Abstract
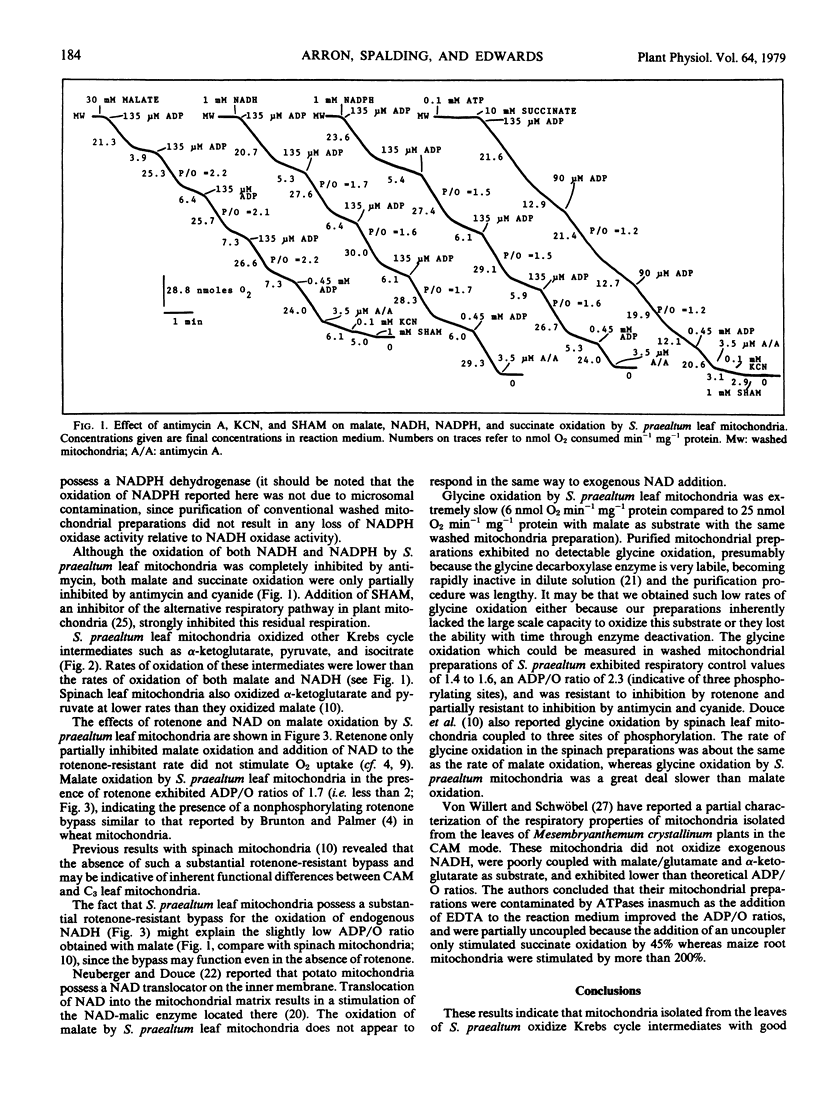
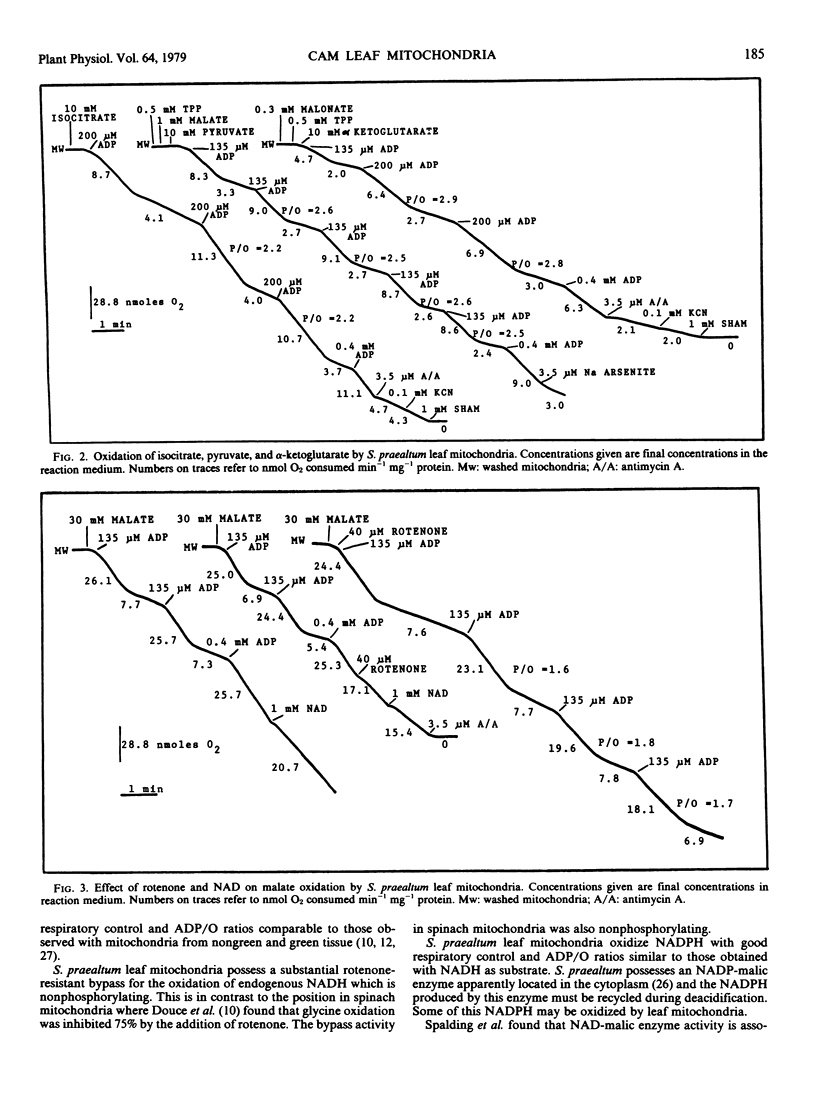
A procedure is described for preparing intact mitochondria from leaves of Sedum praealtum D.C., a plant showing Crassulacean acid metabolism. These mitochondria oxidized malate, pyruvate, α-ketoglutarate, succinate, NADH, NADPH, and isocitrate with good respiratory control and ADP/O ratios better than those observed in mitochondria from other photosynthetic tissues.
Malate oxidation was very resistant to inhibition by rotenone. Glycine oxidation was very slow with poor respiratory control and was resistant to rotenone inhibition. Antimycin A completely inhibited the oxidation of both NADH and NADPH. The oxidation of isocitrate, malate, succinate, and α-ketoglutarate was partially inhibited by antimycin A and cyanide. Overall rates of substrate oxidation were slow on a protein basis, but purification of the mitochondrial preparations on a linear sucrose gradient removed a large amount of nonmitochondrial protein. The original mitochondrial preparations contained little glycolate oxidase activity, and most of this activity was removed by the sucrose gradient.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird I. F., Cornelius M. J., Keys A. J., Whittingham C. P. Adenosine triphosphate synthesis and the natural electron acceptor for synthesis of serine from glycine in leaves. Biochem J. 1972 Jun;128(1):191–192. doi: 10.1042/bj1280191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton C. J., Palmer J. M. Pathways for the oxidation of malate and reduced pyridine nucleotide by wheat mitochondria. Eur J Biochem. 1973 Nov 1;39(1):283–291. doi: 10.1111/j.1432-1033.1973.tb03125.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The oxidation of malate by isolated plant mitochondria. Eur J Biochem. 1972 Apr 24;26(4):499–509. doi: 10.1111/j.1432-1033.1972.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Pyruvate and malate transport and oxidation in corn mitochondria. Plant Physiol. 1977 Apr;59(4):630–635. doi: 10.1104/pp.59.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The Effect of Exogenous Nicotinamide Adenine Dinucleotide on the Oxidation of Nicotinamide Adenine Dinucleotide-linked Substrates by Isolated Plant Mitochondria. Plant Physiol. 1974 Sep;54(3):360–363. doi: 10.1104/pp.54.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUDNUT H. B., Jr, KEY C., JAQUES W. E. Embolism to the right side of the heart. Am Heart J. 1962 Jun;63:743–746. doi: 10.1016/0002-8703(62)90058-3. [DOI] [PubMed] [Google Scholar]
- Jackson C., Dench J., Moore A. L., Halliwell B., Foyer C. H., Hall D. O. Subcellular localisation and identification of superoxide dismutase in the leaves of higher plants. Eur J Biochem. 1978 Nov 15;91(2):339–344. doi: 10.1111/j.1432-1033.1978.tb12685.x. [DOI] [PubMed] [Google Scholar]
- Koeppe D. E., Miller R. J. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by isolated corn mitochondria. Plant Physiol. 1972 Mar;49(3):353–357. doi: 10.1104/pp.49.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laties G. G. The potentiating effect of adenosine diphosphate in the uncoupling of oxidative phosphorylation in potato mitochondria. Biochemistry. 1973 Aug 14;12(17):3350–3355. doi: 10.1021/bi00741a032. [DOI] [PubMed] [Google Scholar]
- Macrae A. R., Moorhouse R. The oxidation of malate by mitochondria isolated from cauliflower buds. Eur J Biochem. 1970 Sep;16(1):96–102. doi: 10.1111/j.1432-1033.1970.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Schmitt M. R., Ku S. B., Edwards G. E. Intracellular Localization of Some Key Enzymes of Crassulacean Acid Metabolism in Sedum praealtum. Plant Physiol. 1979 Apr;63(4):738–743. doi: 10.1104/pp.63.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]


