Abstract
Geographically diverse samples from strawberry exhibiting symptoms of Strawberry Green Petal (SbGP), periwinkle plants with virescence, and blackberry, blueberry, and raspberry plants displaying yellowing and inedible fruits, were assayed for the presence of phytoplasma DNA. PCR targeting the 16S rRNA-encoding gene and chaperonin-60 (cpn60) showed that the plants were infected with phytoplasma subgroup16SrXIII-(A/I)I (SbGP/MPV). To examine the geographic distribution of this pathogen in Mexico, we designed an array of cpn60-targeted molecular diagnostic assays for SbGP/MPV phytoplasma. A fluorescent microsphere hybridization assay was designed that was capable of detecting SbGP/MPV phytoplasma in infected plant tissues, successfully differentiating it from other known phytoplasma cpn60 UT sequences, while identifying a double infection with SbGP/MPV and aster yellows (16SrI) phytoplasma. Two quantitative assays, quantitative real-time PCR (qRT-PCR) and droplet digital PCR (ddPCR), gave similar results in infected samples. Finally, a loop-mediated isothermal amplification (LAMP) assay provided rapid detection of SbGP/MPV phytoplasma DNA. Application of these assays revealed that SbGP/MPV phytoplasma is widely distributed in Central Mexico, with positive samples identified from eleven localities within three states separated by hundreds of kilometres. These results also provide tools for determining the presence and geographic distribution of this pathogen in plant and insect samples in other localities.
Introduction
Phytoplasmas (‘Candidatus Phytoplasma’ spp.) are wall-less bacteria that were first described as mycoplasma-like organisms1 with a small, A-T rich and distinctive genome, that are taxonomically classified as Mollicutes2, 3. Phytoplasmas are insect-vectored plant pathogens that infect a very wide variety of plants, including most crop species, causing developmental alterations leading to leaf-like floral structures and aborted seed production4–6. Detection of phytoplasma infection in plant and insect tissues, along with proper classification and identification, is therefore critical for disease surveillance and control2. However, phytoplasmas are unculturable microorganisms and the detection and classification criteria are based on the use of molecular approaches7. Phytoplasma detection has relied on PCR amplification of targets within and surrounding the 16 S rRNA-encoding gene8. Identification and classification has typically used restriction fragment length polymorphism (RFLP) analysis of this locus9, resulting in the identification of over thirty 16Sr groups described as16SrI – 16SrXXXIII10. However, other genes have been used as additional markers, including the groEL or cpn60 gene11, 12. The cpn60 universal target (cpn60 UT), a sequence of approximately 550 bp, is located within the Cpn60-encoding gene13. This sequence has been identified as a molecular barcode for the domain Bacteria14 and is used as a taxonomic marker to characterize microbial communities15, 16. Furthermore, cpn60 UT has been shown to be a suitable target for the development of highly discriminatory molecular diagnostic assays for various organisms, including phytoplasma12, 17. The cpn60 UT has also been identified as a marker to identify and classify phytoplasmas based on the in silico RFLP analysis of these sequences18.
Strawberry green petal (SbGP) disease affects strawberry plants (Fragaria x ananassa) and is associated with phytoplasmas19. This disease, which was first detected in 1959 in Central Europe20 has since been reported in Canada21, the Czech Republic19 and Italy22, and is typically associated with phytoplasmas of the Aster Yellows (‘Ca. Phytoplasma asteris’, 16SrI) group. The hallmarks of SbGP include the flower petals changing from white to green in color, along with fruits showing green structures that give the appearance of a large green flower. Another characteristic symptom of the disease is the presence of red leaves and the formation of leaves in the fruit, which renders the fruit inedible and not viable for commercial sale. In Australia and the USA, diseases associated with phytoplasma affecting strawberry plants are associated mainly with members of the Aster Yellows group23, 24. In Latin America, SbGP disease was first reported in Argentina and was found to be caused by phytoplasmas of the 16SrVII group25. Phytoplasmas from group 16SrXIII have also been associated with strawberry diseases, specifically strains included in subgroups 16SrXIII-B and 16SrXIII-F24, 26. The group 16SrXIII or Mexican periwinkle virescence was first identified in Catharanthus roseus from Mexico27 and represents a new ‘Ca. Phytoplasma’ species, ‘Ca. Phytoplasma hispanicum’28. Other plants, such as potato, have also been identified as hosts, but members of this phytoplasma group have not been described outside of the Americas29.
We previously determined that Strawberry green petal (SbGP) disease affecting strawberry plants and Mexican periwinkle virescence disease (MPV) affecting periwinkle plants (Catharanthus roseus) in Mexico is associated with the heterogeneous 16SrXIII-(A/I)I phytoplasma30. In this work, we compared the performance of the previously reported PCR targeting phytoplasma cpn60 12 to nested PCR targeting the F2nR2 region31, an accepted standard for the detection of phytoplasma infections. To determine the presence and geographic distribution of this pathogen in Mexican production fields, we developed and applied cpn60 UT-targeted molecular diagnostic assays to 86 samples of symptomatic strawberry, raspberry, blueberry, blackberry, and periwinkle plants sampled in 11 localities within the states of San Luis Potosi, Jalisco, and Michoacan, Mexico. The results reveal the minimum extent of the geographic distribution of this pathogen in Mexico and provide a set of tools for determining the prevalence and distribution of the pathogen in other geographic locations.
Results
Plants affected by 16SrXIII-(A/I)I phytoplasma are found in three Mexican states
In a previous study the presence of the SbGP/MPV phytoplasma [16SrXIII-(A/I)I] was demonstrated in samples of periwinkle from San Luis Potosi and of strawberry from Michoacan, Mexico30. This phytoplasma contains two non-identical copies of the 16S rRNA-encoding locus30. To confirm that the samples from all geographic areas in the present study represented the same strain, F2nR2 sequences were analyzed in samples S07-P-JC and S10-L-JC, which were randomly selected from the samples collected in the state Jalisco. The sequencing showed that clones representing both subgroups 16SrXIII-A and 16SrXIII-I30 were found in each sample (Supplementary Fig. S1). Furthermore, the cpn60 UT sequences determined from 11 strawberry (KY061173 to KY061183), 1 blueberry (KY061168), 4 raspberry (KY061169 to KY061172), and 2 blackberry (KY061184, KY061185) samples were identical to the SbGP/MPV phytoplasma cpn60 UT reported previously30 (e.g. GenBank accession no. KU896201). These results indicate that the phytoplasma affecting the samples was the 16SrXIII-(A/I)I phytoplasma previously identified. The F2nR2 sequences obtained for both samples were deposited to GenBank under the accession numbers: KY061162 and KY061163 for S07-P-JC-clone1, S07-P-JC-clone5, respectively, and KY061164 and KY061165 for S10-L-JC-clone3 and S10-L-JC-clone6, respectively.
Performance of phytoplasma-targeted conventional PCR
DNA extracts were analyzed using pan-phytoplasma PCR with primers targeting the 16S rRNA-encoding locus [P1/Tint8, F2nR231 (direct) PCR, or P1/P732, 33 followed by F2nR2 (nested) PCR], as well as cpn60-targeted universal phytoplasma primers12. Many of the samples, particularly the blueberry, blackberry, and raspberry, were difficult to amplify, requiring dilution optimization in order to generate a PCR product (Fig. 1). A dilution of 1:50 was chosen for all templates analyzed by conventional PCR. Most of the strawberry samples from 2014 were negative using the 16S-targeted P1/Tint assay, although other tissues from these plants were positive by the F2nR2 direct and cpn60-targeted assays (Table 1). Samples from 2015 (all plants) were therefore assayed using F2nR2 (direct and nested) and cpn60-targeted assays.
Figure 1.
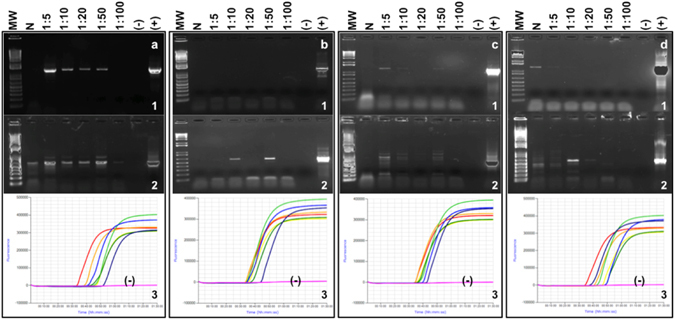
F2nR2-based PCR (1) and cpn60-based PCR (2) and LAMP amplification curves (3) applied to serial dilutions of infected berry plant DNA analyzed in this study. (a) Sample S05-L-MB (strawberry); (b) Bl01-L-JA (blueberry); (c) R05-L-MC (raspberry); (d) Bk02-L-JD (blackberry). No positive controls were included in the LAMP assay (3), and negative (−) in all cases refers to the “no template” control.
Table 1.
Name, origin and phytoplasma status of the tissue samples analyzed in this study.
| Symptomatic Samplesa | Tissue | Sampled inb | Phytoplasma status | ||
|---|---|---|---|---|---|
| PCRc | |||||
| F2nR2d | cpn60 UT | LAMPe | |||
| Strawberry | |||||
| 2014 | |||||
| S26b-GP-MA | Green petal | M-A | + | + | + |
| S26b-P-MA | Fruit peduncle | M-A | NTf,g | NT | + |
| S26b-F-MA | Fruit pulp | M-A | NTg | NT | + |
| S27b-GP-MA | Green petal | M-A | + | + | + |
| S31b-L-MA | Midrib of green leaves with red margin | M-A | + | + | + |
| S31b-GP-MA | Green petal | M-A | NTg | + | + |
| S31b-P-MA | Fruit peduncle | M-A | NTg | NT | + |
| S31b-F-MA | Fruit pulp | M-A | NTg | NT | + |
| S267-GP-MA | Green petal | M-A | NTg | − | − |
| S267-P-MA | Fruit peduncle | M-A | NTg | NT | − |
| S267-F-MA | Fruit pulp | M-A | NTg | NT | − |
| S289-L-MA | Midrib of green leaves with red margin | M-A | + | + | + |
| S289-GP-MA | Green petal | M-A | NTg | NT | − |
| S289-P-MA | Fruit peduncle | M-A | NTg | + | + |
| S289-F-MA | Fruit pulp | M-A | NTg | NT | − |
| 2015 | |||||
| S01-L-MA | Midrib of green leaves with red margin | J-A | − | − | − |
| S01-P-MA | Fruit peduncle | J-A | + | + | + |
| S02-P-JA | Fruit peduncle | J-A | − | − | − |
| S03-P-JB | Fruit peduncle | J-B | − | − | − |
| S04-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S05-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S05-P-MB | Fruit peduncle | M-B | + | + | + |
| S06-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S06-P-MB | Fruit peduncle | M-B | + | − | + |
| S07-P-JC | Fruit peduncle | J-C | + | + | + |
| S08-L-JA | Midrib of green leaves with red margin | J-A | − | − | − |
| S09-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S09-P-MB | Fruit peduncle | M-B | + | + | + |
| S10-L-JC | Midrib of green leaves with red margin | J-C | + | + | + |
| S10-P-JC | Fruit peduncle | J-C | + | + | + |
| S11-L-JC | Midrib of green leaves with red margin | J-C | + | + | + |
| S12-P-JC | Fruit peduncle | J-C | + | + | + |
| S13-L-JC | Midrib of green leaves with red margin | J-C | + | + | + |
| S14-P-JA | Fruit peduncle | J-A | − | − | − |
| S15-L-JA | Midrib of green leaves with red margin | J-A | − | − | + |
| S16-P-JA | Fruit peduncle | J-A | − | − | − |
| S17-P-JA | Fruit peduncle | J-A | − | − | − |
| S18-P-JA | Fruit peduncle | J-A | − | − | − |
| S19-L-JD | Midrib of green leaves with red margin | J-D | + | + | + |
| S19-P-JD | Fruit peduncle | J-D | + | + | + |
| S20-L-JD | Midrib of green leaves with red margin | J-D | − | − | − |
| S21-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S21-P-MB | Fruit peduncle | M-B | + | + | + |
| S22-L-MB | Midrib of green leaves with red margin | M-B | + | − | + |
| S22-P-MB | Fruit peduncle | M-B | + | + | + |
| S23-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S24-P-JB | Fruit peduncle | J-B | − | − | − |
| S25-L-JC | Midrib of green leaves with red margin | J-C | + | + | + |
| S25-P-JC | Fruit peduncle | J-C | + | + | + |
| S26-L-JA | Midrib of green leaves with red margin | J-A | + | + | + |
| S26-P-JA | Fruit peduncle | J-A | − | − | + |
| S27-P-JA | Fruit peduncle | J-A | − | − | − |
| S28-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S29-L-MB | Midrib of green leaves with red margin | M-B | − | − | − |
| S30-P-MB | Fruit peduncle | M-B | − | − | − |
| S31-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S32-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S33-L-MB | Midrib of green leaves with red margin | M-B | + | + | + |
| S34-L-MC | Midrib of green leaves with red margin | M-C | − | − | − |
| S35-L-MD | Midrib of green leaves with red margin | M-D | − | − | + |
| S36-L-ME | Midrib of green leaves with red margin | M-E | − | − | + |
| S37-P-MD | Fruit peduncle | M-D | − | − | − |
| S38-L-MF | Midrib of green leaves with red margin | M-F | − | − | + |
| S39-L-MG | Midrib of green leaves with red margin | M-G | − | − | − |
| S40-L-MH | Midrib of green leaves with red margin | M-H | − | − | − |
| S41-L-JB | Midrib of green leaves with red margin | J-B | + | + | + |
| S41-P-JB | Fruit peduncle | J-B | + | + | + |
| S42-L-JB | Midrib of green leaves with red margin and Fruit peduncle | J-B | + | + | + |
| S43-L-JB | Midrib of green leaves with red margin | J-B | + | + | + |
| Periwinkle | |||||
| 2014 | |||||
| P83-L-SLP | Midrib of green leaves | SLP-A | + | + | + |
| P86-L-SLP | Midrib of green leaves | SLP-A | + | + | + |
| Raspberry | |||||
| 2015 | |||||
| R01-L-MA | Green leaves | M-A | + | + | + |
| R01-P-MA | Fruit peduncle | M-A | + | + | + |
| R02-L-MA | Green leaves | M-A | − | + | + |
| R03-L-MA | Green leaves | M-A | − | + | + |
| R04-L-MB | Green leaves | M-B | − | − | + |
| R05-L-MC | Green leaves | M-C | + | + | + |
| R06-L-MC | Green leaves | M-C | − | + | + |
| R07-L-MC | Green leaves | M-C | − | − | + |
| R08-L-MC | Green leaves | M-C | + | + | + |
| Blueberry | |||||
| 2015 | |||||
| Bl01-L-JA | Yellow leaves | J-A | + | + | + |
| Bl02-L-JB | Leaves showing red margin | J-B | − | − | + |
| Blackberry | |||||
| 2015 | |||||
| Bk01-L-JC | Green leaves | J-C | − | + | + |
| Bk02-L-JD | Green leaves | J-D | + | + | + |
| Bk03-L-MD | Green leaves | M-D | − | + | + |
| Bk04-L-ME | Green leaves dry | M-E | − | − | − |
aSymptoms observed in strawberry and periwinkle plants were previously described30. Blackberry and raspberry plants showed small leaves and green structures in the fruits; blueberry plants showed yellow leaves and leaves with red margins (Figure S1). bMichoacan samples: 20.002°N, 102.3089°W; San Luis Potosi samples: 22.2°N, 100.1°W; Jalisco samples: 20.34°N 103.41°W. A total of 11 commercial farms located among the three states mentioned above were visited to collect the symptomatic samples. cPCR using primers R16F2n/R16R231 to amplify the F2nR2 sequence and H279p/H280p12 to amplify the cpn60 UT sequence. dNested PCR was performed on the samples collected in 2015, with P1/P7 primers in the first reaction32, 33, and R16F2n/R16R2 in the nested reaction31. Samples collected in 2014 were analyzed with direct PCR targeting the F2nR2 fragment. eLAMP using primers and conditions described in Table 1. fNT, not tested. gSample was negative using 16S-targeted primers P1/Tint8.
We compared the performances of the 16S-targeted F2nR2-nested and the direct cpn60-targeted conventional PCR assays. A total of 69 samples including 54 from strawberry, nine from raspberry, two from blueberry and four from blackberry were included in the analysis. 38 samples were positive by F2nR2-nested PCR and 31 were negative. Of the positives, the ~605 bp cpn60 UT amplicon was generated from 36 samples. The sensitivity of the cpn60-targeted phytoplasma PCR was high (95%) when compared to the F2nR2-nested PCR (Table 2), which is consistent with a low false negative rate. The specificity of the cpn60 UT-targeted conventional PCR assay was 87%.
Table 2.
Comparison of the performances of the direct cpn60 UT-targeted and nested PCR assay targeting F2nR2.
| cpn60 UT PCR results | F2nR2 nested PCR results | Total | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 36 | 4 | 40 | |
| Negative | 2 | 27 | 29 | |
| Total | 38 | 31 | 69 | |
| 95%CI | Low | High | ||
| Test sensitivity | 0.947 | 0.071 | 0.876 | 1.018 |
| Test specificity | 0.871 | 0.118 | 0.753 | 0.989 |
Discordant samples were analyzed for the presence of SbGP/MPV phytoplasma DNA. Of the eleven samples that were negative by F2nR2 direct or nested PCR but positive with the direct cpn60-targeted conventional PCR assay, all were positive with the cpn60 UT-targeted LAMP and quantitative PCR assays (Supplementary Table S1). Furthermore, cpn60 UT sequences were successfully determined for five of these samples, which all showed 100% identity to the SbGP/MPV phytoplasma sequence. These results demonstrated that the cpn60-targeted direct PCR performed well compared to the F2nR2-targeted PCR in either format (nested and direct), and that the F2nR2 nested PCR generated more positives compared to the direct PCR (Table 1).
Two samples were identified as false negatives using the cpn60 UT-PCR assay (Supplementary Table S1). These samples were positive by F2nR2 PCR (direct and nested), and were also positive using the cpn60 UT-targeted LAMP and qPCR assays (Supplementary Table S1). Also, one of these samples (S22-L-MB) was positive with the fluorescent microsphere hybridization assay (Supplementary Table S1), which uses essentially the same amplification primers.
Expanded oligonucleotide-coupled fluorescent microsphere hybridization assay for phytoplasmas
The probe for SbGP/MPV phytoplasma (Supplementary Table S2) detected only the target sequence among all of the phytoplasma cpn60 UT sequences that have been reported by our group (Fig. 2). Strong fluorescence signals were observed in the samples consisting of either the cloned SbGP/MPV cpn60 UT plasmid DNA or genomic DNA samples derived from symptomatic strawberry and periwinkle plants (Fig. 2). The average of the MFI readings for all of the other 11 probes using the SbGP cpn60 UT plasmid or genomic templates was not significantly greater than that observed with no template; similarly, no significantly positive MFI signal was observed when the SbGP/MPV probe was used on all of the other templates. These results confirm both that the SbGP/MPV probe reacted only with the desired target DNA (among those tested), and that no other template DNA (among those tested) generated a signal with the SbGP/MPV probe. Applying the fluorescent microsphere hybridization assay to the strawberry samples from 2015 revealed a good correspondence with the results of the cpn60 UT-targeted conventional PCR (Supplementary Table S3). In all cases but one, only the SbGP/MPV probe generated a significantly positive MFI result, demonstrating the capacity of this assay to detect and simultaneously type phytoplasma infections.
Figure 2.
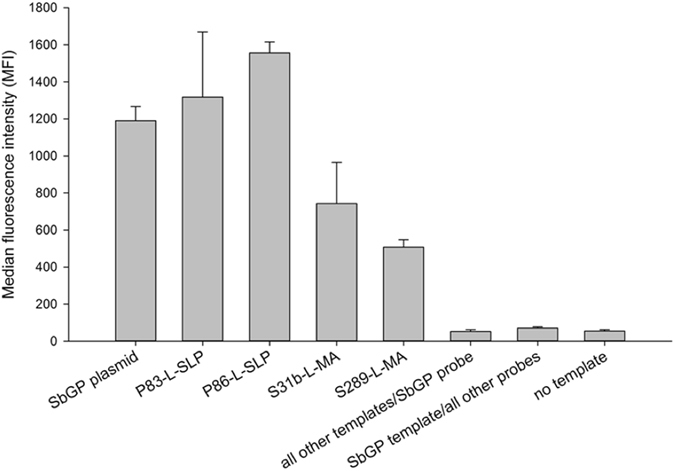
Representative median fluorescence intensities observed using the fluorescent microsphere hybridization assay on cpn60 UT amplicon generated from strawberry and periwinkle DNA templates. “All other templates” are cpn60 UT amplicons generated from AY-Ruta (16SrI-A, ‘Ca. P. asteris’- related strain), SF1 (16SrI-B, ‘Ca. P. asteris’-related strain), CVB, AY-Col (16SrI-C, ‘Ca. P. asteris’-related strain), RS (16SrV-A, ‘Ca. P. ulmi’- related strain), AshY (16SrVII-A, ‘Ca. P. fraxini’-related strain), Cr (16SrIX-H, ‘Ca. P. phoenicium’-related strain), AP (16SrX-A, ‘Ca. P. mali’-related strain), PYLR (16SrX-C, ‘Ca. P. pyri’-related strain), ESFY (16SrX-F, ‘Ca. P. prunorum’-related strain), and BN44948 (16SrXII-A, ‘Ca. P. solani’-related strain). “All other probes” are the probes that specifically target these strains, as previously described12.
Samples S41-L-JB and S41-P-JB, both from the same strawberry plant, showed no significant signal for SbGP/MPV probe, but a significantly positive MFI signal was observed for the AY-SF1 probe12 (16SrI) (Supplementary Table S3). These samples were positive for SbGP/MPV phytoplasma by LAMP and qPCR targeting cpn60 UT (Supplementary Table S3). To determine if this sample contained DNA from both AY and SbGP/MPV phytoplasmas, sequences from individual F2nR2 clones generated from sample S41-L-JB were examined. Phylogenetic analysis of these sequences revealed that one clone clustered with 16SrI-B phytoplasma (KY061166). This clone had >99% nucleotide sequence similarity with MBS phytoplasma (AY265208). Another clone was identified as deriving from SbGP/MPV phytoplasma [16SrXIII-I (KY061167; Supplementary Fig. S1)]. Furthermore, the cpn60 UT sequence obtained from this plant was identified as cpn60 UT I-IIIB (KY061183) subgroup phytoplasma18. A total of 8 clones were examined, all of which corresponded to the AY cpn60 UT I-IIIB subgroup (not shown).
Quantification of SbGP/MPV and AY phytoplasma in plant tissues using qRT-PCR
Dilution optimization for the qRT-PCR assay indicated that PCR inhibition was not a significant factor for detecting and accurately quantifying the target DNA in these samples, as all dilutions analyzed gave similar results (Supplementary Fig. S5). The assay that was implemented for qRT-PCR detection of SbGP/MPV was highly efficient using plasmid standards (Fig. 3a), with a mean PCR efficiency (E) of 1.991 ± 0.005 (n = 3), where 2.0 is theoretical34. In addition, the assay was highly linear (correlation of >0.999 between copies added and copies detected) and accurately determined the number of copies of SbGP/MPV cpn60 in spiked strawberry samples (mean accuracy of 1.42 over 10 samples spanning 5 orders of magnitude; Fig. 3b). The qRT-PCR assay consistently detected samples with as few as 10 copies of SbGP/MPV per assay (Fig. 3a,b). Examining the strawberry and periwinkle samples harvested in 2014, the qRT-PCR assay revealed that nearly all samples analyzed contained very high levels of SbGP/MPV phytoplasma DNA, and that the periwinkle samples contained higher levels than any of the strawberry samples (Tables 3 and S3). The qRT-PCR assay failed to generate a signal using healthy strawberry or periwinkle DNA, and none of the nontarget phytoplasma cpn60 UT plasmids were positive in this assay (Table 3). The strawberry samples from 2014 generally contained high levels of SbGP/MPV phytoplasma in all tissues, particularly in the leaves and fruit peduncle (Table 3). One of the samples, S289-MA, showed high levels of SbGP/MPV phytoplasma DNA in the leaf and fruit peduncle but undetectable levels in the green petal and fruit pulp, consistent with observations made by ddPCR and LAMP (Table 3). Another sample, S267-MA, showed very low levels of SbGP/MPV phytoplasma DNA in the green petal only using qRT-PCR (consistently positive over 4 observations), yet this sample was negative by ddPCR and by LAMP (Table 3).
Figure 3.
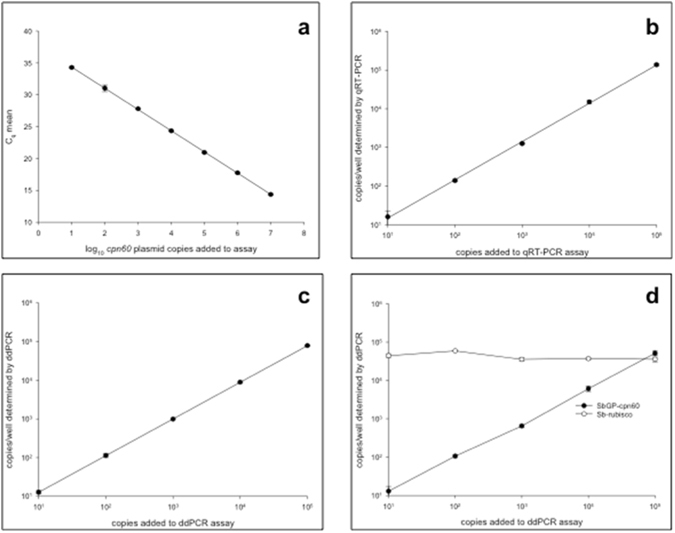
(a) qRT-PCR standard curve for cpn60-targeted SbGP detection assay. PCR efficiency (E) was determined to be >1.99 using E = 10(−1/slope) 32. Results shown are the means of duplicate determinations ± standard deviation. (b,c,d) Quantitative PCR assay (b), qRT-PCR; (c,d), ddPCR) accuracy, linearity, and detection limits determined by relating the number of copies of SbGP cpn60 UT plasmid DNA added to the reaction to the number of copies measured. ddPCR assays were performed in the absence (c); 1-plex) and presence (d); 2-plex) of DNA extracted from a healthy strawberry plant. qRT-PCR assays were performed in the presence of uninfected strawberry DNA. Results shown are the means of duplicate measurements ± standard deviation.
Table 3.
Molecular quantification of SbGP/MPV phytoplasma in infected plant samples obtained in 2014.
| Sample | Method Unit | qRT-PCR genomes/g tissue | ddPCR genomes/g tissue | LAMP Time to positive (Tp), minutes (calcein detection) | |||
|---|---|---|---|---|---|---|---|
| Tissue | Mean (n) | Standard deviation | Mean (n) | Standard deviation | Mean (n) | Standard deviation | |
| S31b-L-MA | Leaf midrib | 4.82 × 108 (10) | 5.48 × 107 | 2.41 × 108 (10) | 2.51 × 107 | 45.88 (2) | 3.01 |
| S31b-GP-MA | Green petal | 2.74 × 109 (2) | 7.58 × 107 | 4.80 × 108 (2) | 4.88 × 107 | 31.88 (2) | 3.00 |
| S31b-P-MA | Fruit peduncle | 4.34 × 108 (2) | 2.01 × 107 | 2.11 × 108 (2) | 2.61 × 107 | 35.0 (3) | 0.43 |
| S31b-F-MA | Fruit pulp | 9.69 × 107 (2) | 1.80 × 107 | 5.48 × 107 (2) | 4.24 × 106 | 45 (2) | 3.54 |
| S289-L-MA | Leaf midrib | 1.34 × 108 (10) | 1.78 × 107 | 7.94 × 107 (9) | 1.14 × 107 | 49.63 (2) | 3.01 |
| S289-GP-MA | Green petal | NDa (2) | ND (2) | ND (2) | |||
| S289-P-MA | Fruit peduncle | 4.24 × 108 (2) | 1.29 × 107 | 7.16 × 107 (2) | 3.61 × 106 | 35.38 (2) | 0.18 |
| S289-F-MA | Fruit pulp | ND (2) | ND (2) | ND (2) | |||
| S26b-GP-MA | Green petal | 1.12 × 109 (2) | 7.44 × 107 | 3.85 × 108 (2) | 7.64 × 106 | 35.38 (2) | 1.24 |
| S26b-P-MA | Fruit peduncle | 1.04 × 109 (2) | 2.90 × 108 | 4.23 × 108 (2) | 6.36 × 106 | 25.25 (2) | 0 |
| S26b-F-MA | Fruit pulp | 3.98 × 108 (2) | 1.22 × 107 | 1.46 × 108 (2) | 1.09 × 107 | 38.38 (2) | 0.18 |
| S27b-GP-MA | Green petal | 4.01 × 109 (2) | 4.07 × 108 | 6.92 × 108 (2) | 1.23 × 108 | 30.88 (2) | 1.59 |
| S267-GP-MA | Green petal | 8.21 × 102 (4) | 4.53 × 102 | ND (2) | ND (2) | ||
| S267-P-MA | Fruit peduncle | ND (2) | ND (2) | ND (2) | |||
| S267-F-MA | Fruit pulp | ND (2) | ND (2) | ND (2) | |||
| P83-L-SLP | Leaf midrib | 4.67 × 109 (10) | 7.72 × 108 | 2.53 × 109 (10) | 1.80 × 108 | 38.42 (3) | 2.31 |
| P86-L-SLP | Leaf midrib | 6.13 × 109 (10) | 8.46 × 108 | 2.82 × 109 (10) | 3.10 × 108 | 37.08 (3) | 2.13 |
| Strawberry (H)b | Leaf | ND (2) | ND (2) | ND (2) | |||
| Periwinkle (H) | Leaf | ND (2) | ND (2) | ND (2) | |||
| Raspberry (H) | Fruit | ND (2) | ND (2) | ND (2) | |||
| Blueberry (H) | Leaf | ND (2) | ND (2) | ND (2) | |||
| Blackberry (H) | Fruit | ND (2) | ND (2) | ND (2) | |||
| Nontargetc | ND (2) | ND (2) | ND (2) | ||||
aND, not detected. bH, DNA from uninfected plant. See Supplementary Fig. S4 for results. cNontarget DNA is a mixture of plasmids consisting of 106 copies each of all of the cpn60 UT fragments from the phytoplasma strains described in Dumonceaux et al.12. See Supplementary Fig. S4 for results on individual templates.
Generally, somewhat lower levels of SbGP/MPV phytoplasma DNA were found in the strawberry samples harvested in 2015, with some samples generating Cq values that were below that of the lowest standard (Supplementary Table S3). Despite showing strong symptoms (Supplementary Fig. S2), the samples obtained from infected raspberry, blueberry, and blackberry also showed relatively low levels of phytoplasma DNA (Supplementary Table S3).
SbGP/MPV phytoplasma DNA was detected in the leaf and peduncle of sample S41-JB (Supplementary Table S3). Since the oligonucleotide-coupled fluorescent microsphere hybridization assay as well as cloning suggested that this plant harbored a double infection with AY and SbGP/MPV phytoplasma, we used a previously described AY (Aster yellows)- specific qRT-PCR assay35 to examine these samples. We also analyzed samples Bl02-L-JB, S03-P-JB, S24-P-JB, S42-L-JB, and S43-L-JB, all of which were collected in the same locality (B) in Jalisco. The AY-qRT-PCR showed that all the samples were positive using both assays. However, only the samples derived from plant S41-JB were quantifiable, with approximately 150-fold higher copy number of AY-phytoplasma compared to SbGP/MPV phytoplasma (Supplementary Table S4).
Quantification of SbGP/MPV phytoplasma using ddPCR
The ddPCR-adapted version of the assay performed similarly to the qRT-PCR assay in serial dilutions of template, except at very low levels of target (Supplementary Fig. S5), suggesting that PCR inhibition was not a significant factor for the quantitative assays. Calibration of the ddPCR assay using known copy numbers of target cpn60 UT plasmids revealed that the assay was highly linear; the “calibration curve” showed a correlation of >0.999 between the number of copies added and the number of copies detected (Fig. 3b). Moreover, the assay was accurate both in the absence (Fig. 3c) and presence (Fig. 3d) of DNA from uninfected strawberry plants, with a mean accuracy of 0.83 across 10 samples spanning 5 orders of magnitude. The ddPCR assay was capable of detecting samples with as few as 10 copies of SbGP per reaction consistently (Fig. 3c,d). Applying the assay to the infected plant DNA extracts showed similar results to the qRT-PCR assay when expressed in terms of phytoplasma genome copies/g tissue extracted (Tables 3 and S3). Similar trends in copy numbers were observed in the samples by ddPCR and qRT-PCR, with P86-L-SLP showing the highest copy number (Tables 3 and S3). Analyzing the reproducibility of the qRT-PCR and ddPCR assays showed that the ddPCR assay had consistently lower measurement error than was observed by qRT-PCR for samples repeated up to 10 times (Table 3; samples S31b-L-MA, S289-L-MA, P83-L-SLP, P86-L-SLP). No detection of any non-target phytoplasma template DNA was observed, nor was any signal generated using uninfected strawberry or periwinkle DNA (Table 3 and Supplementary Fig. S4). ddPCR also revealed the presence of SbGP/MPV phytoplasma in samples S41-JB (L and P) with quantifiable values (Supplementary Table S3). Application of an internal control for the strawberry samples enabled the expression of ddPCR results in terms of fractional abundance (Supplementary Table S3), which corrects for any intersample variation in DNA yield and amplifiability.
Detection of SbGP/MPV phytoplasma using LAMP
Like the qRT-PCR and ddPCR assays, the LAMP assay was negative using DNA from uninfected host plant tissue as well as nontarget phytoplasma cpn60 UT plasmid DNA (Table 3, Fig. 4, and Supplmental Fig. S4). The LAMP assay afforded rapid detection of SbGP/MPV phytoplasma DNA, with positive results observed in as little as 25 minutes using a calcein-based detection system (Supplementary Table S3). To analyze the effect of PCR inhibitors on the detection of SbGP/MPV phytoplasma DNA by LAMP, serial dilutions of samples S05-L-MB, Bl01-L-JA, R05-L-MC, and Bk02-L-JD were examined. Nearly all dilutions, including those that were negative by conventional PCR targeting 16S and cpn60 UT, were positive by LAMP (Fig. 1). Other samples were also shown to amplify robustly across dilutions, like the quantitative PCR-based assays (Supplementary Fig. S5). The LAMP assay also showed an inverse relationship between input copy number and Tp that was linear over several orders of magnitude (Supplementary Fig. S3), with the isothermal detection chemistry affording much more rapid detection with lower measurement error. The LAMP assay in both formats could consistently detect as few as 100 copies of SbGP/MPV phytoplasma DNA, although the calcein-based detection system required slightly more than 60 minutes (the defined assay time) to detect this amount (Supplementary Fig. S3).
Figure 4.
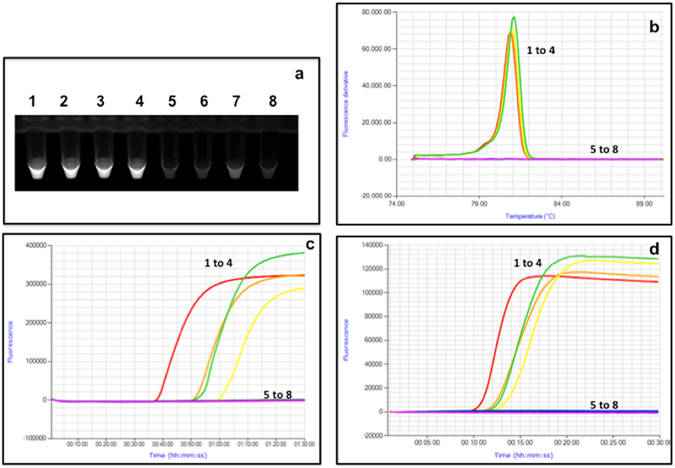
LAMP assay targeting the SbGP/MPVcpn60 gene applied to plant DNA extracts. 1, S31b-L-MA; 2, 289-L-MA; 3, P83-L-SLP; 4, P86-L-SLP; 5, healthy strawberry DNA; 6, healthy C. roseus DNA; 7, mixture of 106 copies each of all non-target plasmid templates (see Table 3); 8, no template control. (a) Reactions were viewed under ultraviolet light using a transilluminator after amplification using the Genie instrument. (b,d) Annealing and Amplification curves respectively, for the same samples using Isothermal detection chemistry. (c) Amplification curves for the same samples using calcein-based detection method.
Using calcein detection chemistry, the 2015 samples were analyzed (undiluted and 1:50 dilutions) with a binomial system by viewing the reactions under ultraviolet light at the end of the assay (e.g. Fig. 4a). The binomial system eliminated the quantitative aspect of the assay as samples with higher levels of SbGP/MPV phytoplasma DNA looked no different from samples with lower levels (Fig. 4a). Using this system, all samples that tested positive for phytoplasma by F2nR2 nested PCR were positive by LAMP, suggesting a perfect concordance on positive samples (Supplementary Table S3). Moreover, 10 symptomatic samples that were F2nR2 negative using conventional PCR were positive using the cpn60 UT-targeted LAMP assay.
The 2015 samples from berry plants were analyzed using the same LAMP primers, but with Isothermal detection chemistry. This facilitated the determination of the time to positive (Tp) for each sample along with a diagnostic annealing temperature that is characteristic of the specific LAMP product generated. The Tp of these samples were very low, with positive results observed in less than 10 minutes in many cases (Supplementary Table S3). The annealing temperature of the SbGP/MPV LAMP product was approximately 81 °C (Supplementary Table S3). The concordance between LAMP results detected using calcein and isothermal detection chemistry was generally very good, although 7 of the 69 samples were positive by calcein and negative by isothermal chemistry while one sample (S36-L-ME) was positive using isothermal chemistry but negative using calcein. All raspberry, blueberry, and blackberry samples analyzed showed perfect agreement between the two detection methods (Supplementary Table S3).
Geographic distribution of SbGP/MPV phytoplasma in central Mexico
Taken together, the provenance of all samples and the results of the quantitative molecular diagnostic assays showed that SbGP/MPV phytoplasma is widespread over an area covering three Mexican states, which range in size from ~60,000 (Michoacan and San Luis Potosi) −80,000 km2 (Jalisco) (Fig. 5). SbGP/MPV phytoplasma was detected in multiple locales in two of these states, with 11 localities represented in the three states. Examination of the quantitative data revealed that samples from some localities tended to show higher levels of SbGP/MPV phytoplasma than others; for example samples from locations A and B within Michoacan were notably higher than samples from other localities in this state (Fig. 5).
Figure 5.
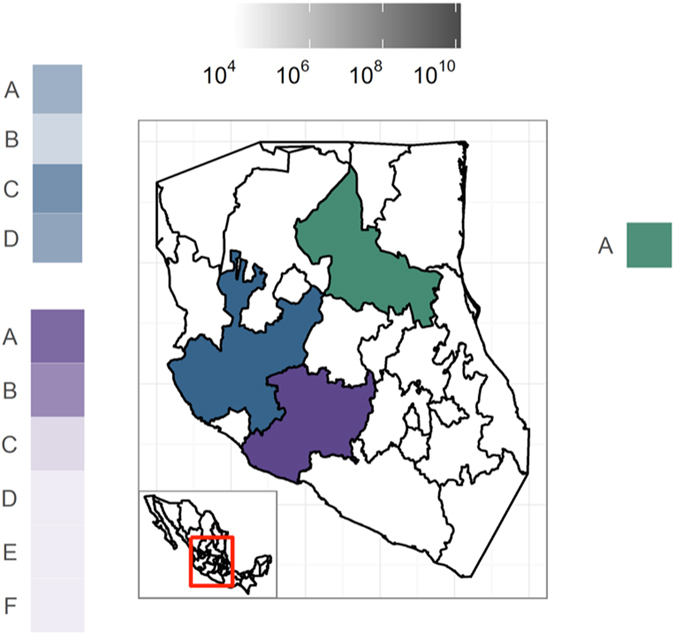
Geographic distribution of SbGP/MPV phytoplasma per farm in central Mexico. Samples from San Luis Potosi (green), Michoacan (blue), and Jalisco (purple) states were examined using qRT-PCR targeting cpn60. Samples from each locality within each state (A-F) are shaded according to the mean number of copies of SbGP/MPV phytoplasma cpn60 per g tissue extracted. To generate the map, data was accessed from the GADM database (Global Administrative Areas 2012). GADM database of Global Administrative Areas, version 2.0. [online] URL: www.gadm.org) and plotted in R (http://www.R-project.org/) using ggplot259.
Discussion
Symptomatic plants collected from different geographic areas in Mexico yielded variable results with the pan-phytoplasma PCR depending on the tissue sampled, with positive results generated from leaves and leaf midribs but negative results from floral parts and fruits (Table 3, Supplementary Table S3). It is well known that the high concentration of phenolic compounds in strawberry and other berry fruits36 can inhibit PCR37, which likely explains why DNA extracted from symptomatic plants using petals, fruit peduncle or fruit as starting material were initially PCR negative in many cases. Analysis using the more inhibitor-tolerant LAMP assay revealed that most of these samples were positive for SbGP/MPV phytoplasma DNA (Table 3, Supplementary Table S3). This suggests that leaves from symptomatic plants are a preferred starting material for initial screening using conventional PCR targeting all phytoplasmas.
A suite of molecular diagnostic assays that target the single-copy cpn60 gene of the SbGP/MPV phytoplasma was designed. Rapid, sequencing-independent detection and typing of phytoplasma infection was provided by the fluorescent microsphere hybridization assay. These results expand the previously reported 11-plex cpn60-based phytoplasma detection array12 to 12-plex, demonstrating the flexibility of this assay format. The assay was also found to be semi-quantitative (Fig. 2 and Table 3), although as an end-point PCR-based method MFI would not be expected to correlate to copy number over a wide dynamic range. The quantitative assays showed very high levels of SbGP/MPV DNA in some of the plant samples, with up to 109 phytoplasma genomes/g tissue in the leaf samples analyzed. Both of the quantitative PCR-based assays gave similar results, but the ddPCR assay had a lower measurement error and appeared to be highly accurate in its determination of the copy number of SbGP/MPV cpn60 genes in the sample. Combined with the fact the ddPCR appears to be more resistant to the effects of PCR inhibitors compared with qRT-PCR38, this indicates that ddPCR may generally be a preferred method for highly accurate quantification of SbGP/MPV phytoplasma genomes in plant DNA extracts. However, this must be countered by the fact that the ddPCR assay is longer, with more operator steps involved, and is more expensive than qRT-PCR, which gives essentially identical results. Moreover, ddPCR has a lower dynamic range than qRT-PCR, which means that strongly positive samples such as some of those sampled here require optimization of the dilution to get the sample into the quantitative range. The use of internal controls in ddPCR requires additional optimizations of the technique to find the balance between detectable phytoplasma and measurable internal control, although it provides the benefit of normalizing the detected pathogen DNA to a known quantity of host DNA and thereby corrects for variability in DNA yields among samples. The choice of quantitative assay therefore depends on instrument availability and operator preference.
Rapid and field-deployable detection of pathogen DNA in samples is a key advantage of the use of LAMP for phytoplasma detection39. LAMP assays targeting many different phytoplasma groups have been described40, 41, some of which target cpn60 17. However, no LAMP assays targeting SbGP/MPV phytoplasmas (16SrXIII) have been described to date. The SbGP/MPV-cpn60 UT-targeted LAMP assay we described was rapid, offering positive results in as little as 9 minutes (isothermal detection chemistry) on strongly positive samples. While isothermal detection chemistry was rapid, linear, and provided a diagnostic annealing temperature for the product, it is more expensive than other detection systems and requires a dedicated instrument to visualize the results. The instrument we used (Genie) is ideally suited for field-based applications, but it is expensive and not necessarily suited to resource-limited settings. However, the LAMP assay equally provides binomial results that are viewable under ultraviolet light when calcein is used as the detection reagent, or under visible light with other detection schemes such as hydroxy naphthol blue42. Like ddPCR, LAMP is typically seen as more resistant to the effects of PCR inhibitors compared to other, PCR-based detection methods43, and LAMP is also normally considered to have an analytical sensitivity that is equal to or better than PCR-based methods44. This fact likely explains why many PCR-negative samples were positive using the LAMP assay. The LAMP assay we have described is likely to detect SbGP/MPV phytoplasma DNA in most symptomatic plant tissues, and probably in positive insects as well since insects accumulate high levels of phytoplasma in the salivary glands6.
The molecular diagnostic assays we employed facilitated the detection of a double infection of some samples with AY and SbGP/MPV phytoplasma. Double infections are not unusual in plants affected by phytoplasmas. Aster yellows phytoplasma and flavescence dorée phytoplasma have been detected affecting the vector Euscelidius variegatus in Italy45, and also in grapevine plants46. In fact, this study is not the first to find phytoplasmas belonging to the aster yellows and Mexican periwinkle phytoplasma groups affecting the same host. Broccoli plants in Brazil were affected by strains from these groups, as well as by phytoplasmas belonging to the 16SrIII ribosomal group47. Usually, the strategy followed to address double infections requires the sequencing of a large number of F2nR2 clones along with the sequencing of a protein-encoding gene30, 47. In this study the double infection was detected through the complementarity of the different SbGP/MPV phytoplasma-cpn60-based molecular diagnostic methods developed, along with previously developed AY phytoplasma cpn60-based specific diagnostic methods. Based on the quantitative results obtained through SbGP/MPV- qRT-PCR and AY- qRT-PCR of samples S41-JB, at least 150 clones would need to be sequenced to identify SbGP/MPV phytoplasma through the sequencing of cpn60 UT clones, which is a laborious and expensive process. The identification of aster yellows in plants affected by strawberry green petal disease is not surprising, since this disease is typically associated with phytoplasmas of the aster yellows (‘Ca. Phytoplasma asteris’, 16SrI) group19–22, 48.
We compared the performance of the cpn60-targeted direct PCR assay to nested PCR targeting F2nR2. A wide variety of PCR primer combinations has been reported in the literature for the detection of phytoplasmas8, 49, and we previously compared the cpn60-targeted direct PCR12 to direct PCR using primers P1/Tint8. More recently, 16S rRNA-encoding gene-targeted primers have been described that are suitable for both conventional and real-time PCR50, but these have not been demonstrated to work with the 16SrXIII group. We therefore chose to compare the cpn60-targeted direct PCR assay to nested PCR targeting the F2nR2 nested PCR, which is commonly used to detect phytoplasma infections. The clinical sensitivity of the cpn60 UT-targeted pan-phytoplasma PCR compared to F2nR2-nested PCR was similar to its previously reported sensitivity compared to the P1/Tint-PCR assay12, but the specificity observed was higher (87.1% compared to F2nR2; 44.4% by comparison to P1/Tint12). These results suggest that P1/Tint primers may have a higher rate of false negative results compared to the phytoplasma cpn60 UT-targeted primers and primers P1/P7 and R16F2n/R16R2. Moreover, the band amplified by P1/Tint does not always correspond to phytoplasma51. The identification of the “false positives” as real positives was achieved through the different SbGP/MPV phytoplasma cpn60 UT-based specific molecular diagnostic methods developed in this study, reinforcing the application of the array of cpn60 UT-based diagnostics presented here.
In this study, blueberry, blackberry, and raspberry plants were identified for the first time as hosts for phytoplasmas in Mexico, and also as hosts for 16SrXIII phytoplasma group worldwide. Recently, two species of ‘Candidatus Phytoplasma’ have been described among the members of the 16SrXIII group, ‘Ca. Phytoplasma hispanicum’28 and ‘Ca. Phytoplasma maliae’52 which have been reported affecting periwinkle27, strawberry24, 26, potato29, papaya53, broccoli47, and china tree54 plants. Among the berry plants analyzed in this study, only strawberry has previously been identified as a host for SbGP/MPV phytoplasma.
Our results also show the minimum extent of the geographic distribution of SbGP/MPV phytoplasma in Central Mexico. One weakness of the study is the lack of the exact position of each farm sampled, but we have demonstrated that the results obtained through the quantitative diagnostic methods can be used to assess the distribution of the phytoplasma in defined geographic areas. Although our study selected symptomatic samples, with an alternative sampling strategy the prevalence of this disease could be easily monitored using the molecular diagnostic assays we describe. The molecular diagnostic assays described in this study can provide timely, accurate information about the identification, characterization, geographic distribution and prevalence of this phytoplasma in plant and insect hosts within the Americas and in other geographic areas.
Materials and Methods
Plant material
Tissues from symptomatic strawberry (Fragaria x ananassa) and periwinkle (Catharanthus roseus)30, along with raspberry (Rubus idaeus), blueberry (Vaccinium corymbosum), and blackberry (Rubus fructicosus) plants with symptoms described in Table 1 and Figure S1 were analyzed. Samples were collected in one locality (A) in San Luis Potosi, four localities in Jalisco (A-D), and 6 localities in Michoacan (A-F) across two growing seasons (November-February), 2014 and 2015. Samples were coded according to the plant, tissue, state, and locality from which they were obtained (Table 1). In total, 69 samples from 48 strawberry plants, 2 samples from 2 periwinkle plants, 9 samples from 8 raspberry plants, 2 samples from 2 blueberry plants, and 4 samples from 4 blackberry plants were collected. DNA was extracted from these samples as previously described51.
Conventional PCR and cloning of PCR products
PCR primers P1/P732, 33 and R16F2n and R16R231, which generate the ~1.2 kb F2nR2 amplicon from all phytoplasmas, were used to amplify the 16S rRNA-encoding locus of the phytoplasma-infected samples analyzed in this study. The primers were used either in a “nested” format with the first step using P1/P7 and the second step using R16F2n/R16R2, or a “direct” format using only the latter primers. For the nested format, the initial PCR (P1/P7) amplified a >1.8-kbp product, which was diluted (1:30) (Supplementary Fig. S6), and used as template in a secondary PCR step with primers R16F2n and R16R2 (Supplementary Fig. S6). In addition, PCR primers designed to amplify the ~600 bp cpn60 UT of all phytoplasmas12 were used to generate cpn60 UT amplicon from the same samples. The sensitivity and specificity of the cpn60 UT primers were determined using the nested F2nR2 amplification as to define positives and negatives as described55.
The F2nR2 amplicon generated from samples S07-P-JC and S10-L-JC was cloned into the vector pGEM-T Easy (Promega, Madison, WI USA) according to the manufacturer’s recommendations, then plasmids were transformed into chemically competent E. coli TOP10 (Life Technologies), and five and three clones were sequenced using plasmid-targeted primers T7/SP6, respectively. The cpn60 UT amplicons from the same samples were generated and sequenced directly with the primers H0279p/H0280p as previously described12. The cpn60 UT from samples S05-L-MB (strawberry), Bl01-L-JA (blueberry), R05-L-MC (raspberry), and Bk02-L-JD (blackberry), were amplified and sequenced directly to confirm the presence of phytoplasmas and identify the phytoplasma affecting the plants. The F2nR2 and the cpn60 UT amplicons generated from sample S41-L-JB were also cloned and sequenced as described above.
Detection and identification of SbGP/MPV phytoplasma using oligonucleotide-coupled microspheres
Methods for probe design and amplicon generation and hybridization have been described in detail12, 56. Briefly, oligonucleotides that distinguish SbGP/MPV phytoplasma cpn60 UT from other phytoplasma cpn60 UT sequences (Supplementary Table S2) were designed using sigoligo57 and PrimerPlex v2.62 (Premier Biosoft, Palo Alto CA). Oligonucleotides were coupled to fluorescent microspheres and hybridized to single-stranded PCR product generated using the cpn60 UT-targeted universal primers for phytoplasma12. Samples with median fluorescence intensities (MFI) that were significantly greater than controls were identified as positive, using duplicate hybridizations and a one-tailed student’s t-test with a significance cut off of 0.05.
Molecular diagnostic assay based on qRT-PCR detection of the SbGP/MPV cpn60
Regions of the SbGP/MPV cpn60 UT that distinguished the target gene from all other described cpn60 UT sequences of ‘Ca. Phytoplasma’ spp. were identified using sigoligo57. These regions were chosen for hydrolysis probe assay design using Beacon Designer v.7.90 (Premier Biosoft, Palo Alto, CA). A primer/probe set was selected based on primer BLAST that was likely to selectively target SbGP/MPV phytoplasma (Supplementary Table S2). All amplification primers and the hydrolysis probe were obtained from Integrated DNA technologies (Coralville, IA). The primer/probe set was evaluated for analytical specificity using 10 ng of template DNA obtained from uninfected strawberry (obtained from a local grocery store) and periwinkle (obtained from control plants maintained in a growth chamber), and a mixture of plasmid DNA consisting of 106 copies each of all of the phytoplasma cpn60 UT sequences previously described12. The final amplification conditions are shown in Supplementary Table S2. For quantification, a set of standards was prepared using plasmid DNA containing the cpn60 UT of SbGP/MPV phytoplasma. Miniprep DNA prepared using a Qiagen miniprep kit (500 ng) was linearized using PstI in a 50 µl digestion volume, then purified using a QiaQuick column (Qiagen). The concentration of linearized, purified plasmid DNA was determined in triplicate using a Qubit instrument (BR kit, Life Technologies), and the mean concentration (ng/µl) was converted to copies/µl using the known length of the plasmid DNA and an approximation of 650 g/mol per base pair. Standards were diluted to concentrations of 107 − 101 copies per 2 µl and used as control templates in qRT-PCR. Reactions were prepared using SsoFast Universal probes supermix (Bio-Rad, Mississauga, ON, Canada) in a 20 µl final volume with 300 nM of each primer and 200 nM of probe. Amplification was carried out using a C1000 thermocycler base with a CFX96 real-time system (Bio-Rad) and reactions were quantified using BioRad CFX manager software (v.3.1). Threshold cycle (Cq) values were converted to copy numbers by interpolation on the standard curve, and the results were corrected to account for sample preparation and dilution to arrive at copy number/g tissue extracted. Positive samples with Cq values below that of the lowest standard were specified as, “detectable but not quantifiable” (DNQ).
ddPCR quantification of SbGP/MPV cpn60
The same primer/probe set used for the qRT-PCR assay was used for ddPCR quantification. Reaction conditions were optimized using gradient PCR with ddPCR supermix for probes (Bio-Rad) including 900 nM each primer and 250 nM of hydrolysis probe in a 20 µl reaction volume with a plasmid standard prepared as described above (103 copies per assay). Final ddPCR conditions are shown in Supplementary Table S2. Template DNA extracted from strawberry and periwinkle samples analyzed by ddPCR was digested prior to amplification using EcoRI at 37 °C for 60 minutes followed by enzyme inactivation at 85 °C for 5 minutes. Samples were then diluted 1:100 and 2 µl used as template for ddPCR. Emulsions were formed using a QX100 droplet generator (Bio-Rad), and amplifications were carried out using a C1000 Touch thermocyler (BioRad). Reactions were analyzed using a QX100 droplet reader (Bio-Rad) and quantified using QuantaSoft v.1.6.6 (Bio-Rad). Results were converted to copy number/g tissue extracted by accounting for sample preparation and dilution. To analyze the samples collected in 2015 a probe targeting strawberry Rubisco gene was developed (Supplementary Table S2), in order to express the results in terms of fractional abundance. Due to the lack of internal control assays, the results obtained from strawberry samples from 2014 along with the blueberry, blackberry, raspberry, periwinkle samples were converted to copy number/g tissue.
Detection of SbGP/MPV cpn60 using LAMP
Amplification primers (Supplementary Table S2) for LAMP were designed using LAMP Designer v. 1.12 (Premier Biosoft, Palo Alto, CA). LAMP conditions were as described for calcein detection43, and a LAMP temperature of 63 °C (1 hour assay time) was used. Alternatively, the same LAMP primers were used with detection using Isothermal Detection Reagent (Prolab Diagnostics, Richmond Hill, ON, Canada) at a temperature of 63 °C for 30 minutes. After amplification, an annealing curve was generated (90 °C–75 °C at 0.05 °C/sec). Reactions were monitored in real time using a Genie II or Genie III instrument (OptiGene, Horsham, UK), and the time to positive (Tp) was reported by the instrument. For binomial (positive/negative) detection, reactions using calcein-based detection were viewed under ultraviolet light using a transilluminator (Bio-Rad Gel Doc). The 2015 samples were analyzed undiluted and diluted 1:50 with 10 mM Tris-Cl, pH 8.5 to examine the effect of co-purifying inhibitors.
Determination of SbGP/MPV-targted assay parameters
The performance characteristics of the molecular diagnostic assays were determined according to established standards55, 58. Analytical specificity was examined by using as template for the LAMP and qPCR assays genomic DNA isolated from plants infected with the various phytoplasma strains described in Dumonceaux et al.12. Analytical specificity was also determined using as template a mixture of 106 copies each of the cloned cpn60 UT amplicons described in Dumonceaux et al.12. The linearity of the quantitative assays was examined by polynomial regression analysis of the Cq or Tp determined over a range of dilutions in a background of uninfected strawberry DNA. Detection limits were inferred by examining the lower levels of detection observed in these assays. Intra-assay precision was determined by performing up to 10 replicates and determining the standard deviation of the calculated results determined for four naturally infected samples. Finally, the performances of the direct PCR-based assays (cpn60 and F2nR2) were compared by scoring the numbers of positive and negative results obtained using the two methods and calculating sensitivity and specificity as described55.
SbGP/MPV phytoplasma distribution in central Mexico
Geospatial data for Mexico was downloaded from the GADM database of Global Administrative Areas (www.gadm.org) and plotted in R (http://www.R-project.org/) using ggplot259. To assess the geographic distribution of SbGP/MPV phytoplasma, the qRT-PCR-determined SbGP/MPV genome copy number/g tissue was expressed on the map generated per locality and state.
Electronic supplementary material
Acknowledgements
This work was supported by the Genomics Research and Development Initiative for the shared priority project on quarantine and invasive species. E.P.L. thanks Agriculture and Agri-Food Canada for the time in T.J.D. laboratory. All authors thank Jennifer Town for generating the map of Mexico.
Author Contributions
The project was conceived by T.J.D., E.P.L., and D.R.M. Field collections by D.R.M. and E.P.L. Molecular assays by T.J.D. and E.P.L. All authors contributed to manuscript writing.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00895-1
Accession codes: DNA sequences determined in the course of this study have been deposited in GenBank (ncbi.nlm.nih.gov) under accession numbers KY061162- KY061185.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Doi Y, Teranaka M, Yora K, Asuyama H. Mycoplasma or PLT-group-like microorganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’-broom, aster yellows, or paulownia witches’-broom. Ann. Phytopathol. Japan. 1967;33:259–266. doi: 10.3186/jjphytopath.33.259. [DOI] [Google Scholar]
- 2.Lee IM, Davis RE, Gundersen-Rindal DE. Phytoplasma: Phytopathogenic mollicutes. Annu Rev Microbiol. 2000;54:221–255. doi: 10.1146/annurev.micro.54.1.221. [DOI] [PubMed] [Google Scholar]
- 3.Zhao, Y., Davis, R. E., Wei, W., Shao, J. & Jomantiene, R. In Genomics of Plant-Associated Bacteria (ed D. C. Gross) Ch. 10, 235–271 (Springer-Verlag, 2014).
- 4.Namba S. Phytoplasmas: A century of pioneering research. J. Gen. Plant Pathol. 2011;77:345–349. doi: 10.1007/s10327-011-0341-y. [DOI] [Google Scholar]
- 5.Maejima K, Oshima K, Namba S. Exploring the phytoplasmas, plant pathogenic bacteria. J. Gen. Plant Pathol. 2014;80:210–221. doi: 10.1007/s10327-014-0512-8. [DOI] [Google Scholar]
- 6.Gasparich GE. Spiroplasmas and phytoplasmas: microbes associated with plant hosts. Biologicals. 2010;38:193–203. doi: 10.1016/j.biologicals.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Murray RGE, Stackebrandt E. Taxonomic note: Implementation of the provisional status Candidatus for incompletely described procaryotes. Int. J. Sys. Bacteriol. 1995;45:186–187. doi: 10.1099/00207713-45-1-186. [DOI] [PubMed] [Google Scholar]
- 8.Smart CD, et al. Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Appl. Environ. Microbiol. 1996;62:2988–2993. doi: 10.1128/aem.62.8.2988-2993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, et al. Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII) Int. J. Syst. Evol. Microbiol. 2009;59:2582–2593. doi: 10.1099/ijs.0.010249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Davis RE. Criteria for phytoplasma 16Sr group/subgroup delineation and the need of a platform for proper registration of new groups and subgroups. Int. J. Syst. Evol. Microbiol. 2016;66:2121–2123. doi: 10.1099/ijsem.0.000999. [DOI] [PubMed] [Google Scholar]
- 11.Mitrović J, et al. The groEL gene as an additional marker for finer differentiation of ‘Candidatus Phytoplasma asteris’-related strains. Ann. Appl. Biol. 2011;159:41–48. doi: 10.1111/j.1744-7348.2011.00472.x. [DOI] [Google Scholar]
- 12.Dumonceaux TJ, Green M, Hammond C, Perez E, Olivier C. Molecular diagnostic tools for detection and differentiation of Phytoplasmas based on chaperonin-60 reveal differences in host plant infection patterns. PLoS ONE. 2014;9:e116039. doi: 10.1371/journal.pone.0116039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh SH, et al. HSP60 gene sequences as universal targets for microbial species identification: studies with coagulase-negative staphylococci. J Clin Microbiol. 1996;34:818–823. doi: 10.1128/jcm.34.4.818-823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Links MG, Dumonceaux TJ, Hemmingsen SM, Hill JE. The chaperonin-60 universal target is a barcode for bacteria that enables de novo assembly of metagenomic sequence data. PLoS One. 2012;7:e49755. doi: 10.1371/journal.pone.0049755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Links MG, et al. Simultaneous profiling of seed-associated bacteria and fungi reveals antagonistic interactions between microorganisms within a shared epiphytic microbiome on Triticum and Brassica seeds. New Phytol. 2014;202:542–553. doi: 10.1111/nph.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenney EA, Ashwell M, Lambert JE, Fellner V. Fecal microbial diversity and putative function in captive western lowland gorillas (Gorilla gorilla gorilla), common chimpanzees (Pan troglodytes), Hamadryas baboons (Papio hamadryas) and binturongs (Arctictis binturong) Int. Zool. 2014;9:557–569. doi: 10.1111/1749-4877.12112. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara K, et al. Rapid and reliable detection of phytoplasma by loop-mediated isothermal amplification targeting a housekeeping gene. J. Gen. Plant Pathol. 2012;78:389–397. doi: 10.1007/s10327-012-0403-9. [DOI] [Google Scholar]
- 18.Pérez-López E, Olivier CY, Luna-Rodríguez M, Dumonceaux TJ. Phytoplasma classification and phylogeny based on in silico and in vitro RFLP analysis of cpn60 universal target sequences. Int. J. Syst. Evol. Microbiol. 2016;66:5600–5613. doi: 10.1099/ijsem.0.000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honetšlegrová JF, Vibio M, Bertaccini A. Electron microscopy and molecular identification of phytoplasmas associated with strawberry green petals in the Czech Republic. Eur. J. Plant Pathol. 1996;102:831–835. doi: 10.1007/BF01877052. [DOI] [Google Scholar]
- 20.Blattny CJ, Blattny CS. A virus green petals disease of Fragaria ananassa. Folia Microbiologica. 1959;4:345–350. doi: 10.1007/BF02929533. [DOI] [Google Scholar]
- 21.Gundersen DE, et al. Genomic diversity and differentiation among phytoplasma strains in 16S rRNA groups I (aster yellows and related phytoplasmas) and III (X-disease and related phytoplasmas) Int. J. Sys. Bacteriol. 1996;46:64–75. doi: 10.1099/00207713-46-1-64. [DOI] [PubMed] [Google Scholar]
- 22.Contaldo N, Mejia JF, Paltrinieri S, Calari A, Bertaccini A. Identification and GroEL gene characterization of green petal phytoplasma infecting strawberry in Italy. Phytopathogenic Mollicutes. 2012;2:59–62. doi: 10.5958/j.2249-4669.2.2.009. [DOI] [Google Scholar]
- 23.Tran-Nguyen L, Blanche KR, Egan B, Gibb KS. Diversity of phytoplasmas in northern Australian sugarcane and other grasses. Plant Pathol. 2000;49:666–679. doi: 10.1046/j.1365-3059.2000.00498.x. [DOI] [Google Scholar]
- 24.Jomantiene R, Davis RE, Maas J, Dally EL. Classification of new phytoplasmas associated with diseases of strawberry in Florida, based on analysis of 16S rRNA and ribosomal protein gene operon sequences. Int. J. Sys. Bacteriol. 1998;48:269–277. doi: 10.1099/00207713-48-1-269. [DOI] [PubMed] [Google Scholar]
- 25.Fernández FD, Conci VC, Kirschbaum DS, Conci LR. Molecular characterization of a phytoplasma of the ash yellows group occurring in strawberry (Fragaria x ananassa Duch.) plants in Argentina. Eur. J. Plant Pathol. 2013;135:1–4. doi: 10.1007/s10658-012-9951-2. [DOI] [Google Scholar]
- 26.Fernández FD, et al. Detection and identification of a novel 16SrXIII subgroup phytoplasma associated with strawberry red leaf disease in Argentina. Int. J. Syst. Evol. Microbiol. 2015;65:2741–2747. doi: 10.1099/ijs.0.000276. [DOI] [PubMed] [Google Scholar]
- 27.Lee IM, Gundersen-Rindal DE, Davis RE, Bartoszyk IM. Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. Int. J. Sys. Bacteriol. 1998;48:1153–1169. doi: 10.1099/00207713-48-4-1153. [DOI] [Google Scholar]
- 28.Davis RE, Harrison NA, Zhao Y, Wei W, Dally EL. ‘Candidatus Phytoplasma hispanicum’, a novel taxon associated with Mexican periwinkle virescence disease of Catharanthus roseus. Int. J. Syst. Evol. Microbiol. 2016;66:3463–3467. doi: 10.1099/ijsem.0.001218. [DOI] [PubMed] [Google Scholar]
- 29.Santos-Cervantes ME, et al. Genetic diversity and geographical distribution of phytoplasmas associated with potato purple top disease in Mexico. Plant Dis. 2010;94:388–395. doi: 10.1094/PDIS-94-4-0388. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-López E, Dumonceaux TJ. Detection and identification of the heterogeneous novel subgroup 16SrXIII-(A/I)I phytoplasma associated with strawberry green petal disease and Mexican periwinkle virescence. Int. J. Syst. Evol. Microbiol. 2016;66:4406–4415. doi: 10.1099/ijsem.0.000726. [DOI] [PubMed] [Google Scholar]
- 31.Gundersen DE, Lee IM. Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathol. Mediterr. 1996;35:144–151. [Google Scholar]
- 32.Schneider, B., Seemuller, E., Smart, C. D. & Kirkpatrick, B. C. In Molecular and Diagnostic Procedures in Mycoplasmology Vol. 1 (eds R. Razin & J. G. Tully) 369-380 (Academic Press, 1995).
- 33.Deng S, Hiruki C. Amplification of 16S rRNA genes from culturable and nonculturable Mollicutes. J. Microbiol. Meth. 1991;14:53–61. doi: 10.1016/0167-7012(91)90007-D. [DOI] [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45–45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivier, C. et al. In Integrated Management of Insect Pests on Canola and Other Brassica Oilseed Crops (ed G.V.P. Reddy) (CABI, 2017).
- 36.Aaby K, Mazur S, Nes A, Skrede G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012;132:86–97. doi: 10.1016/j.foodchem.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Katcher HL, Schwartz I. A distinctive property of Tth DNA polymerase: Enzymatic amplification in the presence of phenol. BioTechniques. 1994;16:90–92. [PubMed] [Google Scholar]
- 38.Racki N, Dreo T, Gutierrez-Aguirre I, Blejec A, Ravnikar M. Reverse transcriptase droplet digital PCR shows high resilience to PCR inhibitors from plant, soil and water samples. Plant Meth. 2014;10:42. doi: 10.1186/s13007-014-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomlinson, J. In Phytoplasma: Methods and Protocols Vol. 938 Methods in Molecular Biology (eds M. Dickinson & J. Hodgetts) 291–300 (Springer, 2013).
- 40.Obura E, Masiga D, Wachira F, Gurja B, Khan ZR. Detection of phytoplasma by loop-mediated isothermal amplification of DNA (LAMP) J. Microbiol. Meth. 2011;84:312–316. doi: 10.1016/j.mimet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Hodgetts J, et al. Development of rapid in-field loop-mediated isothermal amplification (LAMP) assays for phytoplasmas. Bull. Insectol. 2011;64:S41–S42. [Google Scholar]
- 42.Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques. 2009;46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 43.Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protocols. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 44.Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infec. Chemother. 2009;15:62–69. doi: 10.1007/s10156-009-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Amelio R, Marzachi C, Bosco D. Double infection of ‘Candidatus Phytoplasma asteris’ and “flavescence dorée” phytoplasma in the vector Euscelidius variegatus. Bull. Insectol. 2007;60:223–224. [Google Scholar]
- 46.Alma A, et al. Mixed infection of grapevines in northern Italy by phytoplasmas including 16S rRNA RFLP subgroup 16SrI-B strains previously unreported in this host. Plant Dis. 1996;80:418–421. doi: 10.1094/PD-80-0418. [DOI] [Google Scholar]
- 47.Eckstein B, et al. Broccoli Stunt, a New Disease in Broccoli Plants Associated with Three Distinct Phytoplasma Groups in Brazil. J. Phytopathol. 2013;161:442–444. doi: 10.1111/jph.12087. [DOI] [Google Scholar]
- 48.Converse RH, et al. Laboratory detection of viruses and mycoplasmalike organisms in strawberry. Plant Dis. 1988;72:744–749. doi: 10.1094/PD-72-0744. [DOI] [Google Scholar]
- 49.Lee IM, et al. ‘Candidatus Phytoplasma asteris’, a novel phytoplasma taxon associated with aster yellows and related diseases. Int. J. Syst. Evol. Microbiol. 2004;54:1037–1048. doi: 10.1099/ijs.0.02843-0. [DOI] [PubMed] [Google Scholar]
- 50.Ikten C, Ustun R, Catal M, Yol E, Uzun B. Multiplex Real-Time qPCR Assay for Simultaneous and Sensitive Detection of Phytoplasmas in Sesame Plants and Insect Vectors. PLOS ONE. 2016;11:e0155891. doi: 10.1371/journal.pone.0155891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-López E, et al. Maize bushy stunt phytoplasma affects native corn at high elevations in Southeast Mexico. Eur. J. Plant Pathol. 2016;27:963–971. doi: 10.1007/s10658-016-0883-0. [DOI] [Google Scholar]
- 52.Fernández FD, Galdeano E, Kornowski MV, Arneodo J, Conci LR. Description of ‘Candidatus Phytoplasma meliae’, a phytoplasma associated with Chinaberry (Melia azedarach L.) yellowing in South America. Int. J. Syst. Evol. Microbiol. 2016;66:5244–5251. doi: 10.1099/ijsem.0.001503. [DOI] [PubMed] [Google Scholar]
- 53.Melo L, et al. A phytoplasma representative of a new subgroup, 16SrXIII-E, associated with Papaya apical curl necrosis. Eur. J. Plant Pathol. 2013;137:445–450. doi: 10.1007/s10658-013-0267-7. [DOI] [Google Scholar]
- 54.Arneodo JD, et al. Diversity and geographical distribution of phytoplasmas infecting China-tree in Argentina. J. Phytopathol. 2007;155:70–75. doi: 10.1111/j.1439-0434.2007.01181.x. [DOI] [Google Scholar]
- 55.Banoo S, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat.Rev.Microbiol. 2006;4:S21–S31. doi: 10.1038/nrmicro1570. [DOI] [PubMed] [Google Scholar]
- 56.Dumonceaux, T. J., Town, J. R., Hill, J. E., Chaban, B. L. & Hemmingsen, S. M. Multiplex detection of bacteria in complex clinical and environmental samples using oligonucleotide-coupled fluorescent microspheres. J Vis Exp e3344, 10.3791/3344 (2011). [DOI] [PMC free article] [PubMed]
- 57.Zahariev M, Dahl V, Chen W, Levesque CA. Efficient algorithms for the discovery of DNA oligonucleotide barcodes from sequence databases. Mol Ecol Resour. 2009;9(Suppl s1):58–64. doi: 10.1111/j.1755-0998.2009.02651.x. [DOI] [PubMed] [Google Scholar]
- 58.Burd EM. Validation of Laboratory-Developed Molecular Assays for Infectious Diseases. Clin. Microbiol. Rev. 2010;23:550–576. doi: 10.1128/CMR.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


