Abstract
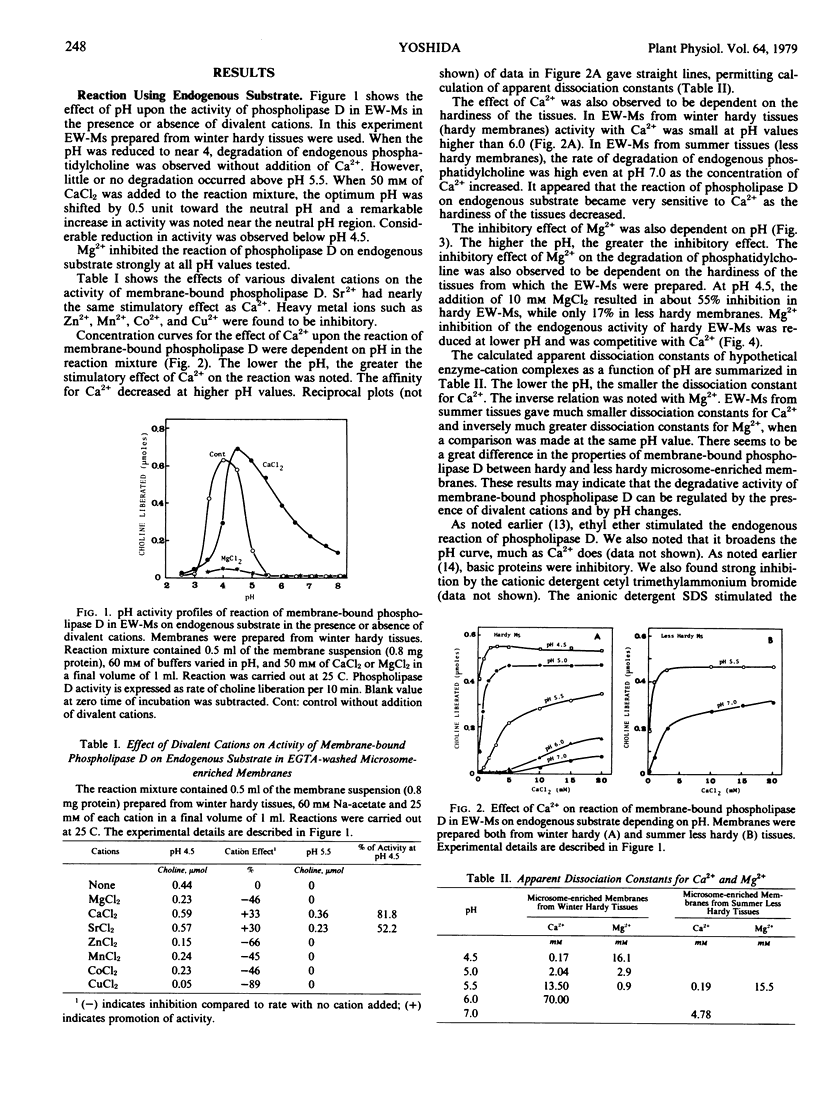
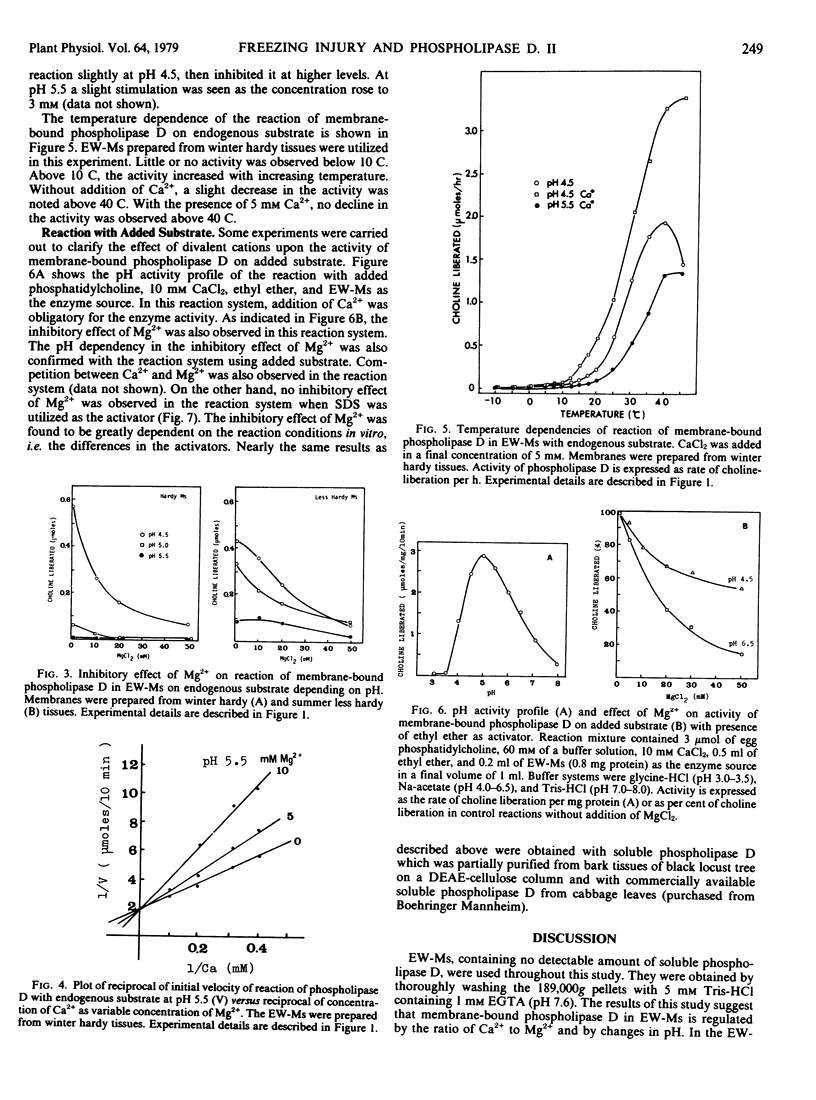
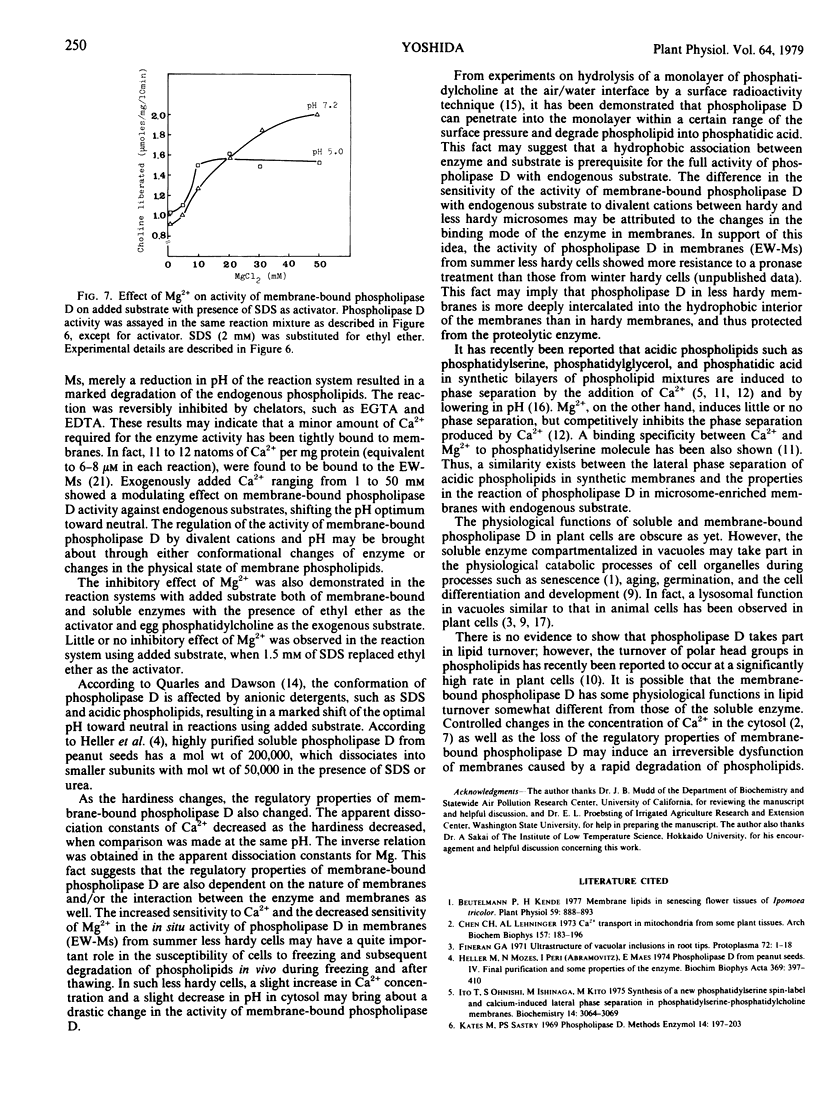
Activity of membrane-bound phospholipase D in microsomes from bark tissues of black locust tree (Robina pseudoacacia L.) was demonstrated to be regulated by a competitive binding of divalent cations. Binding of Ca2+ at high concentrations (1 to 50 millimolar) modified the pH activity profile, shifting the optimum pH by 0.5 unit toward neutral and increasing the activity in the neutral pH. Mg2+, on the other hand, inhibited the reaction of membrane-bound phospholipase D without added Ca2+, and competitively inhibited the Ca2+ stimulation. The regulatory effects of those ions were dependent on pH. Reduction in pH resulted in a decrease in the apparent dissociation constant for Ca2+ and an increase in that for Mg2+. From Lineweaver-Burk double reciprocal plots of Ca2+ and the initial velocity, it was suggested that the binding of Ca2+ in the higher concentration resulted in nearly the same conformational change of enzyme as reduction in pH. Mg2+, on the other hand, counteracted those effects of Ca2+ and lower pH on the enzyme conformation in such a manner as to inactivate. The membrane-bound phospholipase D because more sensitive to Ca2+ and less sensitive to Mg2+ as the hardiness of the tissues decreased. This fact may indicate that some qualitative changes in membranes are involved in the hardiness changes and also in the susceptibility of phospholipid to degradation by phospholipase D in plant cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutelmann P., Kende H. Membrane Lipids in Senescing Flower Tissue of Ipomoea tricolor. Plant Physiol. 1977 May;59(5):888–893. doi: 10.1104/pp.59.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Lehninger A. L. Ca 2+ transport activity in mitochondria from some plant tissues. Arch Biochem Biophys. 1973 Jul;157(1):183–196. doi: 10.1016/0003-9861(73)90404-9. [DOI] [PubMed] [Google Scholar]
- Iot T., Ohnish S., Ishinaga M., Kito M. Synthesis of a new phosphatidylserine spin-label and calcium-induced lateral phase separation in phosphatidylserine-phosphatidylcholine membranes. Biochemistry. 1975 Jul 15;14(14):3064–3069. doi: 10.1021/bi00685a004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S. Phospholipid turnover in soybean tissue cultures. Plant Physiol. 1977 Nov;60(5):754–758. doi: 10.1104/pp.60.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C., Pangborn W., Nir S., Papahadjopoulos D. Specificity of Ca2+ and Mg2+ binding to phosphatidylserine vesicles and resultant phase changes of bilayer membrane structure. Biochim Biophys Acta. 1978 Jan 19;506(2):281–287. doi: 10.1016/0005-2736(78)90398-x. [DOI] [PubMed] [Google Scholar]
- Onishi S., Ito T. Calcium-induced phase separations in phosphatidylserine--phosphatidylcholine membranes. Biochemistry. 1974 Feb 26;13(5):881–887. doi: 10.1021/bi00702a008. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. A shift in the optimum pH of pospholipase D produced by activating long-chain anions. Biochem J. 1969 May;112(5):795–799. doi: 10.1042/bj1120795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The distribution of phospholipase D in developing and mature plants. Biochem J. 1969 May;112(5):787–794. doi: 10.1042/bj1120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The hydrolysis of monolayers of phosphatidyl(Me-14C)choline by phospholipase D. Biochem J. 1969 Jul;113(4):697–705. doi: 10.1042/bj1130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij A. J., de Kruyff B., Ververgaert P. H., Tocanne J. F., van Deenen L. L. The influence of pH, Ca2+ and protein on the thermotropic behaviour of the negatively charged phospholipid, phosphatidylglycerol. Biochim Biophys Acta. 1974 Mar 29;339(3):432–437. doi: 10.1016/0005-2736(74)90171-0. [DOI] [PubMed] [Google Scholar]
- Villiers T. A. Lysosomal activities of the vacuole in damaged and recovering plant cells. Nat New Biol. 1971 Sep 8;233(36):57–58. doi: 10.1038/newbio233057a0. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Freezing Injury and Phospholipid Degradation in Vivo in Woody Plant Cells: III. Effects of Freezing on Activity of Membrane-bound Phospholipase D in Microsome-enriched Membranes. Plant Physiol. 1979 Aug;64(2):252–256. doi: 10.1104/pp.64.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Freezing injury and phospholipid degradation in vivo in woody plant cells: I. Subcellular localization of phospholipase d in living bark tissues of the black locust tree (robinia pseudoacacia L.). Plant Physiol. 1979 Aug;64(2):241–246. doi: 10.1104/pp.64.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Sakai A. Phospholipid degradation in frozen plant cells associated with freezing injury. Plant Physiol. 1974 Mar;53(3):509–511. doi: 10.1104/pp.53.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]


