complex then localizes to the cell surface where it binds to the active site of a MT1-MMP molecule.25–28 This ternary proMMP-2/TIMP-2/MT1-MMP complex then facilitates the cleavage and activation of its bound proMMP-2 to active MMP-2 by another “free” MT1-MMP molecule. This non-inhibitory complex is different from the inhibitory complex of TIMP-2/active MMP-2. It is formed between the C-terminal domain of TIMP-2 and the C-terminal hemopexin of MMP-2, such that both molecules maintain their inhibitory and proteolytic properties, respectively.24,29,30 The activation of MMP-2 on the cell surface allows it to accumulate pericellularly where it could reach marked collagenolytic activity locally in the extracellular space.10 Similarly, the stromelysins MMP-3 and -10 are secreted from the cells as inactive proMMPs, but are then activated on the cell surface. MMPs can also be activated by various physicochemical agents including heat, low pH, thiol-modifying agents such as 4-aminophenylmercuric acetate, mercury chloride, N-ethylmaleimide, oxidized glutathione, sodium dodecyl sulfate, and chaotropic agents. Most of these activators disrupt the cysteine-Zn2+ coordination at the cysteine switch motif of the MMP molecule. Other MMP activators include plasmin which activates MMP-9. Also, both MMP-3 and hypochlorous acid activate MMP-7, and MMP-7 could in turn activate MMP-1.2
MMP expression/activity can also be influenced by hormones, growth factors, and cytokines.31 For example, ovarian sex hormones could affect the expression/activity of various MMPs which could in turn participate in endometrial tissue remodeling and shedding during the menstrual and estrous cycles. Also, increases in estrogen and progesterone as well as vascular endothelial growth factor (VEGF) and placental growth factor during pregnancy could promote the expression/activity of uteroplacental MMPs and in turn facilitate cytotrophoblast tissue invasion and uteroplacental growth and vascularization. MMP expression/activity also increases during the inflammatory process. MMPs are secreted by pro-inflammatory cells and their secretion is promoted by pro-inflammatory cytokines.
MMPs can be regulated by growth factors.32 For example, overexpression of VEGFa in SNU-5 cells increases MMP-2 expression, while downregulation of VEGFa decreases MMP-2 expression.33 Also, platelet derived growth factor-BB (PDGF-BB) increases MMP-2 expression in rat VSMCs, possibly via Rho-associated protein kinase, extracellular signal-regulated kinases (ERK), and phosphorylation of p38 mitogen-activated protein kinase (MAPK).34 Also, in carotid artery plaques, epidermal growth factor (EGF) upregulates MMP-1 and -9 mRNA transcripts and increases MMP-9 activity in VSMCs.35 In contrast, transforming growth factor-β1 (TGF-β1) may downregulate MMPs via a TGF-β1 inhibitory element in the MMP promoter. Interestingly, MMP-2 does not have this element, and therefore may not be affected, or in some instances upregulated, by TGF-β1.
MMP activity is also regulated by endogenous TIMPs. Increased MMP expression/activity or decreased TIMPs could lead to MMP/TIMP imbalance and results in various pathological conditions most notably heart failure, osteoarthritis and cancer.
5. MMP SUBSTRATES
ECM has three main components; fibers, proteoglycans and polysaccharides. Fibers are largely glycoproteins that include collagen, which is the main ECM protein, and elastin, which is not glycosylated and provides plasticity and flexibility to certain tissues such as the arteries, lungs and skin. Laminin is a glycoprotein localized in the basal lamina of the epithelium. Fibronectin is a glycoprotein used by cells to bind to ECM, and can modulate the cytoskeleton to facilitate or hinder cell movement. Proteoglycans have more carbohydrates than proteins, and attract water to keep the ECM hydrated. Proteoglycans also facilitate binding of growth factors to the ECM milieu. Syndecan-1 is a proteoglycan and integral transmembrane protein that bind chemotactic cytokines during the inflammatory process. Other ECM proteins include glycoproteins such as vitronectin, aggrecan, entactin, fibrin and tenascin, and polysaccharides such as hyaluronic acid.2
MMPs play a major role in tissue remodeling by promoting turnover of various ECM proteins including collagens, elastin, gelatin, and other matrix glycoproteins and proteoglycans. Collagen and elastin are essential for the structural integrity of the vascular wall and are important MMP substrates. MMPs break down collagen type I, II, III, IV, V, VI, VII, VIII, IX, X, and XIV with different efficacies. MMP degrades other ECM protein substrates such as aggrecan, entactin, fibronectin, tenascin, laminin, myelin basic protein, and vitronectin (Table 1). While casein is not a physiological MMP substrate, it is digested by several MMPs and, therefore, is used to measure the activity of these MMPs in zymography assays.2
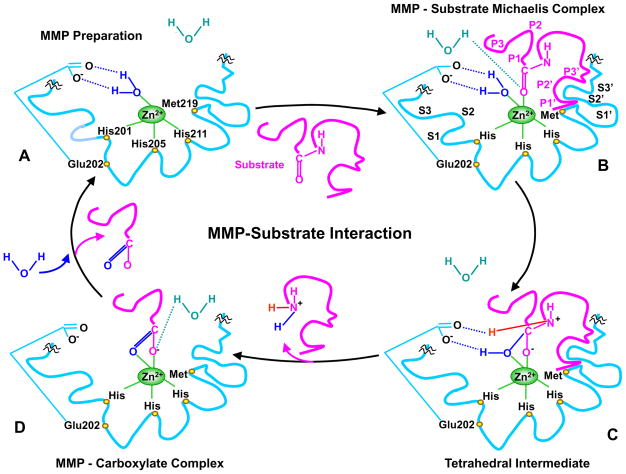
The hemopexin domain may confer most of the MMP substrate specificity.36,37 The hemopexin domain may be essential in the recognition and subsequent catalytic degradation of fibrillar collagen, whereas the catalytic domain may be sufficient in the degradation of non-collagen substrates.10 MMPs catalytic activity generally requires Zn2+ and a water molecule flanked by three conserved histidine residues and a conserved glutamate, with a conserved methionine acting as a hydrophobic base to support the structure surrounding the catalytic Zn2+ in the MMP molecule. During the initial transition states of the MMP-substrate interaction, Zn2+ is penta-coordinated with a substrate’s carbonyl oxygen atom, one oxygen atom from the MMP glutamate-bound water, and the three conserved histidines in the MMP molecule. The Zn2+-bound water then performs a nucleophilic attack on the substrate, resulting in the breakdown of the substrate and the release of a water molecule (Fig. 2).11,38–40 The MMP-substrate interaction may involve alternative transition states whereby Zn2+ is penta-coordinated with a substrate’s carbonyl oxygen atom, two oxygen atoms from the MMP conserved glutamate, and two of the three conserved histidines. One oxygen from glutamate then performs a nucleophilic attack and causes breakdown of the substrate.41 Peptide catalysis and substrate degradation is also influenced by specific subsites or pockets (S) within the MMP molecule that interact with corresponding substituents (P) in the substrates (Fig. 2). The most important pocket for substrate specificity and binding is the MMP S1′ pocket, which is extremely variable and could have a shallow, intermediate, or deep location.11,38,39 MMPs with shallow S1′ pocket include MMP-1 and -7. MMP-2, -9, and -13 have intermediate S1′ pocket, while MMP-3, -8, and -12 have deep S1′ pocket.38 S2′ and S3′ pockets are shallower than S1′ pocket and, therefore, are more exposed to solvents than S1′.39 Second to the S1′ pocket, the S3 pocket may contribute to substrate specificity.6
Fig. 2.
MMP-substrate interaction. MMP-3 is used as an example, and slight variations in the MMP-substrate-interaction and the positions of the conserved His and Glu may occur with other MMPs. Only the MMP catalytic domain is illustrated, and the remaining part of the MMP molecule is truncated by squiggles. A) In the quiescent MMP molecule, the catalytic Zn2+ is supported in the HEXXHXXGXXH-motif by binding to the imidazole rings of the 3 histidines His201, 205, 211. Additionally, the methionine-219 (Met219) in the conserved XBMX Met-turn acts as a hydrophobic base to further support the structure surrounding the catalytic Zn2+. In preparation of MMP for substrate binding, an incoming H2O molecule is polarized between the MMP acidic Zn2+ and basic glutamate-202 (Glu202). B) Using H+ from free H2O, the substrate carbonyl group binds to Zn2+, forming a Michaelis complex. This allows the MMP S1, S2, S3, …Sn pockets on the right side of Zn2+ and the primed S1′, S2′, S3′, …Sn′ pockets on the left side of Zn2+ to confer specific binding to the substrate P1, P2, P3, … Pn and the primed P1′, P2′, P3′, … Pn′ substituents, respectively. The MMP pockets are organized such that the S1 and S3 pockets are located away from the catalytic Zn2+, while the S2 pocket is closer to Zn2+. C) The substrate-bound H2O is freed, the Zn2+-bound O from the Glu-bound H2O executes a nucleophilic attack on the substrate carbon, and the Glu202 extracts a proton from the Glu-bound H2O to form an N-H bond with the substrate N, resulting in a tetrahedral intermediate. D) Freed H2O is taken up again, and the second proton from Glu-bound H2O is transferred to the substrate, forming an additional N-H bond. As a result, the substrate scissile C-N bond breaks, thus releasing the N portion of the substrate while the carboxylate portion of the substrate remains in an MMP-carboxylate complex. Another H2O is taken up, thus releasing the remaining carboxylate portion of the substrate, and the MMP is prepared to attack another substrate (A).
Specific MMPs degrade specific protein substrates. Stromelysin-1 and -2 (MMP-3 and -10) do not cleave interstitial collagen, but degrade other ECM protein substrates and may participate in cleaving certain proMMPs to their active form. Although MMP-3 and -10 have similar substrate specificity, MMP-3 has greater proteolytic efficiency than MMP-10. Stromelysin-3 (MMP-11) is distantly related to stromelysin-1 and -2. MMP-11 does not cleave interstitial collagen, and shows very weak proteolytic activity toward other ECM protein substrates.23 Importantly, different MMPs may cooperate in order to completely degrade a protein substrate. For example, the collagenases MMP-1, -13, and -18 first unwind triple helical collagen and hydrolyze the peptide bonds of fibrillar collagen type I, II and III into ¾ and ¼ fragments.6,42 The resulting single α-chain gelatins are further degraded by the gelatinases MMP-2 and -9 into smaller oligopeptides.36 Gelatinases have three type-II fibronectin repeats in their catalytic domain that allow them to bind not only gelatin, but also collagen and laminin (Fig. 1). Therefore, while MMP-2 is primarily a gelatinase, it can function much like the collagenase MMP-1, albeit in a weaker manner.6 MMP-2 can degrade collagen in two steps; first by inducing a weak interstitial collagenase-like collagen degradation into ¾ and ¼ fragments, then by promoting gelatinolysis using the fibronectin-like domain.43 MMP-9 could also act as a collagenase and gelatinase. As a collagenase, MMP-9 binds the α2 chains of collagen IV with high affinity even when it is inactive, making the substrate readily available.44
6. MMPS, ECM DEGRADATION, AND TISSUE REMODELING
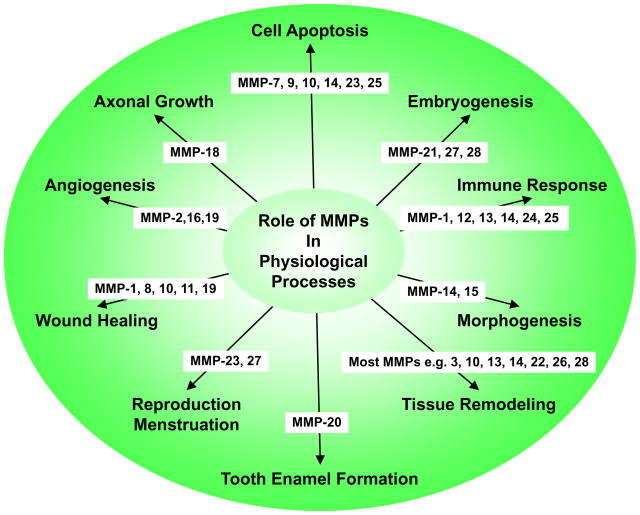
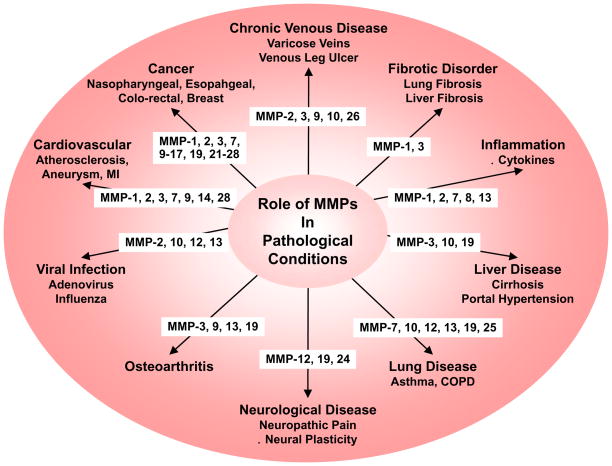
MMPs are important in many biological processes including cell proliferation, migration, and differentiation, remodeling of ECM, and tissue invasion and vascularization (Fig. 3). These biological processes take place multiple times during normal development and organogenesis, but, if not properly balanced, could also contribute to harmful pathological conditions such as cancer, tumor progression and tissue invasion (Fig. 4). MMPs can participate in these processes by several mechanisms including proteolytic cleavage of growth factors so that they become available to cells that are not in direct physical contact, degradation of ECM so that founder cells can move across the tissues into nearby stroma, and regulated receptor cleavage in order to terminate migratory signaling and cell migration.45
Fig. 3.
Representative roles of MMPs in physiological processes.
Fig. 4.
Representative roles of MMPs in pathological conditions. COPD, chronic obstructive pulmonary disease; MI, myocardial infarction
Dynamic modulation of the physical contacts between neighboring cells is integral to epithelial processes such as tissue repair. MMPs participate in tissue repair after acute injury.46 Induction of MMP activity contributes to the disassembly of intercellular junctions and the degradation of ECM, thus overcoming the physical constraint to cell movement.47
MMPs may affect VSMC growth, proliferation, and migration. MMPs induce the release of growth factors by cleaving the growth factor-binding proteins and matrix molecules.48 MMPs can facilitate VSMC proliferation by promoting permissive interactions between VSMCs and components of ECM, possibly via integrin-mediated pathways.49 MMP-1 and -9 increase human aortic SMC migration.50,51 MMP-induced ECM proteolysis can modulate cell-ECM adhesion either by removal of sites of adhesion or by exposing a binding site and in turn facilitate VSMC migration.
Alterations in MMPs expression/activity may be associated with cardiovascular disease. Evidence suggests associations between polymorphisms in MMP-1, 2, 3, 9, and 12 with ischemic stroke incidence, pathophysiology, and clinical outcome. Polymorphisms in the MMP genes can be influenced by racial and ethnic background., and could ultimately affect the presentation of ischemic stroke.52 MMPs also play key roles in the spread of viral infection, inflammation and remodeling of the respiratory airways and tissue fibrosis.53 MMPs may also participate in cancer development, progression, invasiveness and dissemination by promoting a pro-tumorigenic microenvironment and modulating the cell-ECM and cell-to-cell contacts.46 MMPs could break the cell to cell and cell to ECM adhesion, degrade ECM proteins, and promote angiogenesis, and thereby facilitate cancer invasion and metastasis.54
7. MMPS AND CELL SIGNALING
In addition to their role in ECM degradation, immunohistochemical studies have localized MMPs in many cell types. Localization of MMPs in certain cells not only supports that these cells could be a source of the MMPs released in ECM, but also suggests a role of MMP in cell signaling and intracellular pathways. Evidence for MMP-induced signaling pathways has been demonstrated in several tissues including blood vessels.55,56
7.1 MMPs and VSM Function
MMPs may affect VSM contraction mechanisms. VSM contraction is triggered by increases in Ca2+ release from the intracellular stores in the sarcoplasmic reticulum and Ca2+ entry from the extracellular space through different types of Ca2+ channels. We have shown that MMP-2 and -9 do not inhibit phenylephrine-induced contraction of isolated aortic segments incubated in Ca2+-free physiological solution, suggesting that these MMPs do not inhibit the Ca2+ release mechanism from the intracellular stores.57 However, MMP-2 and -9 cause relaxation of phenylephrine-precontracted aortic segments, and inhibit phenylephrine-induced Ca2+ influx.57 Similarly, MMP-2 inhibits Ca2+-dependent contraction mechanisms in isolated segments of rat inferior vena cava (IVC).56 It has been proposed that during substrate degradation MMPs may produce Arg-Gly-Asp (RGD)-containing peptides, which could bind to αvβ3 integrin receptors and inhibit Ca2+ entry into VSM.58 This is unlikely as RGD peptides do not affect IVC contraction.56 The mechanism by which MMPs inhibit Ca2+ entry could involve direct effects on Ca2+ or K+ channels. In rat IVC, MMP-2 induced relaxation is abolished in high KCl depolarizing solution, which prevents K+ ion from moving out of the cell via K+ channels. Importantly, blockade of large conductance Ca2+-activated K+ channels (BKCa) by iberiotoxin inhibits MMP-2 induced IVC relaxation, suggesting that MMP-2 actions may involve activation of BKCa and membrane hyperpolarization, which in turn decreases Ca2+ influx through voltage-gated Ca2+ channels.59 The MMP-induced inhibition of venous tissue Ca2+ influx and contraction may lead to prolonged venous dilation and varicose veins.
While MMP-2 and -9 reduce Ca2+ influx in both arteries and veins,56,57 veins differ from arteries in their structure and function, and the effects of MMPs on the veins should not always be generalized to the arteries. Veins have fewer layers of VSMCs compared to the several layers of VSMCs in the arteries. Also, venous and arterial VSMCs originate from distinct embryonic locations and are exposed to different pressures and hemodynamic effects in the circulation.60 Studies have also shown that MMP-2 expression is higher in cultured VSMCs from human saphenous veins compared with those from human coronary artery. In contrast, MMP-3, -10, -20, and -26 expression is less in saphenous vein than coronary artery VSMCs.60 Interestingly, while some studies suggest that MMP-2 and -9 levels could be similar in cultured saphenous vein and internal mammary artery VSMCs, venous VSMCs exhibit more proliferation, migration and invasion compared to arterial VSMCs.61 These observations make it important to further study the differences in the expression and activity of MMPs in veins versus arteries and in venous versus arterial disease.
7.2 MMPs and Endothelial Cell Function
The endothelium controls vascular tone by releasing relaxing factors including nitric oxide and prostacyclin, and through hyperpolarization of the underlying VSMCs by endothelial-derived hyperpolarizing factor (EDHF).62 MMPs may stimulate protease activated receptors (PARs). PARs 1–4 are G-protein coupled receptors that have been identified in humans and other species. PAR-1 is expressed in VSMCs,63 endothelial cells, and platelets 64 and is coupled to increased nitic oxide production,65 and in turn contributes to vasodilation. MMP-1 has been shown to activate PAR-1.66
EDHF-mediated relaxation may involve the opening of small and intermediate conductance Ca2+-activated K+ channels and hyperpolarization of endothelial cells. Endothelial cell hyperpolarization may spread via myoendothelial gap junctions and cause relaxation of VSMCs. EDHF could also cause hyperpolarization through opening of BKCa in VSM.62 MMP-2 may increase EDHF release and enhance K+ efflux via BKCa, leading to venous tissue hyperpolarization and relaxation.59 In contrast, MMP-3 may impair endothelium-dependent vasodilation,67 making it important to further examine the effects of MMPs on EDHF.
8. SPECIAL ATTRIBUTES OF SPECIFIC MMPS
8.1 Collagenases
Collagenases include MMP-1 (interstitial collagenase), -8 (neutrophil collagenase), -13 and -18. These MMPs play an important role in cleaving fibrillar collagen type I, II and III into characteristic 3/4 and 1/4 fragments. They first unwind triple helical collagen, then hydrolyze the peptide bonds. The MMPs hemopexin domains are essential for cleaving native fibrillar collagen while the catalytic domains are needed for cleaving noncollagen substrates.42,68
MMP-1
MMP-1, also termed collagenase-1 or interstitial collagenase, has a gene locus on chromosome 11q22.3, i.e. MMP-1 is coded on the q arm of chromosome 11. MMP-1 degrades collagen and gelatin. MMP-1 also cleaves proMMP-9 into its active form. As with many other MMPs, the levels of MMP-1 are very low in most cells under physiological conditions, but are upregulated in inflammatory conditions and autoimmune disease.1 Increased levels and activities of MMP-1, -8, and -9 with relatively low levels of TIMP have been identified in slow-to-heal wounds and venous wounds.69 MMP-1 expression is augmented by inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1).70 In cultured human vocal fold fibroblasts, TNF-α inhibits cell proliferation, downregulates TIMP-3 and the mRNA transcript levels for collagen III and fibronectin, and upregulates MMP-1 and -2 expression, resulting in increased MMP/TIMP-3 ratio, which may accelerate wound healing following vocal fold injury.71 MMP-1 may also play a role in the circulatory disturbance and inflammation associated with sudden deafness. In a Korean population, a single nucleotide polymorphism (SNP) of MMP-1 at the promotor region -1607G/2G is associated with increased risk of sudden deafness when compared with the G/2G and G/G genotypes.70
Localized controlled release of anti-fibrogenic factors can prevent tissue fibrosis surrounding biomedical prostheses such as breast implants and vascular stents. In a rabbit ear fibrotic model, topically applied stratifin prevents dermal fibrosis and promotes normal tissue repair by regulating ECM deposition. Studies have tested the anti-fibrogenic effect of a controlled release form of stratifin in the prevention of fibrosis induced by dermal poly(lactic-co-glycolic acid) (PLGA) microsphere/poly(vinyl alcohol) (PVA) hydrogel implants. Controlled release of stratifin from PLGA microsphere/PVA hydrogel implants increased MMP-1 expression in the surrounding tissue, resulted in less collagen deposition, moderated dermal fibrosis and inflammation by reducing collagen deposition, total tissue cellularity and infiltrated CD3(+) immune cells in the surrounding tissue. These stratifin-eluting PLGA/PVA composites may be used as coatings to decrease fibrosis around implanted biomedical prostheses such as breast implants and vascular stents.72 Kynurenic acid is a downstream end product of kynurenine that has antiscarring properties and is unlikely to pass the blood brain barrier or cause central side effects. Studies showed that kynurenic acid did not cause adverse effects on dermal cell viability, and markedly increased the expression of MMP-1 and -3, and suppressed the production of type-I collagen and fibronectin by fibroblasts. The findings suggest that kynurenic acid could be a candidate antifibrogenic agent to improve healing outcome in patients at risk of hypertrophic scarring.73 Kynurenine treatment appears to increase the levels of MMP-1 and -3 expression through activation of the (MAPK)/extracellular signal-regulated kinase (ERK1/2) signaling pathway.74 In human primary chondrocytes, IL-1β-induced activation of p38 MAPK may increase MMP-1 and -13 production and glycosaminoglycan release. Thus, activated p38 could accelerate cartilage breakdown by enhancing the expression of MMP-1 and -13 which promote collagen cleavage, and therefore p38 inhibitors may have chondroprotective effects in osteoarthritis.75
MMP-1 may play a role in cancer development and metastasis. Studies have suggested an association between SNP of MMP-1 -1607 2G/2G and poor prognosis in malignant tumors such as tongue squamous cell carcinoma.76 Also, in patients with invasive well-differentiated thyroid carcinoma, MMP-1 expression correlates with tumor aggressiveness manifested as laryngotracheal invasion, multifocality of the tumor, and the presence of metastases. MMP-1 expression is associated with poor prognosis in esophageal cancer,77 and may serve as a prognostic marker and an indicator for the need for more aggressive surgical intervention.78
MMP-8
MMP-8, also termed collagenase-2 or neutrophil collagenase, has a gene locus on chromosome 11q22.3. MMP-8 was discovered in cDNA library constructed from mRNA extracted from peripheral leukocytes of a patient with chronic granulocytic leukemia. The library was screened with an oligonucleotide probe constructed from the putative Zn2+-binding region of fibroblast collagenase. Eleven positive clones were identified, of which the one bearing the largest insert (2.2 kilobases) was sequenced. From the nucleotide sequence of the 2.2-kb cDNA clone, a 467-amino acid sequence representing the entire coding sequence of the enzyme was deduced.79 Being a collagenase, MMP-8 can cleave interstitial collagens I, II, and III at a site within the triple helical domain about 3/4 down from the N-terminus.10 While some proMMPs are secreted then form heterodimeric complexes bound to TIMPs, e.g. the MMP-2/TIMP-2 complex, secreted proMMP-8 remains in its free form. The proMMP-8 activity is then regulated by proteolytic cleavage by other MMPs such as MMP-3 and -10.80
MMP-8 is the first collagenase to appear during dermal wound-healing and its levels peak earlier than that of MMP-1, supporting time-dependent expression of different MMPs during wound healing.81 Mice deficient in MMP-8 show delayed healing of cutaneous wounds, and increased inflammatory responses, supporting that MMP-8 is a necessary component in dermal wound healing and the regulation of the inflammatory process.82 In a study to assess the temporal relationship between periodontal tissue destruction and activity of collagenase, exudate from inflamed periodontal tissues was collected, and latent and active collagenase activities were measured. It was found that the collagenase activity was derived from neutrophils, and there was an overall 40% increase of pooled active collagenase activity in all subjects with progressive loss of connective tissue. These findings suggest a role of neutrophil collagenase or MMP-8 in the destruction of periodontal connective tissue, and MMP-8 expression in the saliva may be used as a marker of diseases involving connective tissue breakdown and advanced periodontitis.83 MMP-8 can also be detected and analyzed in gingival crevicular fluid using time-resolved immunofluorometric assay, a MMP-8 specific chair-side dip-stick test, a dentoAnalyzer device and ELISA kit. Western immunoblots confirmed that immunofluorometric assay and dentoAnalyzer can detect activated 55 kDa MMP-8 species in periodontitis-affected gingival crevicular fluid.84
MMP-13
MMP-13, also termed collagenase-3, has a gene locus on chromosome 11q22.3. MMP-13 is very efficient in degrading type II collagen. MMP-13 was first thought to be expressed in connective tissue particularly cartilage and developing bone. However, MMP-13 has also been detected in epithelial and neuronal cells. MMP-13 is overexpressed in cartilage tissues of osteoarthritis patients, and increased expression of MMP-13 in chondrocytes may contribute to the development of osteoarthritis.85 MMP-13 has been suggested as a direct target gene of micoRNA miR-411 in chondrocytes. Overexpression of miR-411 inhibits MMP-13 expression, and increases the expression of type II and IV collagen in chondrocytes. In comparison with normal cartilage, osteoarthritis cartilage shows downregulation of miR-411 and increased MMP-13 expression. These findings suggest that miR-411 may regulate MMP-13 expression and ECM remodeling in human chondrocytes, and may be a therapeutic target in treatment of osteoarthritis.86 Low ratio of linoleic acid (n-6)/α-linolenic acid (n-3) polyunsaturated fatty acids reduces MMP-13 expression in inflammatory chondrocytes in vitro and in vivo, and may be a means to control or reduce the symptoms of osteoarthritis. In cultured human chondrocytes low 1:1 and 2:1 n-6/n-3 ratios decreased the mRNA expression and protein levels of MMP-13 without affecting chondrocytes proliferation. In rat model of arthritis produced by injection of Freund’s complete adjuvant, low 1:1 and 2:1 n-6/n-3 dietary ratio reduced paw swelling rate, decreased serum MMP-13 and IL-1 levels, and alleviated cartilage damage.87
MMP-13 may be involved in lung diseases such as acute lung injury, viral infections, and chronic obstructive pulmonary disease. In human small airway epithelial cells, polyinosinic-polycytidylic acid stimulated the secretion of MMP-13, and MMP-13 secretion was abolished by p38 MAPK inhibitor SB304680, phosphoinositide 3-kinase (PI3K) inhibitor LY294002, Janus kinase (JAK) inhibitor I, RNA-activated protein kinase inhibitor, and nuclear factor κB (NFκB) inhibitor Bay 11-7082. Interferon-β (IFN-β) also caused stimulation of MMP-13 secretion that was inhibited by all modulators except Bay 11-7082. It was suggested that MMP-13 secretion was mediated through IFN receptor pathways independently of NFκB and that polyinosinic-polycytidylic acid stimulated IFN secretion in an NFκB-dependent manner, leading to IFN-stimulated MMP-13 secretion from human small airway epithelial cells. MMP-13 inhibitors and MMP-13 siRNA inhibited IFN-stimulated secretion of IFNγ-inducible protein 10 and regulated on activation normal T-cell expressed and secreted (RANTES), suggesting that MMP-13 is involved in the secretion of these virus-induced proinflammatory chemokines. Also, a novel polymorphism was identified in the promoter region of the MMP-13 gene. These observations support that MMP-13 plays a role in defense mechanisms of airway epithelial cells.88
MMP-13 may be involved in ECM degradation in brain astrocytes. Conditioned medium collected from activated microglia increased IL-18 production and enhanced MMP-13 expression in astrocytes. Treatment with recombinant IL-18 increased MMP-13 protein and mRNA levels in astrocytes. Recombinant IL-18 stimulation also increased the enzymatic activity of MMP-13 and the migratory activity of astrocytes, and MMP-13 or pan-MMP inhibitors antagonized IL-18-induced migratory activity of astrocytes. Treatment of astrocytes with recombinant IL-18 led to the phosphorylation of JNK, Akt, or PKC-δ, and treatment of astrocytes with JNK, PI3K/Akt, or PKC-δ inhibitors decreased IL-18-induced migratory activity. These findings suggest that IL-18 is an important regulator of MMP-13 expression and cell migration in astrocytes, likely via JNK, PI3K/Akt, and PKC-δ signaling pathways.89
Liver fibrosis is the final stage of liver diseases that lead to liver failure and cancer and studies have tested whether overexpressing MMP-13 gene in rat liver could prevent liver fibrosis progression. In a rat model of liver fibrosis model established by ligating the bile duct, liver-targeted hydrodynamic gene delivery of a MMP-13 expression vector, containing a CAG promoter-MMP13-IRES-tdTomato-polyA cassette caused a peak in serum level of MMP-13 after 14 days that was sustained for the next 60 days. Hyaluronic acid levels were lower in the treated versus nontreated rats, suggesting therapeutic effect of MMP-13 overexpression. Quantitative analysis of tissues stained with the collagen stain sirius red showed a statistically smaller volume of fibrotic tissue in MMP-13-treated versus nontreated rats. Liver-targeted hydrodynamic delivery of MMP-13 gene could be useful in prevention of liver fibrosis.90
MMP-13 is often overexpressed in tumors and may increase the risk of tumor progression and metastasis. MMP-13 is overexpressed in nasopharyngeal cancer cells and exosomes purified from conditioned medium, as well as plasma of nasopharyngeal cancer patients. Transwell analysis revealed that MMP-13-containing exosomes facilitated the metastasis of nasopharyngeal cancer cells. MMP-13 siRNA reduced the effect of MMP13-containing exosomes on tumor cell metastasis and angiogenesis.91
MMP-18
MMP-18, also termed collagenase-4, has a gene locus on chromosome 12q14. In the 1990s, sequence similarity searching of databases containing expressed sequence tags identified a partial cDNA encoding the 3′ end of a putative novel human MMP. The remaining 5′ end of the MMP cDNA was amplified by PCR from human mammary gland cDNA. The predicted protein product displayed all the structural features characteristic of the MMP family and showed closest identity with MMP-1, -3, -10, and 11, and was designated MMP-18. MMP-18 differs structurally from other MMPs in that its amino acid sequence contains two cleavage sites for activation. MMP-18 mRNA is expressed in several normal human tissues, but is not detected in the brain, skeletal muscle, kidney, liver, or leukocytes.92 MMP-18 is expressed in migrating macrophages.93 Growth of peripheral axons is strongly attracted towards limb buds and skin explants in vitro. Directed axonal growth towards skin explants of Xenopus laevis in matrigel is associated with expression of MMP-18 and other MMPs, and is inhibited by the MMP inhibitors BB-94 and GM6001. Also, forced expression of MMP-18 in COS-7 cell aggregates enhances axonal growth from Xenopus dorsal root ganglia explants. Nidogen is the target of MMPs released by cultured skin in matrigel, whereas other components remain intact. These findings suggest a link between MMP-18 and ECM breakdown in the control of axonal growth.94 Despite its diverse tissue distribution and function, MMP-18 has not been directly linked to a specific pathological condition.
8.2 Gelatinases
Gelatinases include gelatinase A (MMP-2) and gelatinase B (MMP-9). MMP-2 and -9 are structurally similar to other proteinases in the MMP family, but differ in that they have a distinct collagen-binding domain composed of three fibronectin type II tandem repeats in the N-terminus of the catalytic domain, which is needed for gelatin binding.95,96 MMP-2 and -9 have been long recognized as major contributors to proteolytic degradation of ECM. In recent years, a plethora of non-matrix proteins have been identified as gelatinase substrates thus broadening our understanding of these enzymes as proteolytic executors and regulators in various physiological and pathological states including embryonic growth and development, angiogenesis, vascular diseases, inflammation, infective diseases, degenerative diseases of the brain and tumor progression.
MMP-2 and MMP-9 are particularly involved in cancer invasion and metastasis. Gelatin zymography in situ showed increased gelatinolytic activity of MMP-2 and -9 in esophageal squamous cell carcinomas, with different intensities of localization in the tumor nest itself and the stromal cells adjacent to tumor nests.97 Although the effect of broad-spectrum MMP inhibitors in the treatment of cancer has been disappointing in clinical trials, novel mechanisms of gelatinase inhibition have been identified. Inhibition of the association of gelatinases with cell-surface integrins appears to offer highly specific means to target these enzymes without inhibiting their catalytic activity in multiple cell types including endothelial cells, leukocytes, and tumor cells.98
MMP-2
MMP-2, also termed gelatinase-A or type IV collagenase, has a gene locus on chromosome 16q13-q21. MMP-2 cleaves collagen in two phases, the first resembling that of interstitial collagenase, followed by gelatinolysis, which is promoted by the fibronectin-like domain.36,43 The collagenolytic activity of MMP-2 is much weaker than collagenases. However, proMMP-2 is recruited to the cell surface and undergoes autocatalytic cleavage at the cell surface with the support of MT1-MMP/TIMP-2 complex, and therefore accumulates pericellularly and causes marked local collagenolytic activity.6,99 MMP-2 is ubiquitous in many cells and tissues and is involved in a variety of physiological and pathological processes, including angiogenesis, tissue repair, and inflammation. MMP-2 and its inhibitors TIMP-1 and -2, also play a role in tumor invasion and metastasis, and MMP-2/TIMPs imbalance may contribute to tumor progression. The involvement of MMP-2 in cancer has been studied in different malignancies including esophageal cancer.77,100 MMP-2 activity was correlated with lymph node metastasis, and lymphatic and vascular invasion, supporting an important role of MMP-2 in the invasion of esophageal carcinoma.97 MMP-2 levels also correlate with invasiveness of cancer cells and shortened survival independent of major prognostic indicators in patients with primary breast carcinoma.101 MMP-2 may play a role in malignant tumors of the central nervous system, and because of the highly proliferative and aggressive nature of these tumors, current treatments are not been very successful, and new lines of therapy to target MMP-2 have been explored. An adenoviral vector expressing small interfering RNA (siRNA) against the MMP-2 gene was constructed to specifically inhibit MMP-2 expression, and to test its effects on invasion, angiogenesis, tumor growth, and metastasis of A549 lung cancer cells. Adenoviral-mediated MMP-2 siRNA infection of A549 lung cancer cells caused down-regulation of MMP-2, mitigated lung cancer invasion and migration, and reduced tumor cell-induced angiogenesis in vitro. In a mouse model of metastatic lung tumor, treatment of established tumors with adenoviral-mediated MMP-2 siRNA inhibited subcutaneous tumor growth and formation of lung nodules in mice. Adenoviral-mediated MMP-2 siRNA may have a therapeutic potential for lung cancer in part by inhibiting angiogenesis.102
Integrins control a variety of signal transduction pathways central to cell survival, proliferation, and differentiation, and their functions and expression levels are altered in many types of cancer. In a study to examine the mechanisms underlying the involvement of α5β1 integrin in tumor invasion, its expression in MCF-7Dox human breast carcinoma cells was depleted using siRNA. Concomitant to α5β1 integrin depletion, there was a sharp decrease in MMP-2 expression and inhibition of the invasiveness of these cells in vitro. Similar reduction of invasion potential was observed upon siRNA-mediated silencing of the MMP-2 gene. Downregulation of α5β1 integrin was associated with decrease in the amounts of active phosphorylated forms of Akt, ERK1/2 kinases and c-Jun oncoprotein. Also, in MCF-7Dox cells, inhibition of these kinases reduced expression of MMP-2 and c-Jun, and suppressed invasion of the cells in vitro. Co-immunoprecipitation experiments demonstrated that α5β1 integrin interacted with MMP-2 on the surface of MCF-7Dox breast carcinoma cells. These findings suggest that α5β1 integrin controls invasion of breast cancer cells via regulation of MMP-2 expression through signaling pathways involving PI3K, Akt, and ERK kinases and the c-Jun or via direct recruitment of MMP-2 to the cell surface.103
MMP-2 is markedly upregulated in glioblastomas.104 Knockdown of MMP-2 using MMP-2 siRNA in human glioma xenograft cell lines 4910 and 5310 decreased cell proliferation. Cytokine array and Western blotting using tumor-conditioned media displayed modulated secretory levels of various cytokines including granulocyte-macrophage colony-stimulating factor, IL-6, IL-8, IL-10, TMF-α, angiogenin, VEGF and platelet-derived growth factor-BB (PDGF-BB) in MMP-2 knockdown cells. Further, cDNA PCR array suggested potential negative regulation of Janus kinase/Stat3 pathway in MMP-2 knockdown cells. Mechanistically, MMP-2 is involved in complex formation with α5β1 integrin and MMP-2 downregulation inhibited α5β1 integrin-mediated Stat3 phosphorylation and nuclear translocation. Electrophoretic mobility shift assay and chromatin immunoprecipitation assays showed inhibited Stat3 DNA-binding activity and recruitment at CyclinD1 and c-Myc promoters in MMP-2 siRNA-treated cells. MMP-2/α5β1 binding is enhanced in human recombinant MMP-2 treatments, resulting in elevated Stat3 DNA-binding activity and recruitment on CyclinD1 and c-Myc promoters. In vivo experiments in orthotropic tumor model revealed decreased tumor size upon treatment with MMP-2 siRNA. Immunofluorescence studies in tumor sections showed high expression and co-localization of MMP-2/α5β1, which is decreased along with reduced IL-6, phospho-Stat3, CyclinD1, and c-Myc expression levels upon treatment with MMP-2 siRNA. These observations suggest a role of MMP-2/α5β1 interaction in the regulation of α5β1-mediated IL-6/Stat3 signaling and highlight the therapeutic potential of blocking MMP-2/α5β1 interaction in glioma treatment.105
MMP-9
MMP-9 or gelatinase-B is also a type IV collagenase that has a gene locus on chromosome 20q11.2-q13.1. MMP-9 is produced by a variety of cells including epithelial cells, fibroblasts, keratinocytes, osteoblasts, dendritic cells, macrophages, granulocytes, and T-cells. In the house ear institute-organ of Corti 1 choclear cells, IL-1β induces expression of MMP-9 in a dose- and time-dependent manner, and dexamethasone and p38 MAPK inhibitor SB203580 inhibit IL-1β-induced MMP-9 expression/activity.106 MMP-9 plays a key role in inflammatory cell migration and in the destructive behavior of cholesteatoma. However, serum levels of MMP-9 might not correctly reflect the extent of localized tissue inflammation. In a study of patients with cholesteatoma, MMP-9 and TIMP-1 serum levels were similar with those in control group. In contrast, the levels of MMP-9 and TIMP-1 were higher in cholesteatoma tissues than normal skin specimens. These findings suggest better clinical usefulness of MMP-9 and TIMP-1 expression in cholesteatoma tissues than either serum or plasma levels of these proteins and that the higher the expression of MMP-9 the stronger the inflammation-accompanied cholesteatoma.107
Chronic sinonasal inflammation is associated with tissue remodeling and sinonasal osteitis, which could be a marker of refractory disease. Bone real-time polymerase chain reaction (RT-PCR) revealed upregulation of MMP-9 in all patients with chronic rhinosinusitis, but the magnitude of MMP-9 upregulation decreased with severity of osteitis. Mucosa RT-PCR showed upregulation of MMP-9 in moderate/severe osteitis only. The pattern of expression suggests a time- and tissue-dependent role for MMP-9 in the pathophysiology of osteitis.108
In the cornea, galectin-3 is a carbohydrate-binding protein that promotes cell-cell detachment and redistribution of the tight junction protein occludin through its N-terminal polymerizing domain. Galectin-3 initiates cell-cell disassembly by inducing MMP-9 expression in a manner that is dependent on the interaction with and clustering of the extracellular MMP inducer EMMPRIN (CD147, basigin) on the cell surface. Corneas of control mice expressing galectin-3 had a substantial amount of MMP-9 in the migrating epithelia of healing corneas. In contrast, corneas of galectin-3-knockout mice show impairment in MMP-9 expression. These findings suggest a galectin-3-mediated regulatory mechanism for induction of MMP-9 expression and disruption of cell-cell contacts required for cell motility in migrating epithelia.47
MMP-9 is also expressed in migrating macrophages.93 MMP-9 has also been detected in esophageal cancer,77 and gelatin zymography showed a correlation between MMP-9 activity and vascular invasion of esophageal carcinoma.97
8.3 Stromelysins
Stromelysins 1, 2 and 3, also known as MMP-3, -10, and -11, respectively, have the same domain arrangement as collagenases, but do not cleave interstitial collagen. MMP-3 and -10 are similar in structure and substrate specificity, while MMP-11 is distantly related. MMP-3 and MMP-10 digest a number of ECM molecules and participate in proMMP activation, but MMP-11 has very weak activity toward ECM molecules. Also, MMP-3 and -10 are secreted from the cells as inactive proMMP, but MMP-11 is activated intracellularly by furin and secreted from the cells as an active enzyme.23
MMP-3
MMP-3, also known as stromelysin-1, has a gene locus on chromosome 11q22.3. Structurally, MMP-3 possesses some unique characteristics. First, MMP-3 retains protease capability even if the zinc moiety is replaced with cobalt, manganese, cadmium, or nickel ions, but depending on the moiety, the protease activity becomes sensitive to different substrates. Second, MMP-3 has a unique deep active site that transverses the length of the enzyme.31 MMP-3 is well known as a secretory endopeptidase that degrades ECM.109 MMP-3 preferentially cleaves proteins at sites where the first three amino acids following the cleavage site are hydrophobic.31 MMP-3 degrades collagen type II, IV, and IX as well as a variety of proteoglycans, elastin, fibronectin, and laminin. MMP-3 may activate other MMPs necessary for tissue remodeling including MMP-1, -7, and -9.31 MMP-3 has been detected in the nucleus, and human nuclear MMP-3 may function as a trans-regulator of connective tissue growth factor. MMP-3 has also been detected in the nuclei of hepatocytes and may be involved in apoptosis.110 MMP-3 was detected in the nuclei of cultured chondrocytic cells and in normal and osteoarthritic chondrocytes in vivo. Nuclear translocation of externally added recombinant MMP-3, and six putative nuclear localization signals in MMP-3 have been identified. Heterochromatin protein-γ regulates connective tissue growth factor by interacting with MMP-3, and MMP-3 knockdown suppresses connective tissue growth factor expression. These observations suggest that MMP-3 may be involved in the development, tissue remodeling, and pathology of arthritic diseases through regulation of connective tissue growth factor.109
Post-traumatic osteoarthritis is characterized by progressive cartilage degeneration in injured joints, and fibronectin-fragments may degrade cartilage through up-regulating MMPs. Studies have profiled the catabolic events, fibronectin fragmentation and proteinase expression in bovine osteochondral explants following a single blunt impact on cartilage. Impacted cartilage released higher amount of chondrolytic fibronectin-fragments and proteoglycan than non-impacted controls. Those increases coincided with up-regulation of MMP-3 in impacted cartilage, suggesting that post-traumatic osteoarthritis may be propelled by fibronectin-fragments which act as catabolic mediators through up-regulating cartilage-damaging proteinases such as MMP-3.111
In addition to its role in arthritis, MMP-3 may be involved in the development of atherosclerosis, and tumor growth and metastasis.112,113 Seum levels of MMP-3 and VEGF are higher in patients with malignant adrenal incidentalomas than in those with benign ones, and therefore can be used as markers of malignancy of incidentalomas. Also, MMP-3 and VEGF levels decreased after tumor resection in patients with malignant tumors and increased in patients with recurrence, and therefore, could be of prognostic value in these patients.114 MMP-3 activation may also be a key upstream event that leads to induction of mitochondrial reactive oxygen species and NADPH oxidase 1 (Nox1) and eventual dopaminergic neuronal death.115
MMP-10
MMP-10 or stromelysin-2 has a gene locus on chromosome 11q22.3. MMP-10 is a secreted protein that may play a role in pulmonary fibrosis. In patients with idiopathic pulmonary fibrosis, serum levels of MMP-7 and -10 correlate with both the percentage of predicted forced vital capacity and the percentage of predicted diffusing capacity of the lung for carbon. MMP-7 and -10 levels in bronchoalveolar lavage fluid correlate with their corresponding serum levels. Serum MMP-10 predicted clinical deterioration within 6 months and overall survival. In idiopathic pulmonary fibrosis lungs, the expression of MMP-10 was enhanced and localized to the alveolar epithelial cells, macrophages, and peripheral bronchiolar epithelial cells. These findings suggest that MMP-10 may be a useful biomarker of disease severity and prognosis in patients with idiopathic pulmonary fibrosis.116
Respiratory syncytial virus is an important pathogen of bronchiolitis, asthma, and severe lower respiratory tract disease in infants and young children. Studies have investigated the regulation of MMP in respiratory syncytial virus-infected human nasal epithelial cells in vitro. MMP-10 mRNA expression was increased in human nasal epithelial cells after respiratory syncytial virus infection, together with induction of mRNAs of MMP-1, -7, -9, and -19. The amount of MMP-10 released from human nasal epithelial cells was also increased in a time-dependent manner after respiratory syncytial virus infection as is that of chemokine RANTES. The upregulation of MMP-10 was prevented by inhibitors of NF-κB and pan-PKC with inhibition of respiratory syncytial virus replication. Upregulation of MMP-10 was prevented by inhibitors of JAK/STAT, MAPK, and epidermal growth factor (EGF) receptors without inhibition of respiratory syncytial virus replication. In lung tissue of an infant with severe respiratory syncytial virus infection in which a few respiratory syncytial virus antibody-positive macrophages were observed, MMP-10 was expressed at the apical side of the bronchial epithelial cells and alveolar epithelial cells. These findings suggest that MMP-10 induced by respiratory syncytial virus infection in human nasal epithelial cells is regulated via distinct signal transduction pathways with or without relation to virus replication. MMP-10 may play an important role in the pathogenesis of respiratory syncytial virus diseases and may have the potential to be a marker and therapeutic target for respiratory syncytial virus infection.53
MMP-10 may be associated with peripheral arterial disease. Studies have analyzed MMP-10 levels in patients with peripheral arterial disease according to disease severity and cardiovascular risk factors and evaluated the prognostic value of MMP-10 for cardiovascular events and mortality in lower limb arterial disease after a follow-up period of 2 years. Patients with peripheral arterial disease showed increased levels of MMP-10 and decreased levels of TIMP-1 compared with controls. Among patients with peripheral arterial disease, those with critical limb ischemia showed higher levels of MMP-10 compared with those with intermittent claudication, whereas the MMP-10/TIMP-1 ratio remained similar. The univariate analysis showed an association between MMP-10 levels, age, hypertension, and ankle-brachial index in peripheral arterial disease patients. Patients with the highest MMP-10 tertile had an increased incidence of all-cause mortality and cardiovascular mortality. These observations suggest that MMP-10 is associated with severity and poor outcome in peripheral arterial disease.117
MMP-10 is expressed by macrophages and epithelium in response to injury, and its role in wound repair has been examined. In wounds of MMP-10 KO mice, collagen deposition and skin stiffness is increased, with no change in collagen expression or reepithelialization. Increased collagen deposition in MMP-10 KO wounds was accompanied by less collagenolytic activity and reduced expression of specific metallocollagenases, particularly MMP-8 and -13, where MMP-13 was the key collagenase. Ablation and adoptive transfer approaches and cell-based models demonstrated that the MMP-10-dependent collagenolytic activity was a product of alternatively activated (M2) resident macrophages. These observations suggest a role for MMP-10 in controlling the tissue remodeling activity of macrophages and moderating scar formation during wound repair.118
MMP-10 may be involved in pelvic organ prolapse. In a study exploring the correlation between genetic mutations in MMP-10 and susceptibility to pelvic organ prolapse, serum MMP-10 levels were higher in the pelvic organ prolapse group than in the control group. Also, there was a marked difference between the two groups in the distribution frequency of the MMP-10 rs17435959G/C genotype. Patients with parity > 2 and postmenopausal women had elevated serum MMP-10 levels, and patients with parity > 2 and postmenopausal women who carried the G/C + C/C genotype in the MMP-10 gene had an increased risk of pelvic organ prolapse. These observations suggest that the rs17435959 polymorphism of the MMP-10 gene may be associated with an increased risk of pelvic organ prolapse.119
MMP-10 is often expressed in human cancers and could play a role in tumor progression, migration, and invasion. Non-neoplastic oral epithelium does not show MMP-10 expression. MMP-10 may be involved in the transformation of normal oral epithelium to oral verrucous carcinoma and squamous cell carcinoma. MMP-10 expression levels are higher in oral squamous cell carcinoma than verrucous carcinoma, and therefore can be used in the differential diagnosis of the two tumors.120 MMP-10 is limited to epithelial cells and may facilitate tumor cell invasion by targeting collagen, elastin and laminin. Increased MMP-10 expression has been linked to poor clinical prognosis in some cancers. MMP-10 expression is positively correlated with the invasiveness of human cervical and bladder cancers. MMP-10 can regulate tumor cell migration and invasion, and endothelial cell tube formation, and these effects are associated with resistance to apoptosis. Increasing MMP-10 expression stimulates the expression of hypoxia inducible factor HIF-1α and MMP-2 (pro-angiogenic factors) and plasminogen activator inhibitor type 1 (PAI-1) and C-X-C chemokine receptor CXCR2 (pro-metastatic factors). Targeting MMP-10 with siRNA in vivo results in decreased xenograft tumor growth, reduced angiogenesis, and apoptosis. These findings suggest that MMP-10 can play a role in tumor growth and progression, and MMP-10 inhibition may represent a rational strategy for cancer treatment.54
MMP-10 plays a role in liver regeneration. Studies have examined MMP-10 expression and function in human hepatocellular carcinoma and diethylnitrosamine-induced mouse hepatocarcinogenesis. MMP-10 was induced in human and murine hepatocellular carcinoma tissues and cells. MMP10-deficient mice showed less hepatocellular carcinoma incidence, smaller histological lesions, reduced tumor vascularization, and less lung metastases. Importantly, expression of the protumorigenic, C-X-C chemokine receptor-4 (CXCR4), was reduced in diethylnitrosamine-induced hepatocarcinogenesis in MMP10-deficient mice livers. Human hepatocellular carcinoma cells stably expressing MMP-10 had increased CXCR4 expression and migratory capacity. Pharmacological inhibition of CXCR4 reduced MMP10-stimulated hepatocellular carcinoma cell migration. MMP-10 expression in hepatocellular carcinoma cells was induced by hypoxia and the CXCR4 ligand, stromal-derived factor-1 (SDF1), through the ERK1/2 pathway, involving an activator protein 1 site in MMP-10 gene promoter. These findings suggest that MMP-10 contributes to hepatocellular carcinoma development, and participates in tumor angiogenesis, growth, and dissemination. Reciprocal crosstalk between MMP-10 and the CXCR4/SDF1 axis may contribute to hepatocellular carcinoma progression and metastasis.46
MMP-11
MMP-11 or stromelysin-3 has a gene locus on chromosome 22q11.23. MMP-11 was first identified in stromal cells surrounding invasive breast carcinoma, and has been proposed as one of the stroma-derived factors that play a role in the progression of epithelial malignancies.121 Like all other members of the MMP gene family, stromelysin-3 is synthesized as an inactive precursor that must be processed to its mature form in order to express enzymatic activity. However, compared to other MMPs which require activation extracellularly, MMP-11 is secreted in its active form. MMP-11 can be processed directly to its enzymatically active form by an obligate intracellular proteolytic event that occurs within the constitutive secretory pathway. Like other furin-containing MMPs, intracellular activation of MMP-11 is regulated by a 10-amino-acid insert sandwiched between the pro- and catalytic-domains of MMP-11, which is encrypted with an Arg-X-Arg-X-Lys-Arg recognition motif for the Golgi-associated proteinase furin, a mammalian homologue of the yeast Kex2 pheromone convertase. A furin-MMP-11 processing axis not only differentiates the regulation of this enzyme from other non-furin containing MMPs, but also identifies pro-protein convertases as potential targets for therapeutic intervention in matrix-destructive disease states.23
Some of the MMP-11 substrates include laminin receptor and α-1-proteinase inhibitor.1,122 MMP-11 is expressed in tissues undergoing the active remodeling associated with embryonic development, wound healing and tumor invasion.23 MMP-11 may promote tumorigenicity. In breast cancer, MMP-11 is a bad prognosis marker and its expression is associated with a poor clinical outcome.15 In a study investigating the influence of genetic polymorphisms of MMP-11 gene on the susceptibility to oral squamous cell carcinoma in a Taiwanese population, MMP-11 gene polymorphisms exhibited synergistic effects with the environmental factors betel nut chewing and tobacco use on the susceptibility to oral squamous cell carcinoma. Among patients with oral squamous cell carcinoma with betel nut consumption, those who have at least one polymorphic C allele of MMP-11 rs738792 have an increased incidence of lymph node metastasis when compared with patients homozygous for T/T. These observations suggest combined effects of MMP-11 gene polymorphisms and environmental carcinogens in the increased risk for oral squamous cell carcinoma and may be a predictive factor for tumor lymph node metastasis in Taiwanese with oral squamous cell carcinoma.123
MMP-11 levels are elevated in specimens from patients with esophageal squamous cell carcinoma. Patents with esophageal dysplasia also show elevated MMP-11, suggesting that these alterations are early events in esophageal tumorigenesis. In postesophagectomy follow-up, patients with MMP-11 positive TIMP-2 negative carcinoma had shorter disease-free survival compared with patients with other MMP/TIMP profiles. These findings suggest that MMP-11 positive TIMP-2 negative phenotype may be associated with adverse prognosis in patients with esophageal cancer.124 MMP-11 is also overexpressed in sera of cancer patients compared with normal control group, and in tumor tissue specimens from patients with laryngeal, gastric, pancreatic and breast cancer. The presence of MMP-11 in tumor tissues has suggested that it could promote cancer development by inhibiting apoptosis as well as enhancing migration and invasion of cancer cells. However, studies in animal models suggest that MMP-11 may play a negative role against cancer progression by suppressing metastasis.125
In patients with laryngeal squamous cell carcinoma, the expression of MMP-11 mRNA expression and the tumor suppressor gene p14ARF was different in tumor tissues compared with their corresponding adjacent tissues and was associated with several clinical characteristics. Patients with low MMP-11 and high p14ARF expression had better survival compared with those with high MMP-11 and low p14ARF expression. It was suggested that altered expression of MMP-11 and p14ARF in tumors may individually, or in combination, predict poor prognosis of laryngeal squamous cell carcinoma.126
8.4 Matrilysins
Matrilysins include MMP-7 and -26, and they both lack the hemopexin domain and the hinge region.
MMP-7
MMP-7 or matrilysin-1 has a gene locus on chromosome 11q21–q22. Structurally, MMP-7 is one of the smallest MMPs. MMP-7 is expressed by Xenopus embryonic macrophages.93 Common substrates of MMP-7 include proteoglycans, fibronectin, casein, and gelatin types I, II, IV, and V. MMP-7 plays a role in remodeling of tissues involved in development and reproduction such as the uterus, and could play a role in remodeling following tissue injury.31 MMP-7 degrades ECM components, and cleaves cell surface molecules such as Fas–ligand, pro-TNF-α, syndecan-1 and E-cadherin to generate soluble forms.127 MMP-7 can have dual effects on apoptosis, whereby it can induce apoptosis by releasing Fas–ligand or inhibit apoptosis by producing heparin-binding epidermal growth factor.31 MMP-7 acts intracellularly in the intestine to process procryptidins to bactericidal forms.
Studies have examined MMP-2, MMP-7, MMP-9 and TIMP-1 in dysregulated turnover of connective tissue matrices in tonsillar specimens from children with recurrent tonsillitis and undergoing tonsillectomy. MMP-7 level of the superficial part and MMP-9 level at both the superficial and core regions were higher in patients with grade III and IV than patients with grade I and II tonsillar hypertrophy. The balance between MMP-7 and -9 and TIMP-1 activities tended to shift toward the MMP-7 and -9 side with increased tonsillar grade. The presence of MMPs in tonsil tissue suggested a role of degraded ECM proteins in the pathophysiology of chronic tonsillitis. The specific increases in MMP-7 and -9 activities suggest that they are the main promoters of ECM degradation that responded to inflammatory changes in the tonsillar tissue.128 MMP-7 has also been described as a useful biomarker for idiopathic pulmonary fibrosis.116
MMP-7 may play a role in cancer development and metastasis. Serum levels of anti-MMP-7 antibody are higher in patients with oral squamous cell carcinoma, and those with poorly differentiated tumors have more MMP-7 antibody than those with well to moderate tumor. Patients with oral squamous cell carcinoma at late tumor-lymph node-metastasis (TNM) stages (III, IV) and lymph node metastases have higher serum MMP-7 antibody levels than those at earlier stages (I, II). Serum MMP-7 antibody positivity independently predicted poor overall survival in patients with oral squamous cell carcinoma. MMP-7 mRNA and protein expression increased in tumor tissues from patients with oral squamous cell carcinoma and high serum MMP-7 antibody. These findings suggested that serum anti-MMP-7 antibody might be useful as a diagnostic and prognostic biomarker for oral squamous cell carcinoma.129
MMP-26
MMP-26, also known as matrilysin-2 or endometase, has a gene locus on chromosome 11p15. The chromosomal location of the MMP-26 gene shows that it maps to the short arm of chromosome 11, a location distinct from that of other MMP genes.130 The cDNA encoding MMP-26 was cloned from fetal cDNA. The deduced 261-amino-acid sequence is homologous to macrophage metalloelastase. It includes only the minimal characteristic features of the MMP family: a signal peptide, a prodomain and a catalytic domain.131 As with MMP-7, MMP-26 lacks the hemopexin domain, believed to be involved in substrate recognition, and also the hinge region.130 The amino acid sequence of MMP-26 also contains a threonine residue adjacent to the Zn2+-binding site that is a specific feature of matrilysin.130 MMP-26 mRNA is specifically expressed in the placenta and uterus. Recombinant MMP-26 demonstrates proteolytic activity toward several substrates including type IV collagen, β-casein, fibrinogen, fibronectin, gelatin, and vitronectin.130–132 MMP-26 also activates proMMP-9 (gelatinase B).130 MMP-26 mRNA is also detected in human cell lines such as HEK 293 kidney cells and HFB1 lymphoma cells, and is widely expressed in malignant tumors from different sources as well as in multiple tumor cell lines. MMP-26 is also expressed in cancer cells of epithelial origin, including carcinomas of the lung, prostate and breast.132,133 The broad proteolytic activity and distribution of MMP-26 in different cell lines suggest that it may play a role in tissue-remodeling events associated with angiogenesis and tumor progression.130,132
MMP-26 expression may be linked to tumor invasion induced by granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF promotes tumor progression in different tumor models, and is associated with highly angiogenic and invasive tumors. In colon adenocarcinoma, GM-CSF overexpression and treatment reduces tumor cell proliferation and tumor growth in vitro and in vivo, but contributes to tumor progression, tumor invasion into the surrounding tissue, and induction of an activated tumor stroma. Enhanced GM-CSF expression is also associated with a discontinued basement membrane deposition likely due increased expression/activity of MMP-2, -9, and -26. Treatment with GM-CSF blocking antibodies reverses this effect. Expression of MMP-26 is predominantly located in pre- and early-invasive areas suggesting that MMP-26 expression is an early event in promoting GM-CSF dependent tumor invasion.134 Pancreatic adenocarcinoma is recognized for its early aggressive local invasion and high metastatic potential. Patients with metastatic lymph nodes had increased expression of MMP-26 in actual tumor samples, and the putative role of MMP-26 as a marker of metastases warrants further studies.135 MMP-26 is negatively regulated by TIMP-2 and -4, with TIMP-4 being more potent inhibitor of MMP-26 induced tissue remodeling.136
8.5 Membrane-Type MMPs
Membrane-Type MMPs (MT-MMPs) include four transmembrane MMPs, MMP-14, -15, -16 and -24, and two glycosyl-phosphatidylinositol (GPI)-anchored MMPs, MMP-17 and -25 (Table 1).8,9 MT-MMPs have a furin-like pro-protein convertase recognition sequence at the C-terminus of the propeptide. They are activated intracellularly and the active enzymes are expressed on the cell surface. MT-MMPs have membrane anchoring domains and display protease activity at the cell surface, and therefore they are optimal pericellular proteolytic machines.137 All MT-MMPs except MT4-MMP (MMP-17) can activate proMMP-2.13 MT1-MMP (MMP-14) activates proMMP-13 on the cell surface.138
MMP-14
MMP-14 or MT1-MMP has a gene locus on chromosome 14q11-q12. MMP-14 is one of four type I transmembrane proteins (MT1, 2, 3, and 5-MMP or MMP-14, -15, -16, and -24, respectively). Type I MT-MMPs, MT1-, MT2-, MT3-, and MT5-MMPs have about a 20-amino acid cytoplasmic tail following the transmembrane domain.139 MT1-MMP is ubiquitously expressed, binds TIMP-2, activates MMP-2, and stimulates cell migration in various cell types.140 MMP-14 is best known for its collagenolytic activity, digesting type I (guinea pig), II (bovine), and III (human) collagens into characteristic 3/4 and 1/4 fragments. MT1-MMP may also degrade cartilage proteoglycan, fibronectin, laminin-1, vitronectin, α1-proteinase inhibitor and α2-macroglobulin.8 The activity of MT1-MMP on type I collagen is synergistically increased with co-incubation with MMP-2.8 MMP-2 is secreted as a proenzyme (proMMP-2, progelatinase A) which is bound and activated on the surface of normal and tumor cells. MT1-MMP induces activation of proMMP-2. In COS-1 cells, MT1-MMP could induce cell-surface binding of proMMP-2, which is consequently processed to an intermediate form. Processing from the intermediate to the fully active form is dependent on MMP-2 concentration. Thus the MT1-MMP induced cell-surface binding concentrates the MMP-2 intermediate form locally to allow autoproteolytic processing to the fully active MMP-2 form.26 One difference between MT-MMPs and the other MMP family members is the insertion of eight amino acids between strands βII and III in the catalytic domain. In MT1-MMP, the best characterized of these enzymes to date, these residues consist of (163)PYAYIREG(170). Characterization of the activity of the soluble forms toward peptides and fibrinogen revealed that neither mutation nor deletion of residues 163–170 impaired catalytic function, suggesting these residues have little influence on conformation of the active site cleft. On the other hand, characterization of the kinetics of activation of pro-MMP2 with and without its gelatin binding region by the mutants generated have shown that efficient activation of proMMP-2 is, at least in part, through an interaction with residues 163–170 of MT1-MMP.13 Also, in a study using sandwich enzyme linked immunoassay systems, the levels of MMP-1, -2, -13, MT1-MMP, and TIMP-1 were higher in homogenates of human salivary gland carcinomas than non-neoplastic control salivary glands. Gelatin zymography demonstrated that the activation ratio of the MMP-2 zymogen was higher in the carcinomas than in the controls, and the pro-MMP-2 activation correlated directly with the MT1-MMP/TIMP-2 ratio. Immunohistochemistry and in situ zymography demonstrated localization of MMP-2, MT1-MMP, and TIMP-2 to carcinoma cells. These findings suggest that enhanced activation of pro-MMP-2 mediated by MT1-MMP is implicated in tumor invasion and metastasis and that TIMP-2 may regulate pro-MMP-2 activation in salivary gland carcinomas in part by inhibiting MMP-14.141
In another study to examine the relation between expression of MT-MMPs and MMP-2, which is one of the key proteinases in invasion and metastasis of various cancers, all head and neck squamous cell carcinoma cell lines examined consistently expressed MT1-MMP and MMP-2, but not MT2-MMP or MT3-MMP. Also, in the clinical specimens, there was a correlation in coexpression of mRNA and colocalization by immunohistochemistry for MT1-MMP and MMP-2. Relative mRNA expression levels of MT1-MMP and MMP-2 in the carcinoma tissues were higher than those of the control tissues. Both mRNA expression and immunopositivity of MT1-MMP correlated with lymph node metastasis. The localization of MMP-2 closely corresponded to that of MT1-MMP. These observations suggest that MT1-MMP possesses a role as a determinant of lymph node metastasis, and that concurrent expression of MT1-MMP and MMP-2 are involved in progression of head and neck squamous cell carcinoma.142
MT1-MMP could be an important molecular tool for cellular remodeling of the surrounding matrix. MT1-MMP-deficient mice show craniofacial dysmorphism, arthritis, osteopenia, dwarfism, and fibrosis of soft tissues likely due to ablation of a collagenolytic activity that is essential for modeling of skeletal and extraskeletal connective tissues. These observations demonstrate the pivotal function of MT1-MMP in connective tissue metabolism.9 MMP-14 may promote vulnerable plaque morphology in mice, whereas TIMP-3 overexpression is protective. High MMP-14 low TIMP-3 rabbit foam cells are more invasive and more prone to apoptosis than low MMP-14 high TIMP-3 cells. Proinflammatory stimuli increase MMP-14 and decrease TIMP-3 mRNA expression and protein levels in human macrophages. Conversion to foam-cells with oxidized LDL is associated with increased MMP-14 and decreased TIMP-3, independently of inflammatory mediators and partly through post-transcriptional mechanisms. Within atherosclerotic plaques, MMP-14 is prominent in foam-cells with either pro- or anti-inflammatory macrophage markers, whereas TIMP-3 is present in less foamy macrophages and colocalized with CD206. MMP-14 positive macrophages are more abundant whereas TIMP-3 positive macrophages are less abundant in plaques histologically designated as rupture prone. These findings suggest that foam-cells with high MMP-14 low TIMP-3 expression are prevalent in rupture-prone atherosclerotic plaques, independent of pro- or anti-inflammatory activation, and that reducing MMP-14 activity and increasing TIMP-3 could be valid therapeutic approaches to reduce plaque rupture and myocardial infarction.143
MT1-MMP has a major impact on invasive cell migration in both physiological and pathological settings such as immune cell extravasation or metastasis of cancer cells.144 Surface-associated MT1-MMP is able to cleave components of ECM, which is a prerequisite for proteolytic invasive migration. In a study of the mechanisms that regulate MT1-MMP trafficking to and from the cell surface, three members of the RabGTPase family, Rab5a, Rab8a and Rab14 were found to be crucial regulators of MT1-MMP trafficking and function in primary human macrophages. Both overexpressed and endogenous forms show prominent colocalization with MT1-MMP-positive vesicles, whereas expression of mutant constructs, as well as siRNA-induced knockdown, reveal that these RabGTPases are crucial in the regulation of MT1-MMP surface exposure, contact of MT1-MMP-positive vesicles with podosomes, ECM degradation and proteolytic invasion of macrophages. Thus, Rab5a, Rab8a and Rab14 are major regulators of MT1-MMP trafficking and invasive migration of human macrophages, and could be potential targets for manipulation of immune cell invasion.144 Of note, MT1-MMP is overexpressed in malignant tumor tissues, including lung and stomach carcinomas that contain activated MMP-2.145
MMP-15
MMP-15 or MT2-MMP has a gene locus on chromosome 16q13. MMP-15 is an understudied member of the MMP family. Like MT1-MMP, MT2-MMP localizes on the cell surface and mediates the activation of MMP-2,145 which is associated with tumor invasion and metastasis.
MT-MMPs are essential for pericellular matrix remodeling in late stages of development, as well as in growth and tissue homeostasis in postnatal life. A study has examined MT1-MMP and MT2-MMP, and their roles in the process of placental morphogenesis in mice. The fetal portion of the placenta, in particular the labyrinth, displays strong overlapping expression of MT1-MMP and MT2-MMP, which is critical for syncytiotrophoblast formation and in turn for fetal vessels. Disruption of trophoblast syncytium formation leads to developmental arrest with only a few poorly branched fetal vessels entering the labyrinth causing embryonic death at day 11.5. Knockdown of either MT1-MMP or MT2-MMP is crucial during the development of the labyrinth. In contrast, knockdown of MT-MMP activity after labyrinth formation is compatible with development to term and postnatal life. These findings identify essential but interchangeable roles for MT1-MMP or MT2-MMP in placental vasculogenesis, and suggest selective temporal and spatial MMP activity during development of the mouse embryo.146
MMP-15 appears to be upregulated during colorectal tumorigenesis, with different expression patterns. MMP-15 expression level increases from normal mucosa to microadenomas, and immunofluorescence analysis showed a stromal localization of MMP-15 in the early phases of neoplastic transformation.147 The mRNA and protein expression of MMP-14, -15 and -16 are increased in supraglottic carcinoma tissues compared to control adjacent non-neoplastic tissues. Expression of MMP-14, but not MMP-15 and MMP-16, is markedly increased in the T3 and neck nodal metastasis groups compared with the T1–2 group and the group without nodal metastasis. Also, MMP-14 mRNA and protein are higher in tumors at clinical stage III–IV compared with stage I–II tumors. Groups with high MMP-14 protein expression had a poorer prognosis than patients with weak or negative expression of MMP-14. Thus while MMP-15 is expressed, MMP-14 appears to play a more dominant role in the tumor progression and may serve as prognostic factor in patients with supraglottic carcinoma.148
A study examined the relation of expressions of MT1, MT2, and MT3-MMP to the invasion and metastases in laryngeal cancer. The expression of MT1, MT2, and MT3-MMP was higher in laryngeal cancer tissues than those in para-tumorous tissues and had a close relationship with invasive depth. The expression of MT1-MMP was higher in patients with metastatic lymph nodes than in patients without metastatic lymph nodes. Thus MT1, MT2, and MT3-MMP play a role in the progression of laryngeal cancer, MT1-MMP may serve as a reliable marker in estimating invasive and metastatic potency of laryngeal cancer, and suppressing expressions of MT1, MT2, and MT3-MMP may inhibit the invasion and metastases of laryngeal cancer.149
MMP-16
MMP-16 or MT3-MMP has a gene locus on chromosome 8q21.3. MMP-16 is a membrane bound protein with a cytoplasmic tail. As a type I MT-MMP, MMP-16 could trnaform proMMP-2 to active MMP-2 and thereby facilitate tumor invasion. In human cardiomyocyte progenitor cells, MMP-16 may activate MMP-2 and -9, which could in turn facilitate undesired cell migration after targeted cell transplantation and potentially limit the beneficial effects of cardiac regeneration. Treatment with MMP-16 siRNA or an MMP-16 blocking antibody blocked cell migration, suggesting that reducing MMP-16 expression/activity could have beneficial effects in progenitor cell transplantation and cardiac regeneration.150
In patients with melanoma, increased expression of MMP-16 is associated with poor clinical outcome, collagen bundle assembly around tumor cell nests, and lymphatic invasion. In cultured WM852 melanoma cells derived from human melanoma metastasis, silencing of MMP-16 resulted in cell-surface accumulation of the MMP-16 substrate MMP-14 (MT1-MMP) as well as L1CAM cell adhesion molecule. When limiting the activities of these trans-membrane protein substrates toward pericellular collagen degradation, cell junction disassembly, and blood endothelial transmigration, MMP-16 supported nodular-type growth of adhesive collagen-surrounded melanoma cell nests, steering cell collectives into lymphatic vessels. These findings suggest that restricted collagen infiltration and limited mesenchymal invasion are unexpectedly associated with the properties of the most aggressive tumors, and reveal MMP-16 as a putative indicator of adverse melanoma prognosis.151
Other studies have suggested that transforming growth factor-β1 (TGF-β1) is involved in the migration and metastases of bladder cancer by inducing epithelial-mesenchymal transition and upregulation of MMP-16. These findings suggest and an association between TGF-β1, MMP-16 and epithelial-mesenchymal transition, in the setting of tumor invasion and metastasis in bladder cancer.152
MMP-16 enhances invasion of breast cancer cells. In MCF7 breast cancer cells, the antitumoral and antiproliferative compound catalpol reduced MMP-16 activity and cell proliferation, promoted apoptosis and increased the expression of miR-146a. These findings suggested that miR-146a may control the expression of MMP-16, and that catalpol suppresses proliferation and facilitates apoptosis of MCF7 breast cancer cells through upregulating miR146a and downregulating MMP-16 expression.153 Likewise, miR-155 may directly target MMP-16, and in turn reduce MMP-2 and -9 activities and as a result efficiently inhibit migration of human cardiomyocyte progenitor cells, suggesting that miR-155 could be used to improve local retention of progenitor cells after intramyocardial delivery.150
Alveolarization requires coordinated ECM remodeling, and MMPs play an important role in this process. Polymorphisms in MMP genes might affect MMP function in preterm lungs and thus influence the risk of bronchopulmonary dysplasia. In a study in neonates with bronchopulmonary dysplasia 9 single-nucleotide polymorphisms (SNPs) were sought in the MMP-2, MMP-14 and MMP-16 genes. After adjustment for birth weight and ethnic origin, the TT genotype of MMP-16 C/T (rs2664352) and the GG genotype of MMP-16 A/G (rs2664349) were found to protect from bronchopulmonary dysplasia. These genotypes were also associated with a smaller active fraction of MMP-2 and a 3-fold-lower MMP-16 level in tracheal aspirates. Further evaluation of MMP-16 expression during the course of normal human and rat lung development showed relatively low expression during the canalicular and saccular stages and a clear increase in both mRNA and protein levels during the alveolar stage. In newborn rat models of arrested alveolarization the lung MMP-16 mRNA level was less than 50% of normal. These findings suggest that MMP-16 may be involved in the development of lung alveoli, and that MMP-16 polymorphisms may influence the pulmonary expression and function of MMP-16 and the risk of bronchopulmonary dysplasia in premature infants.154
MMP-17
MMP-17 or MT4-MMP has a gene locus in chromosome 12q24.3. MMP-17 is one of six human MT-MMPs, but unlike type I MT-MMPs, and as one of GPI anchor MT-MMPs (MT4-MMP and MT6-MMP, or MMP-17 and -25, respectively) it does not positively regulate proMMP-2 (pro-gelatinase A). In the mid-1990s, MMP-17 was cloned from a human breast carcinoma cDNA library. The isolated cDNA contained an open reading frame 1554 bp long, encoding a polypeptide of 518 amino acids. The predicted amino acid sequence displayed a similar domain organization as other MMPs, including a prodomain with the activation locus, a Zn2+-binding site, and a hemopexin domain. In addition, it contained a C-terminal extension, rich in hydrophobic residues and similar in size to those present in other MT-MMPs. MT4-MMP also contains a nine-residue insertion between the propeptide and the catalytic domain, which is a common feature of MT-MMPs and stromelysin-3. This amino acid sequence insertion ends with the consensus sequence R-X-R/K-R, which seems to be essential for the activation of these proteinases by furin. Unlike MT1-, MT2-, MT3-, and MT5-MMPs which have about a 20-amino acid cytoplasmic tail following the transmembrane domain, and similar to MMP-25, MMP-17 lacks the cytoplasmic tail, and instead, has a GPI anchor, which confers MMP-17 (MT4-MMP) and MMP-25 (MT6-MMP) a unique set of regulatory and functional mechanisms that separates them from the rest of the MMP family.139 MT4-MMP shedding from the cell surface appears to require an endogenous metalloproteinase.139
Discovered almost a decade ago, the body of work on GPI-MT-MMPs today is still limited when compared to other MT-MMPs. Accumulating biochemical and functional evidence also highlights their distinct properties.137 MMP-17 gene is expressed in a variety of human tissues mainly leukocytes, colon, ovary, testis and the brain. The expression of MMP-17 in leukocytes together with its membrane localization suggest that it could be involved in activation of membrane-bound precursors of growth factors or inflammatory mediators such as TNF-α. GPI-MT-MMPs are highly expressed in human cancer, where they are associated with tumor progression. MMP-17 transcripts are detected in all breast cancer cell lines, suggesting a role in tumor development/progression.155
MMP-24
MMP-24 or MT5-MMP maps to chromosome 20q11.2, a region frequently amplified in tumors from diverse sources. A cDNA encoding MT5-MMP was identified and cloned from a human brain cDNA library. The isolated cDNA encoded a polypeptide of 645 amino acids that displayed a similar domain organization as other MMPs, including a predomain with the activation locus, a Zn2+-binding site, and a hemopexin domain. The deduced amino acid sequence contains a C-terminal extension, rich in hydrophobic residues and similar in size to the equivalent domains identified in MT-MMPs. Immunofluorescence and Western blot analysis of COS-7 cells transfected with the isolated cDNA revealed that the encoded protein is localized in the plasma membrane. Northern blot analysis demonstrated that MT5-MMP is predominantly expressed in brain, kidney, pancreas, and lung. In addition, MT5-MMP transcripts were detected at high levels compared to normal brain tissue in a series of brain tumors, including astrocytomas and glioblastomas. MMP-24 can cleave proMMP-2 (progelatinase A) into its active MMP-2 form. The catalytic domain of MT5-MMP, produced in Escherichia coli as a fusion protein with glutathione S-transferase, exhibits a potent proteolytic activity against proMMP-2, leading to the generation of the Mr 62,000 active MMP-2. MT5-MMP may contribute to the activation of proMMP-2 in tumor tissues, in which it is overexpressed, thereby facilitating tumor progression.156
MT5-MMP was also isolated from mouse brain cDNA library. It is predicted to contain a candidate signal sequence, a propeptide region with the highly conserved PRCGVPD sequence, a potential furin recognition motif RRRRNKR, a zinc-binding catalytic domain, a hemopexin-like domain, a 24-residue hydrophobic domain as a potential transmembrane domain, and a short cytosolic domain. MT5-MMP is expressed in a brain-specific manner. It is also highly expressed during embryonic development. In contrast to other MT-MMPs, MT5-MMP tends to shed from cell surface as soluble proteinases, thus offering flexibility as both a cell bound and soluble proteinase for ECM remodeling.157 In relation to its location in the brain, MT5-MMP is co-expressed with N-cadherin in adult neural stem cells and ependymocytes. N-cadherin mediates anchorage of neural stem cells to ependymocytes in the adult murine subependymal zone and in turn modulates their quiescence. Importantly, MT5-MMP regulates adult neural stem cell functional quiescence by cleaving and shedding of the N-cadherin ectodomain, supporting that the proliferative status of stem cells can be dynamically modulated by regulated cleavage of cell adhesion molecules.158
MMP-24 is neuron-specific, and is believed to contribute to neuronal circuit formation and plasticity. MT5-MMP cleaves N-cadherin, a protein critical to synapse stabilization, and studies have shown time- and injury-dependent expression of MT5-MMP and N-cadherin during reactive synaptogenesis following neural injury.159 MMP-24 deficient mice do not develop neuropathic pain with mechanical allodynia and do no show sprouting and invasion of Abeta-fiber after sciatic nerve injury. These findings suggest that MT5-MMP is essential for the development of mechanical allodynia and plays an important role in neuronal plasticity.160
MMP-24 is an essential modulator of neuro-immune interactions in thermal pain stimulation, and a mediator of peripheral thermal nociception and inflammatory hyperalgesia. MT5-MMP is expressed by CGRP-containing peptidergic nociceptors in dorsal root ganglia. MMP-24-deficient mice display enhanced sensitivity to noxious thermal stimuli under basal conditions, but do not develop thermal hyperalgesia during inflammation, a phenotype that appears associated with alterations in N-cadherin-mediated cell-cell interactions between mast cells and sensory fibers. These findings demonstrate an essential role of MT5-MMP in the development of dermal neuro-immune synapses and suggest that it may be a target for pain control.161
In a study investigating the expression of MMPs in different grades of human breast cancer tissues, mRNA expressions of MMP-1, -9, -11, -15, -24 and -25 were upregulated, while MMP-10 and -19 were downregulated in breast cancer compared with normal breast tissues. There was also a tumor grade-dependent increase in MMP-15 and -24 mRNA expression, supporting that MMPs are differentially regulated in breast cancer tissues and that they might play various roles in tumor invasion, metastasis and angiogenesis.162
MMP-25
MMP-25 or MT6-MMP has a gene locus on chromosome 16p13.3. MMP-25 is one of the least studied members of the MMP family.140 MMP25 is a GPI-anchored MMP that is highly expressed in leukocytes and some cancer tissues. Natural MT6-MMP is expressed on the cell surface as a major reduction-sensitive form of ~120 kDa species, likely representing enzyme homodimers held by disulfide bridges. The stem region of MT6-MMP contains three cysteine residues at positions 530, 532, and 534 which may contribute to dimerization. A systematic site-directed mutagenesis study of the Cys residues in the stem region shows that Cys532 is involved in MT6-MMP dimerization by forming an intermolecular disulfide bond. Mutagenesis data also suggest that Cys530 and Cys534 form an intramolecular disulfide bond. Dimerization is not essential for transport of MT6-MMP to the cell surface, partitioning into lipid rafts or cleavage of α1-proteinase inhibitor. Monomeric forms of MT6-MMP exhibited enhanced autolysis and metalloprotease-dependent degradation. These findings suggest that the stem region of MT6-MMP is a dimerization interface, an event whose outcome lends protease stability to the protein.163
MT6-MMP is present in lipid rafts and faces inward in living human polymorphonuclear leukocytes (PMNs), but translocates to the cell surface during neutrophil apoptosis. PMNs express high levels of MT6-MMP. MT6-MMP is present in the membrane, granules and nuclear/endoplasmic reticulum/Golgi fractions of PMNs where it is displayed as a disulfide-linked homodimer of ~120 kDa. Stimulation of PMNs results in secretion of active MT6-MMP into the supernatants. Membrane-bound MT6-MMP, conversely, is located in the lipid rafts of resting PMNs and stimulation does not alter this location. Interestingly, living PMNs do not display MT6-MMP on the cell surface. However, induction of apoptosis induces MT6-MMP relocation on PMNs’ cell surface.164 Because of its localization in PMNs, MMP-25 may play a role in respiratory burst and IL-8 secretion.164
To further assess the biochemical features of MT6-MMP, studies have expressed the MT6-MMP construct tagged with a FLAG tag in breast carcinoma MCF-7 and fibrosarcoma HT1080 cells. Phosphatidylinositol-specific phospholipase C was then used to release MT6-MMP from the cell surface and the solubilized MT6-MMP fractions were characterized. It was found that cellular MT6-MMP partially exists in a complex with TIMP-2. Both TIMP-1 and TIMP-2 are capable of inhibiting the proteolytic activity of MT6-MMP. MT6-MMP does not stimulate cell migration. MT6-MMP, however, generates an adequate level of gelatinolysis of fluorescein isothiocyanate-labeled gelatin and exhibits an intrinsic, albeit low, ability to activate MMP-2. As a result, it is exceedingly difficult to record the activation of MMP-2 by cellular MT6-MMP. Because of its lipid raft localization, cellular MT6-MMP is inefficiently internalized. MT6-MMP is predominantly localized in the cell-to-cell junctions. MT6-MMP has been suggested to play a role in autoimmune multiple sclerosis and cancer, but its physiologically relevant cleavage targets remain to be determined.140 MT6-MMP mRNA expression is elevated in several human cancers including brain (anaplastic astrocytomas and glioblastomas), colon, urothelial, and prostate cancers.137,165 MT6-MMP mRNA expression was identified in colon cancer,165 and immunohistochemical studies confirmed the presence of MT6-MMP in samples of invasive colon cancer.166 While MT6-MMP protein is absent in normal colonic epithelium it is highly expressed in invasive adenocarcinomas in 50 out of 60 cases examined.166
8.6 Other MMPs
Other MMPs include MMP-12, -19, -20, -21, -22, -23, -27, and -28
MMP-12
MMP-12 or macrophage metalloelastase has a gene locus on chromosome 11q22.3. As indicated by its name, MMP-12 degrades elastin and is highly expressed by macrophages and other stromal cells. MMP-12 is essential for macrophage migration,167 and is also found in hypertrophic chondrocytes and osteoclasts.168,169 Interferon-α (IFN-α) is essential for antiviral immunity, but in the absence of MMP-12 or IκBα (encoded by NFKBIA), IFN-α is retained in the cytosol of virus-infected cells and is not secreted, suggesting that the export of IFN-α from virus-infected cells require activated MMP-12 and IκBα. The inability of cells in MMP-12 KO mice to express IκBα and thus export IFN-α makes coxsackievirus type B3 infection lethal and renders respiratory syncytial virus more pathogenic. It has been suggested that after macrophage secretion, MMP-12 is transported into virus-infected cells. In HeLa cells MMP-12 is translocated to the nucleus, where it binds to the NFKBIA promoter, driving NFKBIA transcription, and leading to IFN-α secretion and host protection. On the other hand, extracellular MMP-12 cleaves off the IFN-α receptor 2 binding site of systemic IFN-α, preventing an unchecked immune response. Consistent with a role for MMP-12 in clearing systemic IFN-α, treatment of coxsackievirus type B3-infected wild-type mice with a membrane-impermeable MMP-12 inhibitor elevates systemic IFN-α levels and reduces viral replication in the pancreas while sparing intracellular MMP-12, suggesting that inhibiting extracellular MMP-12 could be a new avenue for antiviral treatment.170
MMP-12 plays a role in airway inflammation and remodeling. MMP-12 expression is increased in the lungs of asthmatic patients. Compound 27 is a potent and selective inhibitor of MMP-12 that is orally efficacious in a mouse model of MMP-12 induced ear-swelling inflammation, and may be a candidate drug for treatment of asthma.171
MMP-12 may affect the blood-brain barrier after cerebral ischemia. In rats subjected to middle cerebral artery occlusion and reperfusion, MMP-12 was upregulated ~31-, 47-, and 66-fold in rats subjected 1-, 2-, or 4-hour ischemia, respectively, followed by 1-day reperfusion. MMP-12 suppression by infusion of nanoparticles of MMP-12 shRNA-expressing plasmid protected the blood-brain barrier integrity by inhibiting the degradation of tight-junction proteins, and reduced the percent Evans blue dye extravasation and infarct size. MMP-12 suppression reduced the levels of the other endogenous proteases tissue-type plasminogen activator and MMP-9, which are key players in blood-brain barrier damage. These findings demonstrate the adverse role of MMP-12 in acute brain damage after ischemic stroke and suggest that MMP-12 suppression could be a therapeutic target for cerebral ischemia.172
Studies have examined possible correlation between the expression of MMPs in the primary tumor of head and neck squamous cell carcinomas and the presence of extracapsular spread in cervical nodes metastasis. MMP-2, -3, -12, and -14 were expressed in 27, 47.5, 55, and 57.5 % of cases, respectively. MMP-12 expression was associated with extracapsular spread and correlated with nodal metastasis. MMP-12 expressed in the primary tumor may be a molecular marker for predicting extracapsular spread in head and neck squamous cell carcinomas patients with metastatic nodal disease.173
MMP-19
MMP-19 or RASI-1 or stromelysin-4 has a gene locus on chromosome 12q14. The catalytic domain of MMP-19 can hydrolyze the basement membrane type IV collagen, laminin, and nidogen, as well as the large tenascin-C isoform, fibronectin, and type I gelatin in vitro, suggesting that MMP-19 is a potent proteinase capable of hydrolyzing a broad range of ECM components. Neither the catalytic domain nor the full-length MMP-19 can degrade triple-helical collagen. Also, the MMP-19 catalytic domain can process proMMP-9 to its active form, but may not activate other latent forms of MMPs such as MMP-1, -2, -3, -13, and -14 in vitro.174
MMP-19 is a potent basement membrane-degrading enzyme that plays a role in tissue remodeling, wound healing and epithelial cell migration by cleaving laminin-5γ2 chain.175–178 Angiogenesis is the process of forming new blood vessels from existing ones and requires degradation of the vascular basement membrane and remodeling of ECM in order to allow endothelial cells to migrate and invade the surrounding tissue. Angiostatin, a proteolytic fragment of plasminogen, is a potent antagonist of angiogenesis that inhibits migration and proliferation of endothelial cells. MMP-19 may exhibit anti-angiogenic effects on endothelial cells by processing human plasminogen in a characteristic cleavage pattern to generate three angiostatin-like fragments with a molecular weight of 35, 38, and 42 kDa, that decrease the phosphorylation of c-met, inhibit the proliferation of human microvascular endothelial cells and reduce formation of capillary-like structures.179
Idiopathic pulmonary fibrosis is a progressive interstitial lung disease characterized by aberrant activation of epithelial cells that induce the migration, proliferation and activation of fibroblasts. The resulting distinctive fibroblastic/myofibroblastic foci are responsible for the excessive ECM production and abnormal lung remodeling. MMP-19-deficient mice develop an exaggerated bleomycin-induced lung fibrosis. Microarray analysis of MMP-19-deficient lung fibroblasts revealed the dysregulation of several profibrotic pathways, including ECM formation, migration, proliferation, and autophagy. Compared with wild-type mice, MMP-19-deficient lung fibroblasts show increased α1 (I) collagen gene and collagen protein levels at baseline and after TGF-β treatment and increased smooth muscle-α actin expression. MMP-19-deficient lung fibroblasts also show an increase in proliferation, transmigration and locomotion over Boyden chambers coated with type I collagen or Matrigel. Thus, in lung fibroblasts, MMP-19 has strong regulatory effects on the synthesis of key ECM components, on fibroblast to myofibroblast differentiation, and in migration and proliferation.180
Bleomycin-induced lung fibrosis was evaluated in MMP-19-deficient and wild-type (WT) mice. Laser capture microscope followed by microarray analysis revealed MMP-19 in hyperplastic epithelial cells adjacent to fibrotic regions. MMP-19-deficient mice showed increased lung fibrotic response to bleomycin compared with WT mice. A549 alveolar epithelial cells transfected with human MMP-19 stimulated wound healing and cell migration, whereas silencing MMP-19 had the opposite effect. Gene expression microarray of transfected A549 cells showed prostaglandin-endoperoxide synthase 2 (PTGS2) as one of the highly induced genes. PTGS2 was overexpressed in idiopathic pulmonary fibrosis lungs and colocalized with MMP-19 in hyperplastic epithelial cells. PTGS2 was increased in bronchoalveolar lavage and lung tissues after bleomycin-induced fibrosis in WT mice, but not MMP-19-deficient mice. Inhibition of MMP-19 by siRNA resulted in reduction of PTGS2 mRNA and protein level. These findings suggest that during lung injury up-regulation of MMP-19 may protect against fibrosis through the induction of PTGS2.181
Liver fibrosis is characterized by the deposition and increased turnover of ECM. MMP-19 is highly expressed in liver, and its role during the development and resolution of liver fibrosis was studied in MMP-19-deficient and wild-type mice exposed to chronic carbon tetrachloride intoxication. Loss of MMP-19 was beneficial during liver injury, as plasma ALT and AST levels, deposition of fibrillar collagen, and phosphorylation of SMAD3, a TGF-ss1 signaling molecule, were reduced. The ameliorated course of the disease in MMP-19-deficient mice likely results from a slower rate of basement membrane destruction and ECM remodeling as the knockout mice maintained higher levels of type IV collagen and lower expression and activation of MMP-2. Liver regeneration upon removal of the toxin was also hastened in MMP-19-deficient mice. MMP-19-deficiency may decrease the development of hepatic fibrosis through decreased replacement of physiological ECM with fibrotic deposits in the beginning of the injury.182
MMP-19 mRNA is widely expressed in the synovium of normal and rheumatoid arthritic patients. MMP-19 cleaves aggrecan and cartilage oligomeric matrix protein, two of the macromolecules characterizing the cartilage ECM, supporting that MMP-19 may participate in the degradation of aggrecan and cartilage oligomeric matrix protein in arthritic disease.175
Patients with a congenital cavitary optic disc anomaly (CODA) have profound excavation of the optic nerve resembling glaucoma. A recent study mapped the gene that causes autosomal-dominant CODA in a large pedigree to a chromosome 12q locus. Comparative genomic hybridization and quantitative PCR analysis of this pedigree identified a 6-Kbp heterozygous triplication upstream of the MMP-19 gene, present in all 17 affected family members, but not normal members. The same 6-Kbp triplication was identified in one of 24 unrelated CODA patients and in none of 172 glaucoma patients. Analysis with a Luciferase assay showed that the 6-Kbp sequence has transcription enhancer activity. A 773-bp fragment of the 6-Kbp DNA segment increased downstream gene expression 8-fold, suggesting that triplication of this sequence may lead to dysregulation of the downstream MMP-19 gene in CODA patients. Immunohistochemical analysis of human donor eyes revealed strong expression of MMP-19 in optic nerve head. These findings suggest that triplication of an enhancer may lead to overexpression of MMP-19 in the optic nerve that causes CODA.183
MMP-19 may play a role in cancer. MMP-19 deficient mice develop diet-induced obesity due to adipocyte hypertrophy, but are less susceptible to skin cancers induced by chemical carcinogens.184 In patients with gallbladder carcinoma loss of expression of the tumor suppressor N-myc downstream-regulated gene 2 (NDRG2) was an independent predictor of decreased survival and was associated with a more advanced T stage, higher cellular grade, and lymphatic invasion. Gallbladder carcinoma cells with loss of NDRG2 expression showed enhanced proliferation, migration, and invasiveness in vitro, and tumor growth and metastasis in vivo. Loss of NDRG2 induced the expression of MMP-19, which regulated the expression of Slug at the transcriptional level. MMP-19-induced Slug, increased the expression of a receptor tyrosine kinase, Axl, which maintained Slug expression through a positive feedback loop, and stabilized epithelial-mesenchymal transition of gallbladder carcinoma cells. NDRG2 could be a favorable prognostic indicator and promising target for therapeutic agents against gallbladder carcinoma, and the effects of NDRG2 could be related to suppression of MMP-19.185
MMP-19 appears to be upregulated during colorectal tumorigenesis, with different expression patterns. Increased MMP-19 mRNA expression and protein levels were observed in the progression of colonic lesions, and MMP-19 staining increased in the normal mucosa-microadenoma-carcinoma sequence.147
MMP-19 may play a role in non-small cell lung cancer. MMP-19 gene expression and protein levels are increased in lung cancer tumors compared with adjacent normal lung tissues. Increased MMP-19 gene expression conferred a poorer prognosis in non-small cell lung cancer. Overexpression of MMP-19 promotes epithelial-mesenchymal transition, migration, and invasiveness in multiple non-small cell lung cancer cell lines. Also, miR-30 isoforms, a microRNA family predicted to target MMP-19, are down-regulated in human lung cancer. Thus MMP-19 may be associated with the development and progression of non-small cell lung cancer and may be a potential biomarker of disease severity and outcome.186
On the other hand, MMP-19 may be one of the MMPs downregulated in the nasopharyngeal carcinoma cell lines. Allelic deletion and promoter hypermethylation may contribute to MMP-19 downregulation. Comparative studies of the wild-type and the catalytically inactive mutant MMP-19 suggest that the catalytic activity of MMP-19 may play a role in antitumor and anti-angiogenesis activities. In the in vivo tumorigenicity assay, MMP-19 transfectants suppress tumor formation in only in the wild-type, but not mutant, nude mice. In the in vitro colony formation assay, WT MMP-19 reduced colony-forming ability of nasopharyngeal carcinoma cell lines, when compared to the inactive mutant. In the tube formation assay of human umbilical vein endothelial cells and human microvascular endothelial cells, secreted WT MMP-19, but not mutant MMP-19, caused reduction of tube-forming ability in endothelial cells and decreased VEGF in conditioned media. The anti-angiogenic activity of WT MMP-19 is correlated with suppression of tumor formation. Thus the catalytic activity of MMP-19 may be essential for its tumor suppressive and anti-angiogenic effects in nasopharyngeal carcinoma.187
MMP-20
MMP-20 is also known as enamelysin. The human enamelysin gene maps to chromosome 11q22, clustered to at least seven other members of the MMP gene family. Enamelysin is a tooth-specific MMP expressed in newly formed tooth enamel.188 MMP-20 is specifically expressed by ameloblasts and odontoblasts of dental papilla, and hence its name—enamelysin. A cDNA encoding MMP-20 was cloned from RNA prepared from human odontoblastic cells. The open reading frame of the cloned cDNA codes for a polypeptide of 483 amino acids. Human enamelysin has a domain organization similar to other MMPs, including a signal peptide, a prodomain with the conserved motif PRCGVPD involved in maintaining enzyme latency, a catalytic domain with a Zn2+-binding site, and a C-terminal fragment similar to the sequence of hemopexin. The calculated molecular mass of human enamelysin is about 54 kDa, which is similar to that of collagenases or stromelysins. However, human MMP-20 lacks a series of structural features distinctive of subfamilies of MMPs. MMP-20 contains a very basic hinge region compared to the hydrophobic hinge region of stromelysins and the acidic hinge region of MMP-19. The full-length human enamelysin cDNA has been expressed in Escherichia coli, and the purified and refolded recombinant protein degrades synthetic peptides used as substrates of MMPs. The recombinant human enamelysin also degrades amelogenin,188 the major protein component of the enamel matrix. On the basis of its degrading activity on amelogenin, and its highly restricted expression to dental tissues, it was suggested that human enamelysin plays a central role in tooth enamel formation.189
Enamelysin is expressed during the early through middle stages of enamel development. The enamel matrix proteins amelogenin, ameloblastin, and enamelin are also expressed during this developmental time period, suggesting that enamelysin may be involved in their hydrolysis. Amelogenin imperfecta is a genetic disorder with defective enamel formation involving mutation at MMP-20 cleavage sites.190 Enamelysin null mice show severe amelogenesis imperfecta tooth phenotype that does not process amelogenin properly, altered enamel matrix and rod pattern, hypoplastic enamel that delaminates from the dentin, and a deteriorating enamel organ morphology as development progresses. These findings support that enamelysin activity is essential for proper enamel development.191
MMP-20 also hydrolyses aggrecan efficiently at the well-described MMP cleavage site between residues Asn(341) and Phe(342). MMP-20 also cleaves cartilage oligomeric matrix protein in a distinctive manner, generating a major proteolytic product of 60 kDa. Due to the unique expression pattern of MMP-20, it may primarily be involved in the turnover of aggrecan and cartilage oligomeric matrix protein during tooth development.175
MMP-21
MMP-21 also known as Xenopus-MMP has a genetic code on chromosome 1,31 in contrast to the normal 11q location of other MMPs. MMP-21 is an MMP with measurable gelatinolytic activity expressed in various fetal and adult tissues, macrophages of granulomatous skin lesions, fibroblasts in dermatofibromas, and basal and squamous cell carcinomas.192,193 MMP-21 may play a role in embryogenesis and tumor progression and could be a target of the Wnt, Pax, and Notch signaling pathways. MMP-21 mRNA was detected in mouse embryos aged 10.5, 12.5, 13.5, and 16.5 days, and in various adult murine organs. In both humans and mice, MMP-21 has been detected in the epithelial cells of developing kidney, intestine, neuroectoderm, and skin, but not in normal adult skin. MMP-21 is present in invasive cancer cells of aggressive basal and squamous cell carcinomas, but not in skin disorders characterized by mere keratinocyte hyperproliferation. TGF-β1 induced MMP-21 in HaCaTs and keratinocytes in vitro. MMP-21 expression is temporally and spatially tightly controlled during development. Unlike many classical MMPs, MMP-21 is present in various normal adult tissues. Among epithelial MMPs, MMP-21 has a unique expression pattern in cancer.193
MMP-21 could be an indicator of poor prognosis for certain types of cancer. Increased MMP-21 expression in metastatic lymph nodes may predict unfavorable prognosis and overall survival for oral squamous cell carcinoma patients with lymphatic metastasis.194 MMP-21 expression is increased in esophageal squamous cell carcinoma, and is associated with tumor invasion, lymph node metastasis, and distant metastasis. Patients with tumors of positive MMP-21 staining tend to have worse overall survival. Multivariate analysis showed that MMP-21 was an independent prognostic factor for overall survival in patients with esophageal squamous cell carcinoma. These findings support a role of MMP-21 in tumor progression and prognosis of human esophageal squamous cell carcinoma.195
MMP-21 expression is higher in colorectal cancer compared with that in normal epithelial tissue. MMP-21 expression correlates with tumor invasion, lymph node metastasis, and distant metastasis of colorectal cancer. MMP-21 may also be an independent prognostic factor in patients with stage II and III colorectal cancer.196
Merkel cell carcinoma is an aggressive cutaneous tumor with increasing incidence and poor outcome, and shows differential expression pattern of MMPs. MMP-28 was observed in tumor cells of 15/44 samples especially in tumors <2 cm in diameter while 21/44 specimens showed MMP-28 in the tumor stroma. Expression of MMP-21 was demonstrated in tumor cells of 13/43 samples. MMP-26 was positive in stromal cells (17/44) and its expression associated with tumors > 2 cm in diameter. Stromal expression of MMP-10 was the most frequent finding of the studied samples (31/44), and MMP-10 was detected also in tumor cells (17/44). Most of the metastatic lymph nodes expressed MMP-10 and MMP-26. MMP-10, MMP-21, and MMP-28 mRNAs and corresponding proteins were basally expressed by the UISO cells. IFN-α and TNF-α downregulated MMP-21 and MMP-28 expression. These findings suggest that MMP-26 expression in stroma is associated with larger tumors with poor prognosis. Expression of MMP-21 and MMP-28 seems to associate with the tumors of less malignant potential. The study also confirms the role of MMP-10 in the pathogenesis of Merkel cell carcinoma.197
Pancreatic adenocarcinoma shows early aggressive local invasion and high metastatic potential, and therefore a low 5-year survival rate. MMP-21 was expressed in well-differentiated cancer cells and occasional fibroblasts, but tended to diminish in intensity from grade I to grade III tumors. All cultured cancer cell lines expressed MMP-21 basally at low levels. MMP-21 expression was induced by epidermal growth factor in PANC-1 cells. Thus MMP-21 may not be a marker of invasiveness, but rather of differentiation, in pancreatic cancer, and may be upregulated by epidermal growth factor.135
MMP-22
MMP-22 also known as chicken-MMP has a gene locus on chromosome 1p36.3. The terminal end of the short arm of human chromosome 1, 1p36.3, is frequently deleted in a number of tumors and is believed to be the location of multiple tumor suppressor genes. MMP-21 and MMP-22 genes have been identified in the Cdc2L1–2 locus, which spans approximately 120 kb on 1p36.3. These genes encode MMPs that contain prepro, catalytic, cysteine-rich, IL-1 receptor-related, and proline-rich domains. Their catalytic domains are most closely related to stromelysin-3 and contain the consensus HEXXH Zn2+-binding region required for enzyme activation, while their cysteine-rich domains appear to be related to a number of human, mouse, and Caenorhabditis elegans MMP sequences. These MMPs lack the highly conserved cysteine residue in the proenzyme domain, the so-called “cysteine switch,” which is involved in the autocatalytic activation of many MMPs. The MMP21/22 genes express multiple mRNAs, some of which are derived by alternative splicing, in a tissue-specific manner.198
MMP-23
MMP-23 or cysteine array (CA)-MMP has a gene locus on chromosome 1p36.3. A cDNA encoding MMP-23 has been cloned from an ovary cDNA library. This protein exhibits sequence similarity with MMPs, but displays a different domain structure. MMP-23 lacks a recognizable signal sequence and has a short prodomain, although it contains a single cysteine residue that can be part of the cysteine-switch mechanism operating for maintaining enzyme latency. Whereas all human MMPs, with the exception of matrilysin, contain four hemopexin-like repeats, the C-terminal domain of MMP-23 is considerably shortened and shows no sequence similarity to hemopexin. MMP-23 is devoid of structural features distinctive of the diverse MMP subclasses, including the specific residues located close to the Zn2+-binding site in collagenases, the transmembrane domain of membrane-type MMPs, or the fibronectin-like domain of gelatinases. MMP-23 is unique among MMPs as it lacks the cysteine switch motif in the propeptide, and the hemopexin domain is substituted by cysteine-rich immunoglobulin-like domains.199
MMPs are either secreted or membrane anchored via a type I transmembrane domain or a GPI linkage. Lacking either membrane-anchoring mechanism, MMP-23 was reported to be expressed as a cell-associated protein. MMP-23 is expressed as an integral membrane zymogen with an N-terminal signal anchor, and secreted as a fully processed mature enzyme. MMP-23 is a type II membrane protein regulated by a single proteolytic cleavage for both its activation and secretion.14 MMP-23 is predominantly expressed in ovary, testis, and prostate, suggesting that it may have a specialized role in reproductive processes.199 Gene expression of MMP-23 is elevated and may promote invasiveness in MDA-MB-231 breast cancer cells.200
MMP-27
MMP-27 is a human MMP-22 homolog with a gene locus on chromosome 11q24. MMP-27 is classified as a stromelysin and holds 51.6% structural homology with MMP-10. MMP-27 is a poorly characterized and barely secreted MMP. Sequence comparison suggests that a C-terminal extension includes a potential transmembrane domain as in some MT-MMPs. Subcellular fractionation and confocal microscopy suggest retention of endogenous MMP-27 or recombinant rMMP-27 in the endoplasmic reticulum with locked exit across the intermediate compartment. Conversely, truncated rMMP-27 without C-terminal extension accessed downstream secretory compartments in endoplasmic reticulum intermediate compartment and Golgi and was constitutively secreted. Neither endogenous nor recombinant MMP-27 partitioned in the detergent phase after Triton X-114 extraction, indicating that MMP-27 is not an integral membrane protein. Due to its unique C-terminal extension, which does not lead to stable membrane insertion, MMP-27 is efficiently stored within the endoplasmic reticulum until it is ready to be released.201
MMP-27 is expressed in B-lymphocytes and is overexpressed in cultured human lymphocytes treated with anti-(IgG/IgM).202 MMP-27 is expressed in CD163+/CD206+ M2 macrophages in the cycling human endometrium and in superficial endometriotic lesions. MMP-27 mRNA is detected throughout the menstrual cycle. MMP-27 mRNA levels are increased from the proliferative to the secretory phase, to peak during the menstrual phase. MMP-27 is immunolocalized in large isolated cells scattered throughout the stroma and around blood vessels: these cells are most abundant at menstruation and are identified by immunofluorescence as CD45(+), CD163(+) and CD206(+) macrophages. CD163(+) macrophages are abundant in endometriotic lesions, and co-labelling for CD206 and MMP-27 is observed in ovarian or peritoneal endometriotic lesions. Thus MMP-27 is expressed in a subset of endometrial macrophages related to menstruation and in ovarian and peritoneal endometriotic lesions.203 Several MMPs show a stronger expression in breast cancer tissue compared to normal breast tissue. Of those, expression of MMP-27 is related to tumor grade since it is higher in G3 compared to G2 tissue samples. MDA-MB-468 breast cancer cell line show the strongest mRNA and protein expression for most of the MMPs studied. MMP-27 may be involved breast cancer development and tumor progression.204
MMP-28
MMP-28 or epilysin has a gene locus on chromosome 17q21.1. MMP-28 shows high expression in the epidermis. Epilysin was first cloned from the human keratinocyte and testis cDNA libraries.205,206 Like most MMPs, the deduced 520-amino-acid sequence of MMP-28 includes a signal peptide, a prodomain with an unusual cysteine-switch PRCGVTD motif followed by the furin activation sequence RRKKR, a Zn2+-binding catalytic domain with an HEIGHTLGLTH sequence, a hinge-region and a hemopexin-like domain, but no transmembrane sequence. Within the cysteine-switch, MMP-28 contains a threonine residue at position 94, instead of a proline as in most MMPs. Also, compared to the 10–12 amino acid stretch in other MMPs, a longer 22 residues follows the cysteine-switch before the furin cleavage region. The MMP-28 gene uniquely mapped to chromosome 17q11.2 includes 8 exons and 7 introns, and 5 exons are spliced at sites not used by other MMPs. Also, exon 4 is alternatively spliced to a transcript that does not encode the N-terminal half of the catalytic domain. Recombinant epilysin degrades casein in zymography assay, and its proteolytic activity is inhibited by EDTA and the MMP inhibitor batimastat. Immunohistochemical staining showed epilysin in the basal and suprabasal epidermis of intact skin. In injured skin, epilysin staining is seen in basal keratinocytes both at and some distance from the wound edge, a pattern distinct from that of other MMPs expressed during tissue repair. Epilysin is expressed at high levels in testis and at lower levels in lungs, heart, intestine, colon, placenta, and brain. MMP-28 may function in several tissues both in tissue homeostasis and in repair.205,206
The broad range of expression in normal adult and fetal tissues and in carcinomas suggests important roles for MMP-28.207 Epilysin is expressed in a number of normal tissues, suggestive of functions in tissue homeostasis. The mRNA expression of MMP-28 was highest in healthy tissues when compared to subjects with chronic periodontitis and aggressive periodontitis. The elevated MMP-28 level in healthy tissues support that it may be involved in normal tissue homeostasis and remodeling, and its decreased levels could serve as a biomarker for periodontal health.208
MMP-28 transcript and protein are expressed in rhesus monkey placenta during early pregnancy. MMP-28 mRNA expression was shown by in situ hybridization after day 12 of pregnancy, and both the syncytial and the cytotrophoblastic cell layers of placental villi, the cytotrophoblast cells of the trophoblastic column, and the extravillous trophoblast cells of trophoblastic shell were primary producers of MMP-28 transcript. Expression of MMP-28 mRNA was undetectable in the endovascular trophoblast cells, decidual cells, luminal and glandular epithelium, arterioles, and myometrium. The restricted distribution pattern of MMP-28 in the villous and extravillous trophoblasts during rhesus monkey early pregnancy suggests a potential role in trophoblast invasion associated with embryo implantation.209
MMP-28 may regulate the inflammatory and ECM responses in cardiac aging. In a mouse model of myocardial infarction (MI) of the left ventricle induced by permanent coronary artery ligation, MMP-28 expression was decreased post-MI, and its cell source shifted from myocytes to macrophages. In MMP-28 KO mice, MMP-28 deletion increased day 7 mortality because of increased cardiac rupture post-MI. MMP-28 KO mice exhibited larger left ventricular volumes, worse left ventricular dysfunction, worse left ventricular remodeling index, and increased lung edema. Plasma MMP-9 levels were unchanged in the MMP-28 KO mice but increased in wild-type mice at day 7 post-MI. The mRNA levels of inflammatory and ECM proteins were attenuated in the infarct regions of MMP-28 KO mice, indicating reduced inflammatory and ECM responses. M2 macrophage activation was impaired in MMP-28 KO mice. MMP-28 deletion also led to decreased collagen deposition and fewer myofibroblasts. Collagen cross-linking was impaired as a result of decreased expression and activation of lysyl oxidase in the infarcts of MMP-28 KO mice. These findings suggest that MMP-28 deletion aggravated MI-induced left ventricular dysfunction and rupture as a result of defective inflammatory response and scar formation by suppressing M2 macrophage activation.210
Studies have examined the cellular location and putative function of MMP-19, MMP-26 (matrilysin-2), and MMP-28 (epilysin), in normal, inflammatory, and malignant conditions of the intestine in tissue specimens from patients with ulcerative colitis and archival tissue samples of ischemic colitis, Crohn’s disease, ulcerative colitis, colon cancer, and healthy intestine. Unlike many classical MMPs, MMP-19, -26, and -28 were all expressed in normal intestine. In inflammatory bowel disease, MMP-19 was expressed in nonmigrating enterocytes and shedding epithelium. MMP-26 was detected in migrating enterocytes, unlike MMP-28. In colon carcinomas, MMP-19 and MMP-28 expression was downregulated in tumor epithelium. Staining for MMP-26 revealed a meshwork-like pattern between cancer islets, which was absent from most dedifferentiated areas. These findings suggest that MMP-19 is involved in epithelial proliferation and MMP-26 in enterocyte migration, while MMP-28 expression is not associated with inflammatory and destructive changes seen in inflammatory bowel disease. In contrast to previously characterized MMPs, MMP-19 and MMP-28 are downregulated during malignant transformation of the colon and may play a prominent role in tissue homeostasis.211
MMP-28 is also elevated in cartilage from patients with osteoarthritis and rheumatoid arthritis.212,213
9. MMP/TIMP Ratio
TIMPs are endogenous, naturally occurring MMP inhibitors that bind MMPs in a 1:1 stoichiometry.6,11 Four homologous TIMPs, TIMP-1, -2, -3 and -4, have been identified. TIMP-1 and -3 are glycoproteins, while TIMP-2 and -4 do not contain carbohydrates. TIMPs have poor specificity for a given MMP, and each TIMP can inhibit multiple MMPs with different efficacies.214–216 A change in either TIMP or MMP levels could alter the MMP/TIMP ratio and cause a net change in specific MMP activity.
10. CONCLUDING REMARKS
MMPs are involved in many biological processes and could be important biomarkers for cardiovascular disease, musculoskeletal disorders, and cancer. One challenge to understanding the role of specific MMPs in pathological conditions is that studies often focus on few MMPs or TIMPs, and it is important not to generalize the findings to other MMPs and TIMPs. Because tissue remodeling is a dynamic process, an increase in one MMP in a certain region may be paralleled by a decrease of other MMPs in other regions. Also, because of the differences in the proteolytic activities of MMPs towards different substrates, the activities of MMPs may vary during the course of disease. This makes it important to examine different MMPs and TIMPs in various tissue regions and at different stages of the disease. Another challenge is that the topology of MMPs is well conserved, making it difficult to design highly specific MMP inhibitors. Endogenous TIMPs are not very specific and often inhibit multiple MMPs. Likewise, synthetic MMP inhibitors have poor selectivity and many biologic actions, and therefore often cause side effects.217 New synthetic MMP inhibitors are being developed, and their effectiveness in cardiovascular disease and cancer needs to be examined. Another strategy is to develop specific approaches to target MMPs locally in the vicinity of a localized pathology, and thus minimize undesirable systemic effects.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-111775). Dr. N. Cui was a visiting scholar from the Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, P.R. China, and a recipient of scholarship from the China Scholarship Council. Dr. M. Hu was a visiting scholar from the Department of Cadiovascular Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, P. R. China.
ABBREVIATIONS
- BKCa
large conductance Ca2+-activated K+ channel
- CXCR
C-X-C chemokine receptor
- ECM
extracellular matrix
- EDHF
endothelium-derived hyperpolarizing factor
- EMMPRIN
extracellular matrix metalloproteinase inducer
- ERK
extracellular signal-regulated kinase
- GPI
glycosyl phosphatidylinositol
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HIF
hypoxia-inducible factor
- IL
interleukin
- IFN
Interferon
- IVC
inferior vena cava
- MAPK
mitogen-activated protein kinase
- MI
myocardial infarction
- miR
microRNA
- MMP
matrix metalloproteinase
- MT-MMP
membrane-type MMP
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAR
protease activated receptor
- PMNs
polymorphonuclear leukocytes
- PDGF
platelet-derived growth factor
- PI3K
phosphoinositide 3-kinase
- RGD
Arg-Gly-Asp
- siRNA
small interfering RNA
- SNP
single nucleotide polymorphism
- TGF-β
transforming growth factor-β
- TIMP
tissue inhibitor of metalloproteinases
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VSM
vascular smooth muscle
- VSMC
vascular smooth muscle cell
- Zn2+
zinc
Footnotes
CONFLICT OF INTEREST
None
References
- 1.Mittal R, Patel AP, Debs LH, Nguyen D, Patel K, Grati M, Mittal J, Yan D, Chapagain P, Liu XZ. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. Journal of cellular physiology. 2016;231(12):2599–2621. doi: 10.1002/jcp.25430. [DOI] [PubMed] [Google Scholar]
- 2.Kucukguven A, Khalil RA. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets. 2013;14(3):287–324. [PMC free article] [PubMed] [Google Scholar]
- 3.MacColl E, Khalil RA. Matrix Metalloproteinases as Regulators of Vein Structure and Function: Implications in Chronic Venous Disease. The Journal of pharmacology and experimental therapeutics. 2015;355(3):410–428. doi: 10.1124/jpet.115.227330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochemical pharmacology. 2008;75(2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42(3):113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 8.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272(4):2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 9.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 10.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 11.Bode W, Fernandez-Catalan C, Grams F, Gomis-Ruth FX, Nagase H, Tschesche H, Maskos K. Insights into MMP-TIMP interactions. Ann N Y Acad Sci. 1999;878:73–91. doi: 10.1111/j.1749-6632.1999.tb07675.x. [DOI] [PubMed] [Google Scholar]
- 12.Bode W, Gomis-Ruth FX, Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993;331(1–2):134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- 13.English WR, Holtz B, Vogt G, Knauper V, Murphy G. Characterization of the role of the “MT-loop”: an eight-amino acid insertion specific to progelatinase A (MMP2) activating membrane-type matrix metalloproteinases. The Journal of biological chemistry. 2001;276(45):42018–42026. doi: 10.1074/jbc.M107783200. [DOI] [PubMed] [Google Scholar]
- 14.Pei D, Kang T, Qi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J Biol Chem. 2000;275(43):33988–33997. doi: 10.1074/jbc.M006493200. [DOI] [PubMed] [Google Scholar]
- 15.Gall AL, Ruff M, Kannan R, Cuniasse P, Yiotakis A, Dive V, Rio MC, Basset P, Moras D. Crystal structure of the stromelysin-3 (MMP-11) catalytic domain complexed with a phosphinic inhibitor mimicking the transition-state. J Mol Biol. 2001;307(2):577–586. doi: 10.1006/jmbi.2001.4493. [DOI] [PubMed] [Google Scholar]
- 16.Saito S, Trovato MJ, You R, Lal BK, Fasehun F, Padberg FT, Jr, Hobson RW, 2nd, Duran WN, Pappas PJ. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase-1 in chronic venous insufficiency. J Vasc Surg. 2001;34(5):930–938. doi: 10.1067/mva.2001.119503. [DOI] [PubMed] [Google Scholar]
- 17.Seizer P, May AE. Platelets and matrix metalloproteinases. Thromb Haemost. 2013;110(5):903–909. doi: 10.1160/TH13-02-0113. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg. 2008;48(2):447–456. doi: 10.1016/j.jvs.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansilvestri-Morel P, Fioretti F, Rupin A, Senni K, Fabiani JN, Godeau G, Verbeuren TJ. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clin Sci (Lond) 2007;112(4):229–239. doi: 10.1042/CS20060170. [DOI] [PubMed] [Google Scholar]
- 21.Woodside KJ, Hu M, Burke A, Murakami M, Pounds LL, Killewich LA, Daller JA, Hunter GC. Morphologic characteristics of varicose veins: possible role of metalloproteinases. J Vasc Surg. 2003;38(1):162–169. doi: 10.1016/s0741-5214(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18(6):690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 23.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375(6528):244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 24.Morgunova E, Tuuttila A, Bergmann U, Tryggvason K. Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc Natl Acad Sci U S A. 2002;99(11):7414–7419. doi: 10.1073/pnas.102185399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. The Journal of biological chemistry. 1995;270(10):5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 26.Sato H, Takino T, Kinoshita T, Imai K, Okada Y, Stetler Stevenson WG, Seiki M. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP) FEBS Lett. 1996;385(3):238–240. doi: 10.1016/0014-5793(96)00389-4. [DOI] [PubMed] [Google Scholar]
- 27.Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, Crabbe T, Clements J, d’Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. The Journal of biological chemistry. 1998;273(2):871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 28.Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJ, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP) The Journal of biological chemistry. 1998;273(2):1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]
- 29.Kolkenbrock H, Orgel D, Hecker-Kia A, Noack W, Ulbrich N. The complex between a tissue inhibitor of metalloproteinases (TIMP-2) and 72-kDa progelatinase is a metalloproteinase inhibitor. Eur J Biochem. 1991;198(3):775–781. doi: 10.1111/j.1432-1033.1991.tb16080.x. [DOI] [PubMed] [Google Scholar]
- 30.Fridman R, Bird RE, Hoyhtya M, Oelkuct M, Komarek D, Liang CM, Berman ML, Liotta LA, Stetler-Stevenson WG, Fuerst TR. Expression of human recombinant 72 kDa gelatinase and tissue inhibitor of metalloproteinase-2 (TIMP-2): characterization of complex and free enzyme. Biochem J. 1993;289(Pt 2):411–416. doi: 10.1042/bj2890411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15(6):2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48(9):4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 33.Mao D, Zhang Y, Lu H, Zhang H. Molecular basis underlying inhibition of metastasis of gastric cancer by anti-VEGFa treatment. Tumour Biol. 2014 doi: 10.1007/s13277-014-2095-6. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y, Sun YW, Lin HS, Su WM, Fang Y, Zhao Y, Wei XQ, Qin YH, Kohama K, Gao Y. Platelet-derived growth factor-BB induces matrix metalloproteinase-2 expression and rat vascular smooth muscle cell migration via ROCK and ERK/p38 MAPK pathways. Mol Cell Biochem. 2014;393(1–2):255–263. doi: 10.1007/s11010-014-2068-5. [DOI] [PubMed] [Google Scholar]
- 35.Rao VH, Kansal V, Stoupa S, Agrawal DK. MMP-1 and MMP-9 regulate epidermal growth factor-dependent collagen loss in human carotid plaque smooth muscle cells. Physiol Rep. 2014;2(2):e00224. doi: 10.1002/phy2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson ML, Atkinson SJ, Knauper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503(2–3):158–162. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 37.Suenaga N, Mori H, Itoh Y, Seiki M. CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase. Oncogene. 2005;24(5):859–868. doi: 10.1038/sj.onc.1208258. [DOI] [PubMed] [Google Scholar]
- 38.Park HI, Jin Y, Hurst DR, Monroe CA, Lee S, Schwartz MA, Sang QX. The intermediate S1′ pocket of the endometase/matrilysin-2 active site revealed by enzyme inhibition kinetic studies, protein sequence analyses, and homology modeling. J Biol Chem. 2003;278(51):51646–51653. doi: 10.1074/jbc.M310109200. [DOI] [PubMed] [Google Scholar]
- 39.Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta. 2010;1803(1):72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Pelmenschikov V, Siegbahn PE. Catalytic mechanism of matrix metalloproteinases: two-layered ONIOM study. Inorg Chem. 2002;41(22):5659–5666. doi: 10.1021/ic0255656. [DOI] [PubMed] [Google Scholar]
- 41.Manzetti S, McCulloch DR, Herington AC, van der Spoel D. Modeling of enzyme-substrate complexes for the metalloproteases MMP-3, ADAM-9 and ADAM-10. J Comput Aided Mol Des. 2003;17(9):551–565. doi: 10.1023/b:jcam.0000005765.13637.38. [DOI] [PubMed] [Google Scholar]
- 42.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23(15):3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270(11):5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 44.Olson MW, Toth M, Gervasi DC, Sado Y, Ninomiya Y, Fridman R. High affinity binding of latent matrix metalloproteinase-9 to the alpha2(IV) chain of collagen IV. J Biol Chem. 1998;273(17):10672–10681. doi: 10.1074/jbc.273.17.10672. [DOI] [PubMed] [Google Scholar]
- 45.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11(11):S37–43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Irigoyen O, Latasa MU, Carotti S, Uriarte I, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini S, Benito P, Ladero JM, Rodriguez JA, Prieto J, Orbe J, Paramo JA, Fernandez-Barrena MG, Berasain C, Avila MA. Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C-X-C chemokine receptor 4 axis. Hepatology. 2015;62(1):166–178. doi: 10.1002/hep.27798. [DOI] [PubMed] [Google Scholar]
- 47.Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci. 2014;127(Pt 14):3141–3148. doi: 10.1242/jcs.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Chalothorn D, Jackson LF, Lee DC, Faber JE. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ Res. 2004;95(10):989–997. doi: 10.1161/01.RES.0000147962.01036.bb. [DOI] [PubMed] [Google Scholar]
- 49.Morla AO, Mogford JE. Control of smooth muscle cell proliferation and phenotype by integrin signaling through focal adhesion kinase. Biochem Biophys Res Commun. 2000;272(1):298–302. doi: 10.1006/bbrc.2000.2769. [DOI] [PubMed] [Google Scholar]
- 50.Shi ZD, Ji XY, Berardi DE, Qazi H, Tarbell JM. Interstitial flow induces MMP-1 expression and vascular SMC migration in collagen I gels via an ERK1/2-dependent and c-Jun-mediated mechanism. Am J Physiol Heart Circ Physiol. 2010;298(1):H127–135. doi: 10.1152/ajpheart.00732.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin UH, Suh SJ, Chang HW, Son JK, Lee SH, Son KH, Chang YC, Kim CH. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem. 2008;104(1):15–26. doi: 10.1002/jcb.21599. [DOI] [PubMed] [Google Scholar]
- 52.Chang JJ, Stanfill A, Pourmotabbed T. The Role of Matrix Metalloproteinase Polymorphisms in Ischemic Stroke. Int J Mol Sci. 2016;17(8) doi: 10.3390/ijms17081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirakawa S, Kojima T, Obata K, Okabayashi T, Yokota S, Nomura K, Obonai T, Fuchimoto J, Himi T, Tsutsumi H, Sawada N. Marked induction of matrix metalloproteinase-10 by respiratory syncytial virus infection in human nasal epithelial cells. J Med Virol. 2013;85(12):2141–2150. doi: 10.1002/jmv.23718. [DOI] [PubMed] [Google Scholar]
- 54.Zhang G, Miyake M, Lawton A, Goodison S, Rosser CJ. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14:310. doi: 10.1186/1471-2407-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, Davies AH, Khalil RA. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. Journal of vascular surgery. 2011;53(3):764–773. doi: 10.1016/j.jvs.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2010;159(2):755–764. doi: 10.1016/j.jss.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40(5):1001–1010. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 58.Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, Trzeciakowski JP, Davis MJ, Davis GE, Meininger GA. alpha(4)beta(1) Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90(4):473–480. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- 59.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–380. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng DX, Spin JM, Tsalenko A, Vailaya A, Ben-Dor A, Yakhini Z, Tsao P, Bruhn L, Quertermous T. Molecular signatures determining coronary artery and saphenous vein smooth muscle cell phenotypes: distinct responses to stimuli. Arterioscler Thromb Vasc Biol. 2006;26(5):1058–1065. doi: 10.1161/01.ATV.0000208185.16371.97. [DOI] [PubMed] [Google Scholar]
- 61.Turner NA, Ho S, Warburton P, O’Regan DJ, Porter KE. Smooth muscle cells cultured from human saphenous vein exhibit increased proliferation, invasion, and mitogen-activated protein kinase activation in vitro compared with paired internal mammary artery cells. J Vasc Surg. 2007;45(5):1022–1028. doi: 10.1016/j.jvs.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 62.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26(6):1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 63.McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, 2nd, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91(1):94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 65.Garcia JG, Patterson C, Bahler C, Aschner J, Hart CM, English D. Thrombin receptor activating peptides induce Ca2+ mobilization, barrier dysfunction, prostaglandin synthesis, and platelet-derived growth factor mRNA expression in cultured endothelium. J Cell Physiol. 1993;156(3):541–549. doi: 10.1002/jcp.1041560313. [DOI] [PubMed] [Google Scholar]
- 66.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 67.Lee HY, You HJ, Won JY, Youn SW, Cho HJ, Park KW, Park WY, Seo JS, Park YB, Walsh K, Oh BH, Kim HS. Forkhead factor, FOXO3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2008;28(2):302–308. doi: 10.1161/ATVBAHA.107.150664. [DOI] [PubMed] [Google Scholar]
- 68.Nagase H, Fushimi K. Elucidating the function of non catalytic domains of collagenases and aggrecanases. Connect Tissue Res. 2008;49(3):169–174. doi: 10.1080/03008200802151698. [DOI] [PubMed] [Google Scholar]
- 69.Ayuk SM, Abrahamse H, Houreld NN. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in relation to Photobiomodulation. J Diabetes Res. 2016;2016:2897656. doi: 10.1155/2016/2897656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nam SI, Yu GI, Kim HJ, Park KO, Chung JH, Ha E, Shin DH. A polymorphism at -1607 2G in the matrix metalloproteinase-1 (MMP-1) increased risk of sudden deafness in Korean population but not at -519A/G in MMP-1. Laryngoscope. 2011;121(1):171–175. doi: 10.1002/lary.21334. [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Thibeault SL. Role of tumor necrosis factor-alpha in wound repair in human vocal fold fibroblasts. Laryngoscope. 2010;120(9):1819–1825. doi: 10.1002/lary.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rahmani-Neishaboor E, Hartwell R, Jalili R, Jackson J, Brown E, Ghahary A. Localized controlled release of stratifin reduces implantation-induced dermal fibrosis. Acta Biomater. 2012;8(10):3660–3668. doi: 10.1016/j.actbio.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 73.Poormasjedi-Meibod MS, Hartwell R, Kilani RT, Ghahary A. Anti-scarring properties of different tryptophan derivatives. PloS one. 2014;9(3):e91955. doi: 10.1371/journal.pone.0091955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Kilani RT, Rahmani-Neishaboor E, Jalili RB, Ghahary A. Kynurenine increases matrix metalloproteinase-1 and -3 expression in cultured dermal fibroblasts and improves scarring in vivo. J Invest Dermatol. 2014;134(3):643–650. doi: 10.1038/jid.2013.303. [DOI] [PubMed] [Google Scholar]
- 75.Wada Y, Shimada K, Sugimoto K, Kimura T, Ushiyama S. Novel p38 mitogen-activated protein kinase inhibitor R-130823 protects cartilage by down-regulating matrix metalloproteinase-1,-13 and prostaglandin E2 production in human chondrocytes. Int Immunopharmacol. 2006;6(2):144–155. doi: 10.1016/j.intimp.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Shimizu Y, Kondo S, Shirai A, Furukawa M, Yoshizaki T. A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx. 2008;35(3):381–389. doi: 10.1016/j.anl.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Murray GI, Duncan ME, O’Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185(3):256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 78.Mizrachi A, Koren R, Hadar T, Yaniv E, Morgenstern S, Shvero J. Expression of MMP-1 in invasive well-differentiated thyroid carcinoma. Eur Arch Otorhinolaryngol. 2011;268(1):131–135. doi: 10.1007/s00405-010-1343-7. [DOI] [PubMed] [Google Scholar]
- 79.Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mainardi CL. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. The Journal of biological chemistry. 1990;265(20):11421–11424. [PubMed] [Google Scholar]
- 80.Knauper V, Murphy G, Tschesche H. Activation of human neutrophil procollagenase by stromelysin 2. Eur J Biochem. 1996;235(1–2):187–191. doi: 10.1111/j.1432-1033.1996.00187.x. [DOI] [PubMed] [Google Scholar]
- 81.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6(2):127–134. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- 82.Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C, Puente XS. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21(10):2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. Journal of periodontal research. 1995;30(1):23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 84.Sorsa T, Hernandez M, Leppilahti J, Munjal S, Netuschil L, Mantyla P. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral Dis. 2010;16(1):39–45. doi: 10.1111/j.1601-0825.2009.01603.x. [DOI] [PubMed] [Google Scholar]
- 85.Dalvie D, Cosker T, Boyden T, Zhou S, Schroeder C, Potchoiba MJ. Metabolism distribution and excretion of a matrix metalloproteinase-13 inhibitor, 4-[4-(4-fluorophenoxy)-benzenesulfonylamino]tetrahydropyran-4-carboxylic acid hydroxyamide (CP-544439), in rats and dogs: assessment of the metabolic profile of CP-544439 in plasma and urine of humans. Drug Metab Dispos. 2008;36(9):1869–1883. doi: 10.1124/dmd.108.022566. [DOI] [PubMed] [Google Scholar]
- 86.Wang G, Zhang Y, Zhao X, Meng C, Ma L, Kong Y. MicroRNA-411 inhibited matrix metalloproteinase 13 expression in human chondrocytes. American journal of translational research. 2015;7(10):2000–2006. [PMC free article] [PubMed] [Google Scholar]
- 87.Yu H, Li Y, Ma L, Meng H, Bai X, Fan Z, Yu F, Guo A. A low ratio of n-6/n-3 polyunsaturated fatty acids suppresses matrix metalloproteinase 13 expression and reduces adjuvant-induced arthritis in rats. Nutr Res. 2015;35(12):1113–1121. doi: 10.1016/j.nutres.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 88.Mashimo Y, Sakurai-Yageta M, Watanabe M, Arima T, Morita Y, Inoue Y, Sato K, Nishimuta T, Suzuki S, Watanabe H, Hoshioka A, Tomiita M, Yamaide A, Kohno Y, Okamoto Y, Shimojo N, Hata A, Suzuki Y. Induction of the Matrix Metalloproteinase 13 Gene in Bronchial Epithelial Cells by Interferon and Identification of its Novel Functional Polymorphism. Inflammation. 2016;39(3):949–962. doi: 10.1007/s10753-015-0291-1. [DOI] [PubMed] [Google Scholar]
- 89.Chen JH, Tsai CH, Lin HY, Huang CF, Leung YM, Lai SW, Tsai CF, Chang PC, Lu DY, Lin C. Interlukin-18 Is a Pivot Regulatory Factor on Matrix Metalloproteinase-13 Expression and Brain Astrocytic Migration. Mol Neurobiol. 2016;53(9):6218–6227. doi: 10.1007/s12035-015-9529-z. [DOI] [PubMed] [Google Scholar]
- 90.Abe H, Kamimura K, Kobayashi Y, Ohtsuka M, Miura H, Ohashi R, Yokoo T, Kanefuji T, Suda T, Tsuchida M, Aoyagi Y, Zhang G, Liu D, Terai S. Effective Prevention of Liver Fibrosis by Liver-targeted Hydrodynamic Gene Delivery of Matrix Metalloproteinase-13 in a Rat Liver Fibrosis Model. Mol Ther Nucleic Acids. 2016;5:e276. doi: 10.1038/mtna.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.You Y, Shan Y, Chen J, Yue H, You B, Shi S, Li X, Cao X. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106(12):1669–1677. doi: 10.1111/cas.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cossins J, Dudgeon TJ, Catlin G, Gearing AJ, Clements JM. Identification of MMP-18, a putative novel human matrix metalloproteinase. Biochemical and biophysical research communications. 1996;228(2):494–498. doi: 10.1006/bbrc.1996.1688. [DOI] [PubMed] [Google Scholar]
- 93.Tomlinson ML, Garcia-Morales C, Abu-Elmagd M, Wheeler GN. Three matrix metalloproteinases are required in vivo for macrophage migration during embryonic development. Mech Dev. 2008;125(11–12):1059–1070. doi: 10.1016/j.mod.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Tonge D, Zhu N, Lynham S, Leclere P, Snape A, Brewer A, Schlomann U, Ferdous T, Tennyson C, Bartsch JW, Ward M, Pizzey J. Axonal growth towards Xenopus skin in vitro is mediated by matrix metalloproteinase activity. Eur J Neurosci. 2013;37(4):519–531. doi: 10.1111/ejn.12075. [DOI] [PubMed] [Google Scholar]
- 95.Steffensen B, Wallon UM, Overall CM. Extracellular matrix binding properties of recombinant fibronectin type II-like modules of human 72-kDa gelatinase/type IV collagenase. High affinity binding to native type I collagen but not native type IV collagen. The Journal of biological chemistry. 1995;270(19):11555–11566. doi: 10.1074/jbc.270.19.11555. [DOI] [PubMed] [Google Scholar]
- 96.Shipley JM, Doyle GA, Fliszar CJ, Ye QZ, Johnson LL, Shapiro SD, Welgus HG, Senior RM. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. The Journal of biological chemistry. 1996;271(8):4335–4341. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- 97.Koyama H, Iwata H, Kuwabara Y, Iwase H, Kobayashi S, Fujii Y. Gelatinolytic activity of matrix metalloproteinase-2 and -9 in oesophageal carcinoma; a study using in situ zymography. Eur J Cancer. 2000;36(16):2164–2170. doi: 10.1016/s0959-8049(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 98.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755(1):37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Overall CM, Tam E, McQuibban GA, Morrison C, Wallon UM, Bigg HF, King AE, Roberts CR. Domain interactions in the gelatinase A.TIMP-2.MT1-MMP activation complex. The ectodomain of the 44-kDa form of membrane type-1 matrix metalloproteinase does not modulate gelatinase A activation. The Journal of biological chemistry. 2000;275(50):39497–39506. doi: 10.1074/jbc.M005932200. [DOI] [PubMed] [Google Scholar]
- 100.Augoff K, Grabowski K, Rabczynski J, Kolondra A, Tabola R, Sikorski AF. Expression of decorin in esophageal cancer in relation to the expression of three isoforms of transforming growth factor-beta (TGF-beta1, -beta2, and -beta3) and matrix metalloproteinase-2 activity. Cancer Invest. 2009;27(4):443–452. doi: 10.1080/07357900802527221. [DOI] [PubMed] [Google Scholar]
- 101.Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br J Cancer. 2003;89(7):1270–1275. doi: 10.1038/sj.bjc.6601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chetty C, Bhoopathi P, Joseph P, Chittivelu S, Rao JS, Lakka S. Adenovirus-mediated small interfering RNA against matrix metalloproteinase-2 suppresses tumor growth and lung metastasis in mice. Mol Cancer Ther. 2006;5(9):2289–2299. doi: 10.1158/1535-7163.MCT-06-0169. [DOI] [PubMed] [Google Scholar]
- 103.Morozevich G, Kozlova N, Cheglakov I, Ushakova N, Berman A. Integrin alpha5beta1 controls invasion of human breast carcinoma cells by direct and indirect modulation of MMP-2 collagenase activity. Cell Cycle. 2009;8(14):2219–2225. doi: 10.4161/cc.8.14.8980. [DOI] [PubMed] [Google Scholar]
- 104.Gu G, Xia H, Hu Q, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Chen H, Gao X, Chen J. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials. 2013;34(1):196–208. doi: 10.1016/j.biomaterials.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 105.Kesanakurti D, Chetty C, Dinh DH, Gujrati M, Rao JS. Role of MMP-2 in the regulation of IL-6/Stat3 survival signaling via interaction with alpha5beta1 integrin in glioma. Oncogene. 2013;32(3):327–340. doi: 10.1038/onc.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nam SI, Kwon TK. Dexamethasone inhibits interleukin-1beta-induced matrix metalloproteinase-9 expression in cochlear cells. Clin Exp Otorhinolaryngol. 2014;7(3):175–180. doi: 10.3342/ceo.2014.7.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olszewska E, Matulka M, Mroczko B, Pryczynicz A, Kemona A, Szmitkowski M, Mierzwinski J, Pietrewicz T. Diagnostic value of matrix metalloproteinase 9 and tissue inhibitor of matrix metalloproteinases 1 in cholesteatoma. Histol Histopathol. 2016;31(3):307–315. doi: 10.14670/HH-11-677. [DOI] [PubMed] [Google Scholar]
- 108.Detwiller KY, Smith TL, Mace JC, Trune DR, Sautter NB. Steroid-independent upregulation of matrix metalloproteinase 9 in chronic rhinosinusitis patients with radiographic evidence of osteitis. Int Forum Allergy Rhinol. 2013;3(5):364–368. doi: 10.1002/alr.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eguchi T, Kubota S, Kawata K, Mukudai Y, Uehara J, Ohgawara T, Ibaragi S, Sasaki A, Kuboki T, Takigawa M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol Cell Biol. 2008;28(7):2391–2413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Si-Tayeb K, Monvoisin A, Mazzocco C, Lepreux S, Decossas M, Cubel G, Taras D, Blanc JF, Robinson DR, Rosenbaum J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am J Pathol. 2006;169(4):1390–1401. doi: 10.2353/ajpath.2006.060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ding L, Guo D, Homandberg GA, Buckwalter JA, Martin JA. A single blunt impact on cartilage promotes fibronectin fragmentation and upregulates cartilage degrading stromelysin-1/matrix metalloproteinase-3 in a bovine ex vivo model. J Orthop Res. 2014;32(6):811–818. doi: 10.1002/jor.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J. 1995;73(3):209–215. doi: 10.1136/hrt.73.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang YN, Yan HQ, Huang XB, Wang YN, Li Q, Gao FG. Interleukin 6 trigged ataxia-telangiectasia mutated activation facilitates lung cancer metastasis via MMP-3/MMP-13 up-regulation. Oncotarget. 2015;6(38):40719–40733. doi: 10.18632/oncotarget.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kolomecki K, Stepien H, Bartos M, Kuzdak K. Usefulness of VEGF, MMP-2, MMP-3 and TIMP-2 serum level evaluation in patients with adrenal tumours. Endocr Regul. 2001;35(1):9–16. [PubMed] [Google Scholar]
- 115.Choi DH, Kim JH, Seo JH, Lee J, Choi WS, Kim YS. Matrix metalloproteinase-3 causes dopaminergic neuronal death through Nox1-regenerated oxidative stress. PloS one. 2014;9(12):e115954. doi: 10.1371/journal.pone.0115954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sokai A, Handa T, Tanizawa K, Oga T, Uno K, Tsuruyama T, Kubo T, Ikezoe K, Nakatsuka Y, Tanimura K, Muro S, Hirai T, Nagai S, Chin K, Mishima M. Matrix metalloproteinase-10: a novel biomarker for idiopathic pulmonary fibrosis. Respir Res. 2015;16:120. doi: 10.1186/s12931-015-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martinez-Aguilar E, Gomez-Rodriguez V, Orbe J, Rodriguez JA, Fernandez-Alonso L, Roncal C, Paramo JA. Matrix metalloproteinase 10 is associated with disease severity and mortality in patients with peripheral arterial disease. Journal of vascular surgery. 2015;61(2):428–435. doi: 10.1016/j.jvs.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Rohani MG, McMahan RS, Razumova MV, Hertz AL, Cieslewicz M, Pun SH, Regnier M, Wang Y, Birkland TP, Parks WC. MMP-10 Regulates Collagenolytic Activity of Alternatively Activated Resident Macrophages. J Invest Dermatol. 2015;135(10):2377–2384. doi: 10.1038/jid.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang H, Zhang ZQ, Wang SZ, Lu JL, Wang XL, Zhang ZY. Association of matrix metalloproteinase-10 polymorphisms with susceptibility to pelvic organ prolapse. The journal of obstetrics and gynaecology research. 2015;41(12):1972–1981. doi: 10.1111/jog.12809. [DOI] [PubMed] [Google Scholar]
- 120.Kadeh H, Saravani S, Heydari F, Keikha M, Rigi V. Expression of Matrix Metalloproteinase-10 at Invasive Front of Squamous Cell Carcinoma and Verrucous Carcinoma in the Oral Cavity. Asian Pac J Cancer Prev. 2015;16(15):6609–6613. doi: 10.7314/apjcp.2015.16.15.6609. [DOI] [PubMed] [Google Scholar]
- 121.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- 122.Fiorentino M, Fu L, Shi YB. Mutational analysis of the cleavage of the cancer-associated laminin receptor by stromelysin-3 reveals the contribution of flanking sequences to site recognition and cleavage efficiency. Int J Mol Med. 2009;23(3):389–397. doi: 10.3892/ijmm_00000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin CW, Yang SF, Chuang CY, Lin HP, Hsin CH. Association of matrix metalloproteinase-11 polymorphisms with susceptibility and clinicopathologic characteristics for oral squamous cell carcinoma. Head Neck. 2015;37(10):1425–1431. doi: 10.1002/hed.23771. [DOI] [PubMed] [Google Scholar]
- 124.Sharma R, Chattopadhyay TK, Mathur M, Ralhan R. Prognostic significance of stromelysin-3 and tissue inhibitor of matrix metalloproteinase-2 in esophageal cancer. Oncology. 2004;67(3–4):300–309. doi: 10.1159/000081331. [DOI] [PubMed] [Google Scholar]
- 125.Zhang X, Huang S, Guo J, Zhou L, You L, Zhang T, Zhao Y. Insights into the distinct roles of MMP-11 in tumor biology and future therapeutics (Review) Int J Oncol. 2016;48(5):1783–1793. doi: 10.3892/ijo.2016.3400. [DOI] [PubMed] [Google Scholar]
- 126.Li Z, Ding S, Zhong Q, Li G, Zhang Y, Huang XC. Significance of MMP11 and P14(ARF) expressions in clinical outcomes of patients with laryngeal cancer. Int J Clin Exp Med. 2015;8(9):15581–15590. [PMC free article] [PubMed] [Google Scholar]
- 127.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 128.Acioglu E, Yigit O, Alkan Z, Server EA, Uzun H, Gelisgen R. The role of matrix metalloproteinases in recurrent tonsillitis. Int J Pediatr Otorhinolaryngol. 2010;74(5):535–539. doi: 10.1016/j.ijporl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 129.Jiang T, Xie P, Liu H. Circulating Anti-Matrix Metalloproteinase-7 Antibodies May Be a Potential Biomarker for Oral Squamous Cell Carcinoma. J Oral Maxillofac Surg. 2015;74(3):650–657. doi: 10.1016/j.joms.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 130.Uria JA, Lopez-Otin C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60(17):4745–4751. [PubMed] [Google Scholar]
- 131.de Coignac AB, Elson G, Delneste Y, Magistrelli G, Jeannin P, Aubry JP, Berthier O, Schmitt D, Bonnefoy JY, Gauchat JF. Cloning of MMP-26. A novel matrilysin-like proteinase. Eur J Biochem. 2000;267(11):3323–3329. doi: 10.1046/j.1432-1327.2000.01363.x. [DOI] [PubMed] [Google Scholar]
- 132.Marchenko GN, Ratnikov BI, Rozanov DV, Godzik A, Deryugina EI, Strongin AY. Characterization of matrix metalloproteinase-26, a novel metalloproteinase widely expressed in cancer cells of epithelial origin. Biochem J. 2001;356(Pt 3):705–718. doi: 10.1042/0264-6021:3560705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marchenko ND, Marchenko GN, Weinreb RN, Lindsey JD, Kyshtoobayeva A, Crawford HC, Strongin AY. Beta-catenin regulates the gene of MMP-26, a novel metalloproteinase expressed both in carcinomas and normal epithelial cells. Int J Biochem Cell Biol. 2004;36(5):942–956. doi: 10.1016/j.biocel.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 134.Gutschalk CM, Yanamandra AK, Linde N, Meides A, Depner S, Mueller MM. GM-CSF enhances tumor invasion by elevated MMP-2, -9, and -26 expression. Cancer Med. 2013;2(2):117–129. doi: 10.1002/cam4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bister V, Skoog T, Virolainen S, Kiviluoto T, Puolakkainen P, Saarialho-Kere U. Increased expression of matrix metalloproteinases-21 and -26 and TIMP-4 in pancreatic adenocarcinoma. Mod Pathol. 2007;20(11):1128–1140. doi: 10.1038/modpathol.3800956. [DOI] [PubMed] [Google Scholar]
- 136.Zhao YG, Xiao AZ, Park HI, Newcomer RG, Yan M, Man YG, Heffelfinger SC, Sang QX. Endometase/matrilysin-2 in human breast ductal carcinoma in situ and its inhibition by tissue inhibitors of metalloproteinases-2 and -4: a putative role in the initiation of breast cancer invasion. Cancer Res. 2004;64(2):590–598. doi: 10.1158/0008-5472.can-03-1932. [DOI] [PubMed] [Google Scholar]
- 137.Sohail A, Sun Q, Zhao H, Bernardo MM, Cho JA, Fridman R. MT4-(MMP17) and MT6-MMP (MMP25), A unique set of membrane-anchored matrix metalloproteinases: properties and expression in cancer. Cancer Metastasis Rev. 2008;27(2):289–302. doi: 10.1007/s10555-008-9129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271(29):17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 139.Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. The Journal of biological chemistry. 1999;274(48):34260–34266. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- 140.Radichev IA, Remacle AG, Shiryaev SA, Purves AN, Johnson SL, Pellecchia M, Strongin AY. Biochemical characterization of the cellular glycosylphosphatidylinositol-linked membrane type-6 matrix metalloproteinase. The Journal of biological chemistry. 2010;285(21):16076–16086. doi: 10.1074/jbc.M110.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kayano K, Shimada T, Shinomiya T, Nakai S, Hisa Y, Aoki T, Seiki M, Okada Y. Activation of pro-MMP-2 mediated by MT1-MMP in human salivary gland carcinomas: possible regulation of pro-MMP-2 activation by TIMP-2. J Pathol. 2004;202(4):403–411. doi: 10.1002/path.1541. [DOI] [PubMed] [Google Scholar]
- 142.Imanishi Y, Fujii M, Tokumaru Y, Tomita T, Kanke M, Kanzaki J, Kameyama K, Otani Y, Sato H. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000;31(8):895–904. doi: 10.1053/hupa.2000.9756. [DOI] [PubMed] [Google Scholar]
- 143.Johnson JL, Jenkins NP, Huang WC, Di Gregoli K, Sala-Newby GB, Scholtes VP, Moll FL, Pasterkamp G, Newby AC. Relationship of MMP-14 and TIMP-3 expression with macrophage activation and human atherosclerotic plaque vulnerability. Mediators Inflamm. 2014;2014:276457. doi: 10.1155/2014/276457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiesner C, El Azzouzi K, Linder S. A specific subset of RabGTPases controls cell surface exposure of MT1-MMP, extracellular matrix degradation and three-dimensional invasion of macrophages. J Cell Sci. 2013;126(Pt 13):2820–2833. doi: 10.1242/jcs.122358. [DOI] [PubMed] [Google Scholar]
- 145.Sato H, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem. 1996;119(2):209–215. doi: 10.1093/oxfordjournals.jbchem.a021223. [DOI] [PubMed] [Google Scholar]
- 146.Szabova L, Son MY, Shi J, Sramko M, Yamada SS, Swaim WD, Zerfas P, Kahan S, Holmbeck K. Membrane-type MMPs are indispensable for placental labyrinth formation and development. Blood. 2010;116(25):5752–5761. doi: 10.1182/blood-2009-10-249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sena P, Mariani F, Marzona L, Benincasa M, Ponz de Leon M, Palumbo C, Roncucci L. Matrix metalloproteinases 15 and 19 are stromal regulators of colorectal cancer development from the early stages. Int J Oncol. 2012;41(1):260–266. doi: 10.3892/ijo.2012.1441. [DOI] [PubMed] [Google Scholar]
- 148.Zhang H, Liu M, Sun Y, Lu J. MMP-14 can serve as a prognostic marker in patients with supraglottic cancer. Eur Arch Otorhinolaryngol. 2009;266(9):1427–1434. doi: 10.1007/s00405-009-0943-6. [DOI] [PubMed] [Google Scholar]
- 149.Sun YN, Li Y. Expression of mRNA for membrane-type 1, 2, and 3 matrix metalloproteinases in human laryngeal cancer. Chin Med Sci J. 2004;19(3):170–173. [PubMed] [Google Scholar]
- 150.Liu J, van Mil A, Aguor EN, Siddiqi S, Vrijsen K, Jaksani S, Metz C, Zhao J, Strijkers GJ, Doevendans PA, Sluijter JP. MiR-155 inhibits cell migration of human cardiomyocyte progenitor cells (hCMPCs) via targeting of MMP-16. J Cell Mol Med. 2012;16(10):2379–2386. doi: 10.1111/j.1582-4934.2012.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tatti O, Gucciardo E, Pekkonen P, Holopainen T, Louhimo R, Repo P, Maliniemi P, Lohi J, Rantanen V, Hautaniemi S, Alitalo K, Ranki A, Ojala PM, Keski-Oja J, Lehti K. MMP16 Mediates a Proteolytic Switch to Promote Cell-Cell Adhesion, Collagen Alignment, and Lymphatic Invasion in Melanoma. Cancer Res. 2015;75(10):2083–2094. doi: 10.1158/0008-5472.CAN-14-1923. [DOI] [PubMed] [Google Scholar]
- 152.Chen MF, Zeng F, Qi L, Zu XB, Wang J, Liu LF, Li Y. Transforming growth factorbeta1 induces epithelialmesenchymal transition and increased expression of matrix metalloproteinase16 via miR200b downregulation in bladder cancer cells. Mol Med Rep. 2014;10(3):1549–1554. doi: 10.3892/mmr.2014.2366. [DOI] [PubMed] [Google Scholar]
- 153.Liu C, Wu F, Liu Y, Meng C. Catalpol suppresses proliferation and facilitates apoptosis of MCF-7 breast cancer cells through upregulating microRNA-146a and downregulating matrix metalloproteinase-16 expression. Mol Med Rep. 2015;12(5):7609–7614. doi: 10.3892/mmr.2015.4361. [DOI] [PubMed] [Google Scholar]
- 154.Hadchouel A, Decobert F, Franco-Montoya ML, Halphen I, Jarreau PH, Boucherat O, Martin E, Benachi A, Amselem S, Bourbon J, Danan C, Delacourt C. Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. PloS one. 2008;3(9):e3188. doi: 10.1371/journal.pone.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Puente XS, Pendas AM, Llano E, Velasco G, Lopez-Otin C. Molecular cloning of a novel membrane-type matrix metalloproteinase from a human breast carcinoma. Cancer Res. 1996;56(5):944–949. [PubMed] [Google Scholar]
- 156.Llano E, Pendas AM, Freije JP, Nakano A, Knauper V, Murphy G, Lopez-Otin C. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase a overexpressed in brain tumors. Cancer Res. 1999;59(11):2570–2576. [PubMed] [Google Scholar]
- 157.Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. The Journal of biological chemistry. 1999;274(13):8925–8932. doi: 10.1074/jbc.274.13.8925. [DOI] [PubMed] [Google Scholar]
- 158.Porlan E, Marti-Prado B, Morante-Redolat JM, Consiglio A, Delgado AC, Kypta R, Lopez-Otin C, Kirstein M, Farinas I. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat Cell Biol. 2014;16(7):629–638. doi: 10.1038/ncb2993. [DOI] [PubMed] [Google Scholar]
- 159.Warren KM, Reeves TM, Phillips LL. MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J Neurotrauma. 2012;29(10):1922–1940. doi: 10.1089/neu.2012.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Komori K, Nonaka T, Okada A, Kinoh H, Hayashita-Kinoh H, Yoshida N, Yana I, Seiki M. Absence of mechanical allodynia and Abeta-fiber sprouting after sciatic nerve injury in mice lacking membrane-type 5 matrix metalloproteinase. FEBS Lett. 2004;557(1–3):125–128. doi: 10.1016/s0014-5793(03)01458-3. [DOI] [PubMed] [Google Scholar]
- 161.Folgueras AR, Valdes-Sanchez T, Llano E, Menendez L, Baamonde A, Denlinger BL, Belmonte C, Juarez L, Lastra A, Garcia-Suarez O, Astudillo A, Kirstein M, Pendas AM, Farinas I, Lopez-Otin C. Metalloproteinase MT5-MMP is an essential modulator of neuro-immune interactions in thermal pain stimulation. Proc Natl Acad Sci U S A. 2009;106(38):16451–16456. doi: 10.1073/pnas.0908507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Benson CS, Babu SD, Radhakrishna S, Selvamurugan N, Ravi Sankar B. Expression of matrix metalloproteinases in human breast cancer tissues. Dis Markers. 2013;34(6):395–405. doi: 10.3233/DMA-130986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao H, Sohail A, Sun Q, Shi Q, Kim S, Mobashery S, Fridman R. Identification and role of the homodimerization interface of the glycosylphosphatidylinositol-anchored membrane type 6 matrix metalloproteinase (MMP25) The Journal of biological chemistry. 2008;283(50):35023–35032. doi: 10.1074/jbc.M806553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fortin CF, Sohail A, Sun Q, McDonald PP, Fridman R, Fulop T. MT6-MMP is present in lipid rafts and faces inward in living human PMNs but translocates to the cell surface during neutrophil apoptosis. Int Immunol. 2010;22(8):637–649. doi: 10.1093/intimm/dxq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Velasco G, Cal S, Merlos-Suarez A, Ferrando AA, Alvarez S, Nakano A, Arribas J, Lopez-Otin C. Human MT6-matrix metalloproteinase: identification, progelatinase A activation, and expression in brain tumors. Cancer Res. 2000;60(4):877–882. [PubMed] [Google Scholar]
- 166.Sun Q, Weber CR, Sohail A, Bernardo MM, Toth M, Zhao H, Turner JR, Fridman R. MMP25 (MT6-MMP) is highly expressed in human colon cancer, promotes tumor growth, and exhibits unique biochemical properties. The Journal of biological chemistry. 2007;282(30):21998–22010. doi: 10.1074/jbc.M701737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A. 1996;93(9):3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kerkela E, Bohling T, Herva R, Uria JA, Saarialho-Kere U. Human macrophage metalloelastase (MMP-12) expression is induced in chondrocytes during fetal development and malignant transformation. Bone. 2001;29(5):487–493. doi: 10.1016/s8756-3282(01)00595-6. [DOI] [PubMed] [Google Scholar]
- 169.Hou P, Troen T, Ovejero MC, Kirkegaard T, Andersen TL, Byrjalsen I, Ferreras M, Sato T, Shapiro SD, Foged NT, Delaisse JM. Matrix metalloproteinase-12 (MMP-12) in osteoclasts: new lesson on the involvement of MMPs in bone resorption. Bone. 2004;34(1):37–47. doi: 10.1016/j.bone.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 170.Marchant DJ, Bellac CL, Moraes TJ, Wadsworth SJ, Dufour A, Butler GS, Bilawchuk LM, Hendry RG, Robertson AG, Cheung CT, Ng J, Ang L, Luo Z, Heilbron K, Norris MJ, Duan W, Bucyk T, Karpov A, Devel L, Georgiadis D, Hegele RG, Luo H, Granville DJ, Dive V, McManus BM, Overall CM. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014;20(5):493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- 171.Li W, Li J, Wu Y, Rancati F, Vallese S, Raveglia L, Wu J, Hotchandani R, Fuller N, Cunningham K, Morgan P, Fish S, Krykbaev R, Xu X, Tam S, Goldman SJ, Abraham W, Williams C, Sypek J, Mansour TS. Identification of an orally efficacious matrix metalloprotease 12 inhibitor for potential treatment of asthma. J Med Chem. 2009;52(17):5408–5419. doi: 10.1021/jm900809r. [DOI] [PubMed] [Google Scholar]
- 172.Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK. Matrix Metalloproteinase-12 Induces Blood-Brain Barrier Damage After Focal Cerebral Ischemia. Stroke. 2015;46(12):3523–3531. doi: 10.1161/STROKEAHA.115.011031. [DOI] [PubMed] [Google Scholar]
- 173.Kim JM, Kim HJ, Koo BS, Rha KS, Yoon YH. Expression of matrix metalloproteinase-12 is correlated with extracapsular spread of tumor from nodes with metastasis in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2013;270(3):1137–1142. doi: 10.1007/s00405-012-2161-x. [DOI] [PubMed] [Google Scholar]
- 174.Stracke JO, Hutton M, Stewart M, Pendas AM, Smith B, Lopez-Otin C, Murphy G, Knauper V. Biochemical characterization of the catalytic domain of human matrix metalloproteinase 19. Evidence for a role as a potent basement membrane degrading enzyme. The Journal of biological chemistry. 2000;275(20):14809–14816. doi: 10.1074/jbc.275.20.14809. [DOI] [PubMed] [Google Scholar]
- 175.Stracke JO, Fosang AJ, Last K, Mercuri FA, Pendas AM, Llano E, Perris R, Di Cesare PE, Murphy G, Knauper V. Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP) FEBS Lett. 2000;478(1–2):52–56. doi: 10.1016/s0014-5793(00)01819-6. [DOI] [PubMed] [Google Scholar]
- 176.Sadowski T, Dietrich S, Muller M, Havlickova B, Schunck M, Proksch E, Muller MS, Sedlacek R. Matrix metalloproteinase-19 expression in normal and diseased skin: dysregulation by epidermal proliferation. J Invest Dermatol. 2003;121(5):989–996. doi: 10.1046/j.1523-1747.2003.12526.x. [DOI] [PubMed] [Google Scholar]
- 177.Sadowski T, Dietrich S, Koschinsky F, Sedlacek R. Matrix metalloproteinase 19 regulates insulin-like growth factor-mediated proliferation, migration, and adhesion in human keratinocytes through proteolysis of insulin-like growth factor binding protein-3. Mol Biol Cell. 2003;14(11):4569–4580. doi: 10.1091/mbc.E03-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sadowski T, Dietrich S, Koschinsky F, Ludwig A, Proksch E, Titz B, Sedlacek R. Matrix metalloproteinase 19 processes the laminin 5 gamma 2 chain and induces epithelial cell migration. Cell Mol Life Sci. 2005;62(7–8):870–880. doi: 10.1007/s00018-005-4478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brauer R, Beck IM, Roderfeld M, Roeb E, Sedlacek R. Matrix metalloproteinase-19 inhibits growth of endothelial cells by generating angiostatin-like fragments from plasminogen. BMC Biochem. 2011;12:38. doi: 10.1186/1471-2091-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jara P, Calyeca J, Romero Y, Placido L, Yu G, Kaminski N, Maldonado V, Cisneros J, Selman M, Pardo A. Matrix metalloproteinase (MMP)-19-deficient fibroblasts display a profibrotic phenotype. American journal of physiology. Lung cellular and molecular physiology. 2015;308(6):L511–522. doi: 10.1152/ajplung.00043.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu G, Kovkarova-Naumovski E, Jara P, Parwani A, Kass D, Ruiz V, Lopez-Otin C, Rosas IO, Gibson KF, Cabrera S, Ramirez R, Yousem SA, Richards TJ, Chensny LJ, Selman M, Kaminski N, Pardo A. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am J Respir Crit Care Med. 2012;186(8):752–762. doi: 10.1164/rccm.201202-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jirouskova M, Zbodakova O, Gregor M, Chalupsky K, Sarnova L, Hajduch M, Ehrmann J, Jirkovska M, Sedlacek R. Hepatoprotective effect of MMP-19 deficiency in a mouse model of chronic liver fibrosis. PloS one. 2012;7(10):e46271. doi: 10.1371/journal.pone.0046271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hazlewood RJ, Roos BR, Solivan-Timpe F, Honkanen RA, Jampol LM, Gieser SC, Meyer KJ, Mullins RF, Kuehn MH, Scheetz TE, Kwon YH, Alward WL, Stone EM, Fingert JH. Heterozygous triplication of upstream regulatory sequences leads to dysregulation of matrix metalloproteinase 19 in patients with cavitary optic disc anomaly. Hum Mutat. 2015;36(3):369–378. doi: 10.1002/humu.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pendas AM, Folgueras AR, Llano E, Caterina J, Frerard F, Rodriguez F, Astudillo A, Noel A, Birkedal-Hansen H, Lopez-Otin C. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol Cell Biol. 2004;24(12):5304–5313. doi: 10.1128/MCB.24.12.5304-5313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee DG, Lee SH, Kim JS, Park J, Cho YL, Kim KS, Jo DY, Song IC, Kim N, Yun HJ, Park YJ, Lee SJ, Lee HG, Bae KH, Lee SC, Shim S, Kim YM, Kwon YG, Kim JM, Lee HJ, Min JK. Loss of NDRG2 promotes epithelial-mesenchymal transition of gallbladder carcinoma cells through MMP-19-mediated Slug expression. J Hepatol. 2015;63(6):1429–1439. doi: 10.1016/j.jhep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 186.Yu G, Herazo-Maya JD, Nukui T, Romkes M, Parwani A, Juan-Guardela BM, Robertson J, Gauldie J, Siegfried JM, Kaminski N, Kass DJ. Matrix metalloproteinase-19 promotes metastatic behavior in vitro and is associated with increased mortality in non-small cell lung cancer. Am J Respir Crit Care Med. 2014;190(7):780–790. doi: 10.1164/rccm.201310-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chan KC, Ko JM, Lung HL, Sedlacek R, Zhang ZF, Luo DZ, Feng ZB, Chen S, Chen H, Chan KW, Tsao SW, Chua DT, Zabarovsky ER, Stanbridge EJ, Lung ML. Catalytic activity of Matrix metalloproteinase-19 is essential for tumor suppressor and anti-angiogenic activities in nasopharyngeal carcinoma. International journal of cancer. Journal international du cancer. 2011;129(8):1826–1837. doi: 10.1002/ijc.25855. [DOI] [PubMed] [Google Scholar]
- 188.Ryu OH, Fincham AG, Hu CC, Zhang C, Qian Q, Bartlett JD, Simmer JP. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res. 1999;78(3):743–750. doi: 10.1177/00220345990780030601. [DOI] [PubMed] [Google Scholar]
- 189.Llano E, Pendas AM, Knauper V, Sorsa T, Salo T, Salido E, Murphy G, Simmer JP, Bartlett JD, Lopez-Otin C. Identification and structural and functional characterization of human enamelysin (MMP-20) Biochemistry. 1997;36(49):15101–15108. doi: 10.1021/bi972120y. [DOI] [PubMed] [Google Scholar]
- 190.Barron LA, Giardina JB, Granger JP, Khalil RA. High-salt diet enhances vascular reactivity in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension. 2001;38(3 Pt 2):730–735. doi: 10.1161/01.hyp.38.3.730. [DOI] [PubMed] [Google Scholar]
- 191.Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, Bartlett JD. Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. The Journal of biological chemistry. 2002;277(51):49598–49604. doi: 10.1074/jbc.M209100200. [DOI] [PubMed] [Google Scholar]
- 192.Skoog T, Ahokas K, Orsmark C, Jeskanen L, Isaka K, Saarialho-Kere U. MMP-21 is expressed by macrophages and fibroblasts in vivo and in culture. Exp Dermatol. 2006;15(10):775–783. doi: 10.1111/j.1600-0625.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 193.Ahokas K, Lohi J, Illman SA, Llano E, Elomaa O, Impola U, Karjalainen-Lindsberg ML, Saarialho-Kere U. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-beta1 in keratinocytes. Lab Invest. 2003;83(12):1887–1899. doi: 10.1097/01.lab.0000106721.86126.39. [DOI] [PubMed] [Google Scholar]
- 194.Pu Y, Wang L, Wu H, Feng Z, Wang Y, Guo C. High MMP-21 expression in metastatic lymph nodes predicts unfavorable overall survival for oral squamous cell carcinoma patients with lymphatic metastasis. Oncol Rep. 2014;31(6):2644–2650. doi: 10.3892/or.2014.3124. [DOI] [PubMed] [Google Scholar]
- 195.Zhao Z, Yan L, Li S, Sun H, Zhou Y, Li X. Increased MMP-21 expression in esophageal squamous cell carcinoma is associated with progression and prognosis. Med Oncol. 2014;31(8):91. doi: 10.1007/s12032-014-0091-8. [DOI] [PubMed] [Google Scholar]
- 196.Wu T, Li Y, Liu X, Lu J, He X, Wang Q, Li J, Du X. Identification of high-risk stage II and stage III colorectal cancer by analysis of MMP-21 expression. J Surg Oncol. 2011;104(7):787–791. doi: 10.1002/jso.21970. [DOI] [PubMed] [Google Scholar]
- 197.Suomela S, Koljonen V, Skoog T, Kukko H, Bohling T, Saarialho-Kere U. Expression of MMP-10, MMP-21, MMP-26, and MMP-28 in Merkel cell carcinoma. Virchows Arch. 2009;455(6):495–503. doi: 10.1007/s00428-009-0856-1. [DOI] [PubMed] [Google Scholar]
- 198.Gururajan R, Grenet J, Lahti JM, Kidd VJ. Isolation and characterization of two novel metalloproteinase genes linked to the Cdc2L locus on human chromosome 1p36.3. Genomics. 1998;52(1):101–106. doi: 10.1006/geno.1998.5401. [DOI] [PubMed] [Google Scholar]
- 199.Velasco G, Pendas AM, Fueyo A, Knauper V, Murphy G, Lopez-Otin C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274(8):4570–4576. doi: 10.1074/jbc.274.8.4570. [DOI] [PubMed] [Google Scholar]
- 200.Hegedus L, Cho H, Xie X, Eliceiri GL. Additional MDA-MB-231 breast cancer cell matrix metalloproteinases promote invasiveness. Journal of cellular physiology. 2008;216(2):480–485. doi: 10.1002/jcp.21417. [DOI] [PubMed] [Google Scholar]
- 201.Cominelli A, Halbout M, N’Kuli F, Lemoine P, Courtoy PJ, Marbaix E, Tyteca D, Henriet P. A unique C-terminal domain allows retention of matrix metalloproteinase-27 in the endoplasmic reticulum. Traffic. 2014;15(4):401–417. doi: 10.1111/tra.12149. [DOI] [PubMed] [Google Scholar]
- 202.Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington CJ, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126(Pt 12):2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 203.Cominelli A, Gaide Chevronnay HP, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. Matrix metalloproteinase-27 is expressed in CD163+/CD206+ M2 macrophages in the cycling human endometrium and in superficial endometriotic lesions. Molecular human reproduction. 2014;20(8):767–775. doi: 10.1093/molehr/gau034. [DOI] [PubMed] [Google Scholar]
- 204.Kohrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer. 2009;9:188. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276(13):10134–10144. doi: 10.1074/jbc.M001599200. [DOI] [PubMed] [Google Scholar]
- 206.Saarialho-Kere U, Kerkela E, Jahkola T, Suomela S, Keski-Oja J, Lohi J. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J Invest Dermatol. 2002;119(1):14–21. doi: 10.1046/j.1523-1747.2002.01790.x. [DOI] [PubMed] [Google Scholar]
- 207.Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265(1–2):87–93. doi: 10.1016/s0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- 208.Padmavati P, Savita S, Shivaprasad BM, Kripal K, Rithesh K. mRNA expression of MMP-28 (Epilysin) in gingival tissues of chronic and aggressive periodontitis patients: a reverse transcriptase PCR study. Dis Markers. 2013;35(2):113–118. doi: 10.1155/2013/653982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li QL, Illman SA, Wang HM, Liu DL, Lohi J, Zhu C. Matrix metalloproteinase-28 transcript and protein are expressed in rhesus monkey placenta during early pregnancy. Molecular human reproduction. 2003;9(4):205–211. doi: 10.1093/molehr/gag028. [DOI] [PubMed] [Google Scholar]
- 210.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circulation research. 2013;112(4):675–688. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bister VO, Salmela MT, Karjalainen-Lindsberg ML, Uria J, Lohi J, Puolakkainen P, Lopez-Otin C, Saarialho-Kere U. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal and inflamed intestine and colon cancer. Dig Dis Sci. 2004;49(4):653–661. doi: 10.1023/b:ddas.0000026314.12474.17. [DOI] [PubMed] [Google Scholar]
- 212.Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50(1):131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 213.Momohara S, Okamoto H, Komiya K, Ikari K, Takeuchi M, Tomatsu T, Kamatani N. Matrix metalloproteinase 28/epilysin expression in cartilage from patients with rheumatoid arthritis and osteoarthritis: comment on the article by Kevorkian et al. Arthritis Rheum. 2004;50(12):4074–4075. doi: 10.1002/art.20799. author reply 4075. [DOI] [PubMed] [Google Scholar]
- 214.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115(Pt 19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 215.Batra J, Robinson J, Soares AS, Fields AP, Radisky DC, Radisky ES. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: binding studies and crystal structure. J Biol Chem. 2012;287(19):15935–15946. doi: 10.1074/jbc.M112.341156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Meng Q, Malinovskii V, Huang W, Hu Y, Chung L, Nagase H, Bode W, Maskos K, Brew K. Residue 2 of TIMP-1 is a major determinant of affinity and specificity for matrix metalloproteinases but effects of substitutions do not correlate with those of the corresponding P1′ residue of substrate. J Biol Chem. 1999;274(15):10184–10189. doi: 10.1074/jbc.274.15.10184. [DOI] [PubMed] [Google Scholar]
- 217.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]