Abstract
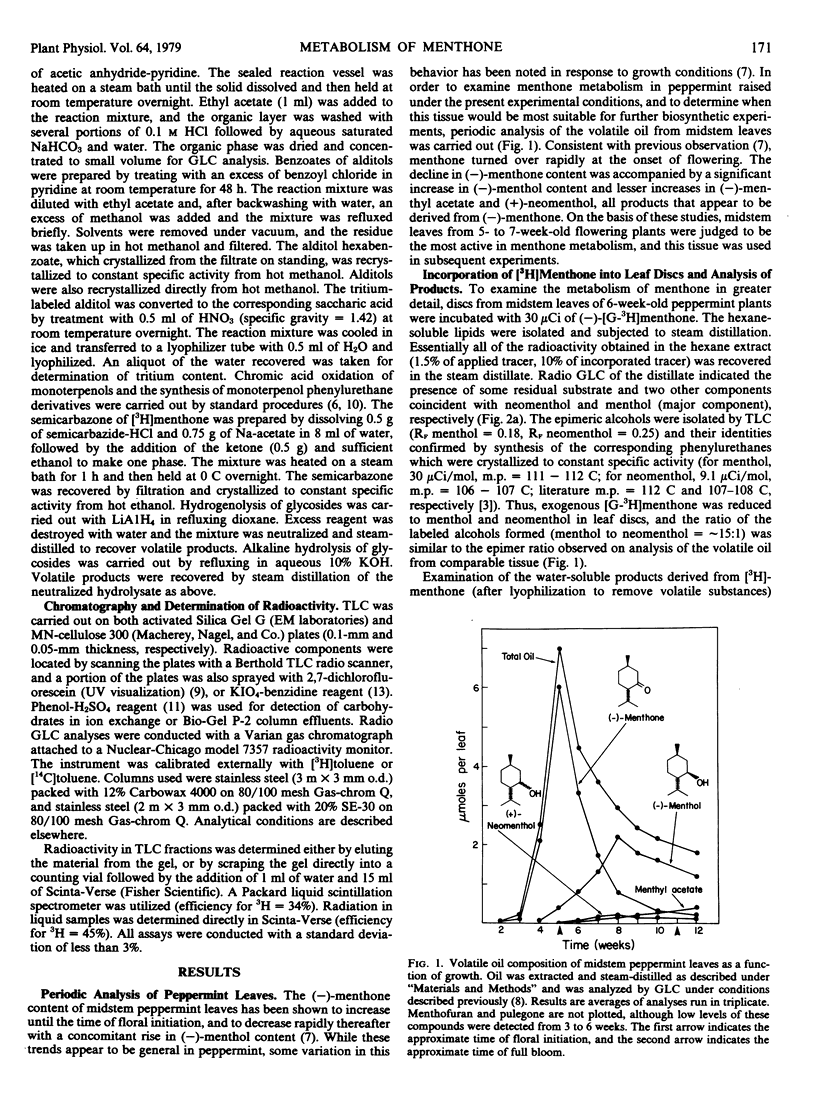
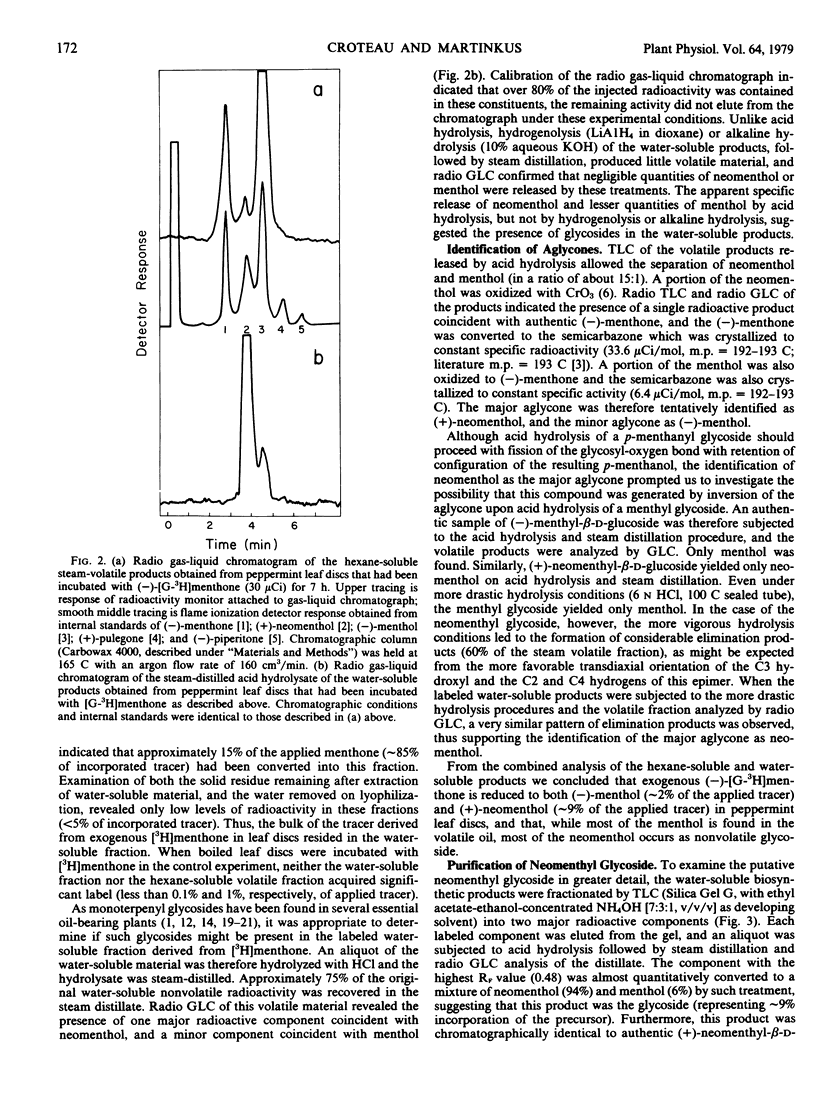
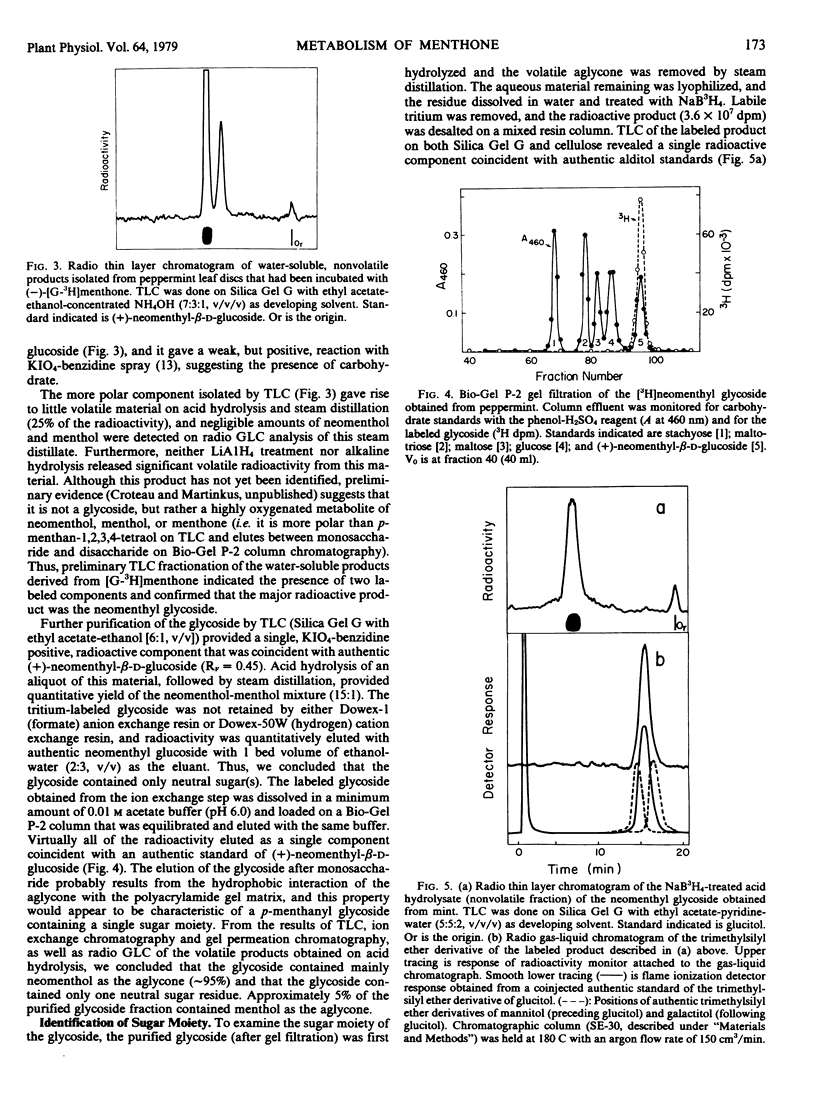
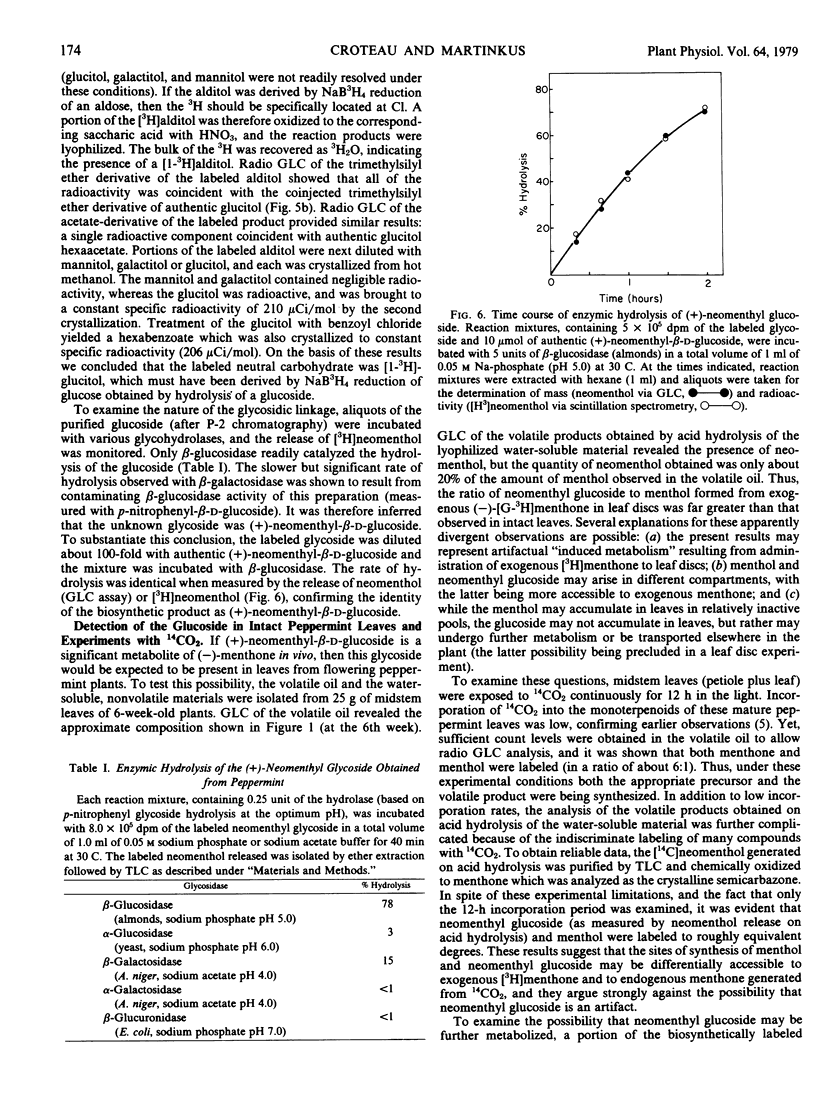
(−)-Menthone, the major monoterpene component of the essential oil of maturing peppermint (Mentha piperita L.) leaves (6 micromoles per leaf) is rapidly metabolized at the onset of flowering with a concomitant rise in the level of (−)-menthol (to about 2 micromoles per leaf). Exogenous (−)-[G-3H]menthone is converted into (−)-[3H]menthol as the major steam-volatile product in leaf discs in flowering peppermint (10% of incorporated tracer); however, the major portion of the incorporated tracer (86%) resided in the nonvolatile metabolites of (−)-[G-3H]menthone. Acid hydrolysis of the nonvolatile material released over half of the radioactivity to the steamvolatile fraction, and the major component of this fraction was identified as (+)-neomenthol by radiochromatographic analysis and by synthesis of crystalline derivatives, thus suggesting the presence of a neomenthyl glycoside. Thin layer chromatography, ion exchange chromatography, and gel permeation chromatography on Bio-Gel P-2 allowed the purification of the putative neomenthyl glycoside, and these results suggested that the glycoside contained a single, neutral sugar residue. Hydrolysis of the purified glycoside, followed by reduction of the resulting sugar moiety with NaB3H4, generated a single labeled product that was subsequently identified as glucitol by radio gas-liquid chromatography of both the hexatrimethylsilyl ether and hexaacetate derivative, and by crystallization to constant specific radioactivity of both the alditol and the corresponding hexabenzoate. These results, along with studies on the hydrolysis of the glycoside by specific glycosidases, strongly suggest that (+)-neomenthyl-β-d-glucoside is a major metabolite of (−)-menthone in flowering peppermint. This is the first report on the occurrence of a neomenthyl glycoside, and the first evidence implicating glycosylation as an early step in monoterpene catabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTAILE J., LOOMIS W. D. Biosynthesis of terpenes. II. The site and sequence of terpene formation in peppermint. Biochim Biophys Acta. 1961 Aug 19;51:545–552. doi: 10.1016/0006-3002(61)90612-6. [DOI] [PubMed] [Google Scholar]
- Burbott A. J., Loomis W. D. Evidence for metabolic turnover of monoterpenes in peppermint. Plant Physiol. 1969 Feb;44(2):173–179. doi: 10.1104/pp.44.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Hooper C. L. Metabolism of Monoterpenes: Acetylation of (-)-Menthol by a Soluble Enzyme Preparation from Peppermint (Mentha piperita) Leaves. Plant Physiol. 1978 May;61(5):737–742. doi: 10.1104/pp.61.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Karp F. Enzymatic synthesis of camphor from neryl pyrophosphate by a soluble preparation from sage (Salvia officinalis). Biochem Biophys Res Commun. 1976 Sep 20;72(2):440–447. doi: 10.1016/s0006-291x(76)80062-9. [DOI] [PubMed] [Google Scholar]
- Lang E., Hörster H. An Zucker gebundene reguläre Monoterpene Teil II Untersuchungen zur Olbidung und Akkumulation ätherischer Ole in Ocimum basilicum Zellkulturen. Planta Med. 1977 Feb;31(2):112–118. doi: 10.1055/s-0028-1097501. [DOI] [PubMed] [Google Scholar]
- REITSEMA R. H., CRAMER F. J., SCULLY N. J., CHORNEY W. Essential oil synthesis in mint. J Pharm Sci. 1961 Jan;50:18–21. doi: 10.1002/jps.2600500104. [DOI] [PubMed] [Google Scholar]
- Skopp K., Hörster H. An Zucker gebundene reguläre Monoterpene, teil I Thymol-und carvacrolglykoside in thymus vulgaris. Planta Med. 1976 May;29(3):208–215. doi: 10.1055/s-0028-1097653. [DOI] [PubMed] [Google Scholar]


