Abstract
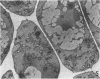
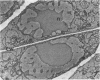
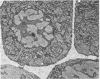
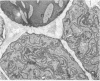
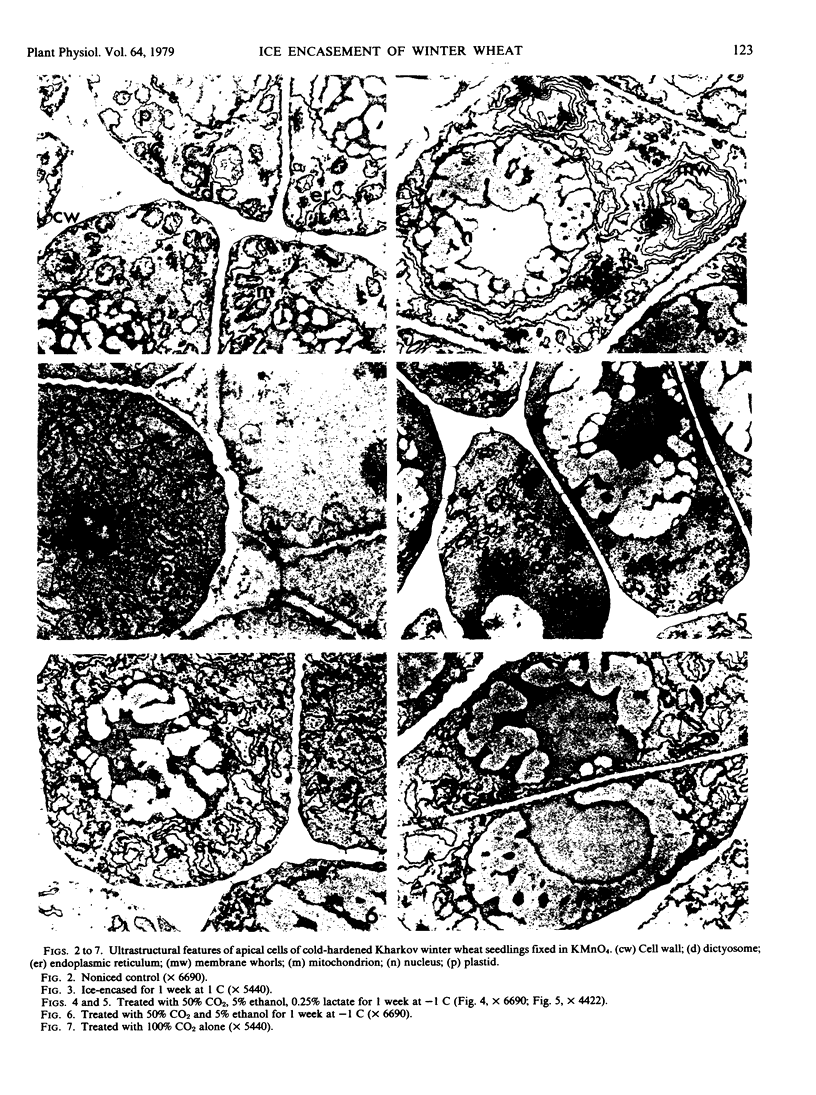
Ice encasement damages cold-hardened winter wheat without major disruption of cellular organelles. CO2 accumulates during total ice encasement to higher levels in Kharkov than in less hardy Fredrick wheat. Partial ice encasement and exposure to a nitrogen atmosphere at -1 C allows greater CO2 accumulation but neither treatment is as damaging as total ice encasement. Lactic acid accumulates to low levels only during the 1st day of encasement and thereafter remains constant. Exposure of plants to a combination of 50% CO2 and 5% ethanol reduces survival, with a cultivar difference similar to that found in ice-encased plants. Plants in CO2 and ethanol show a proliferation of membranes and nuclear condensation similar to that in cells of ice-encased plants. Permeability increases markedly in the presence of CO2 and ethanol, to levels similar to or greater than those of iced plants. Ethanol alone does not increase permeability but in combination with CO2 raises permeability of the less hardy Fredrick, although not of Kharkov, but reduces survival of both cultivars. A comparison of the endogenous levels of ethanol, CO2, and lactic acid at the 50% kill point of plants due to ice encasement or due to externally supplied metabolite indicates that only CO2 accumulates to independently toxic levels. Permeability and ultrastructural evidence suggest that CO2 and ethanol in combination are the agents reducing plant viability during ice encasement.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. J., Pomeroy M. K. Mitochondrial Activity and Ethanol Accumulation in Ice-encased Winter Cereal Seedlings. Plant Physiol. 1977 Jun;59(6):1174–1177. doi: 10.1104/pp.59.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. W. Carbon dioxide and senescence in cotton plants. Plant Physiol. 1975 Mar;55(3):515–519. doi: 10.1104/pp.55.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z. Induced changes in permeability of plant cell membranes to water. Plant Physiol. 1972 Apr;49(4):602–606. doi: 10.1104/pp.49.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z., Reinhold L. Rapid Changes in Permeability of Cell Membranes to Water Brought About by Carbon Dioxide & Oxygen. Plant Physiol. 1962 Jul;37(4):481–486. doi: 10.1104/pp.37.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The interrelationship of membrane and protein structure in the functioning of the (Na + = K + )-activated ATPase. Biochim Biophys Acta. 1972 Jun 20;266(3):613–624. doi: 10.1016/0006-3002(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosawa K. Studies on the effects of alcohols on membrane water permeability of Nitella. Protoplasma. 1975;86(1-3):243–252. doi: 10.1007/BF01275634. [DOI] [PubMed] [Google Scholar]
- Pomeroy M. K., Andrews C. J. Metabolic and Ultrastructural Changes in Winter Wheat during Ice Encasement Under Field Conditions. Plant Physiol. 1978 May;61(5):806–811. doi: 10.1104/pp.61.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. S., Hossack J. A., Rose A. H. Plasma-membrane lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Arch Microbiol. 1978 Jun 26;117(3):239–245. doi: 10.1007/BF00738541. [DOI] [PubMed] [Google Scholar]