Abstract
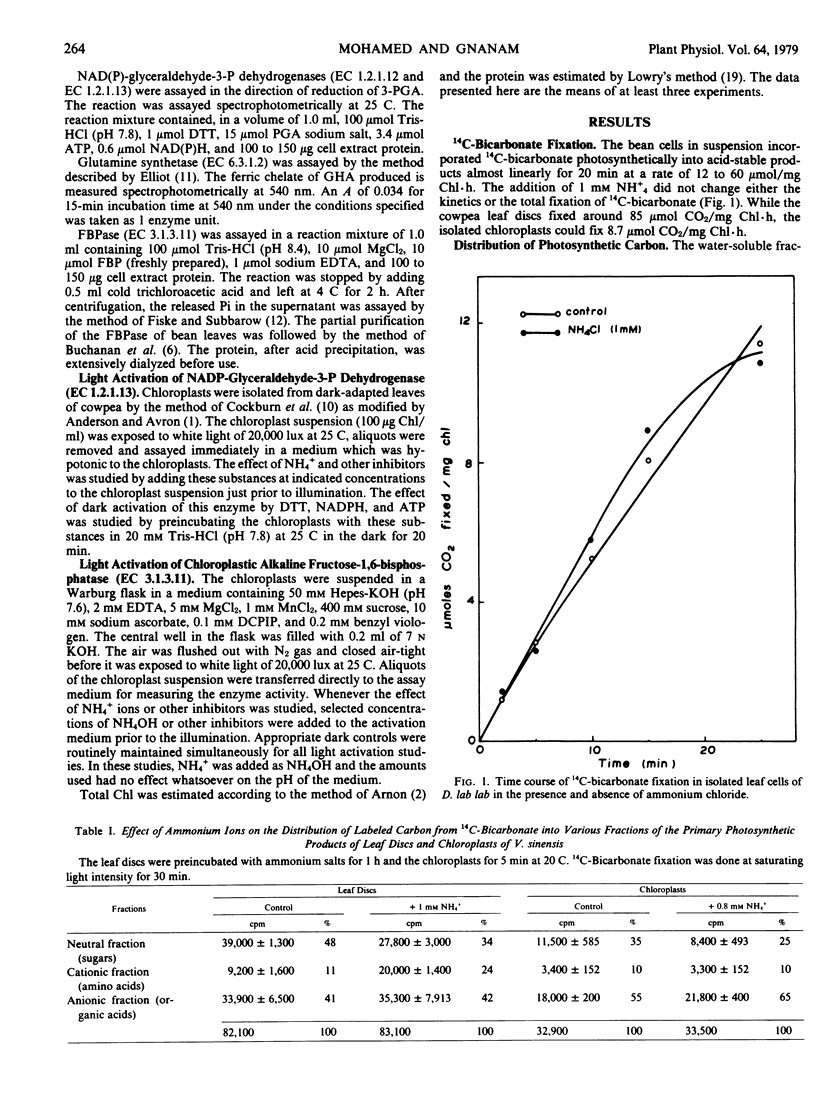
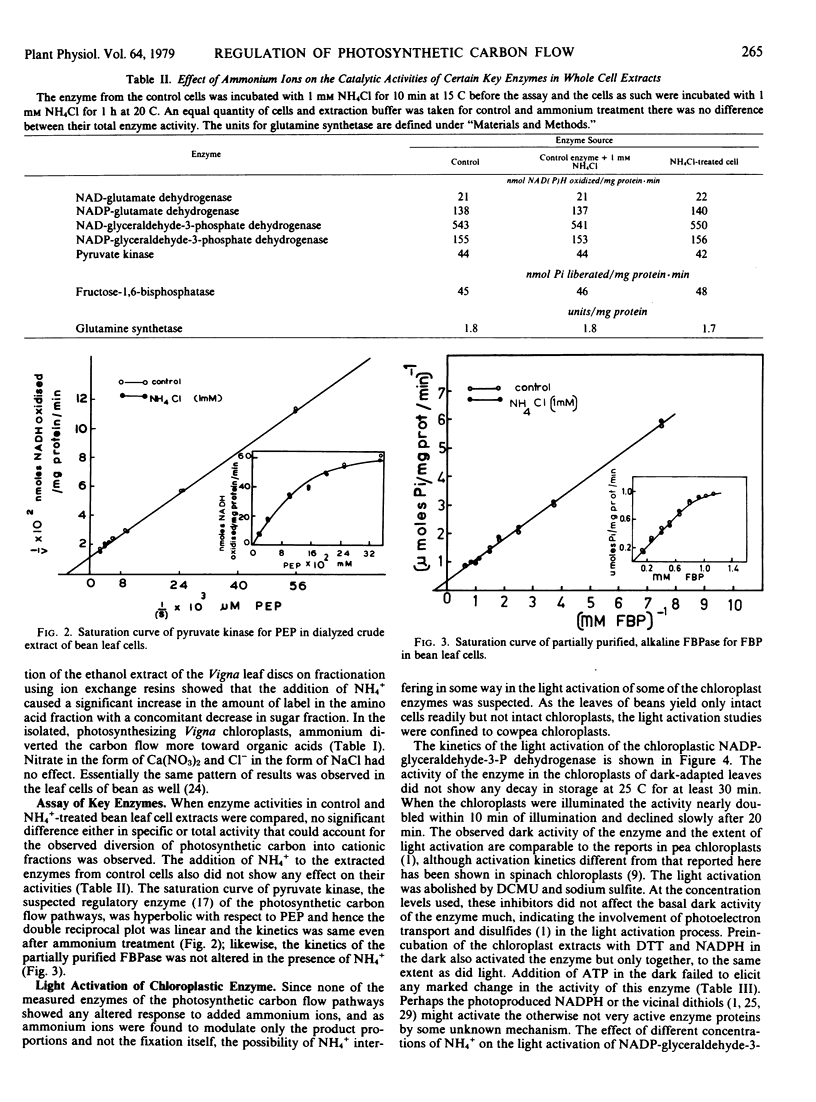
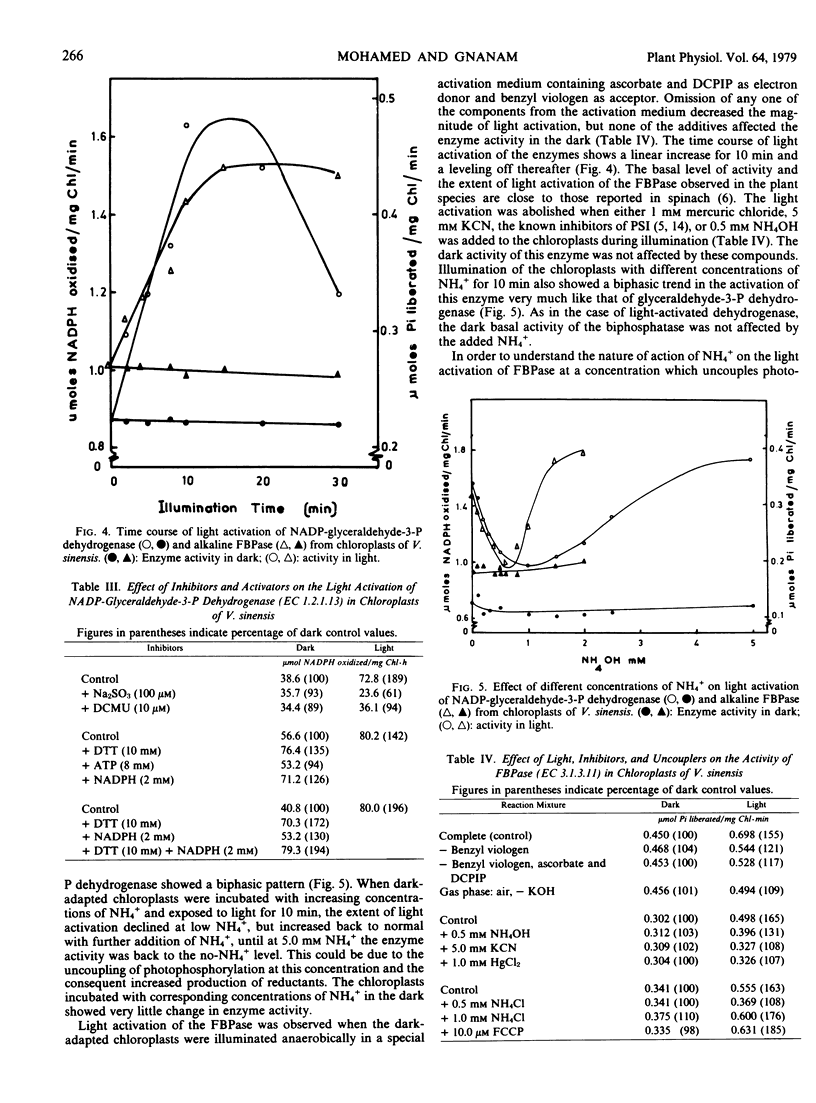
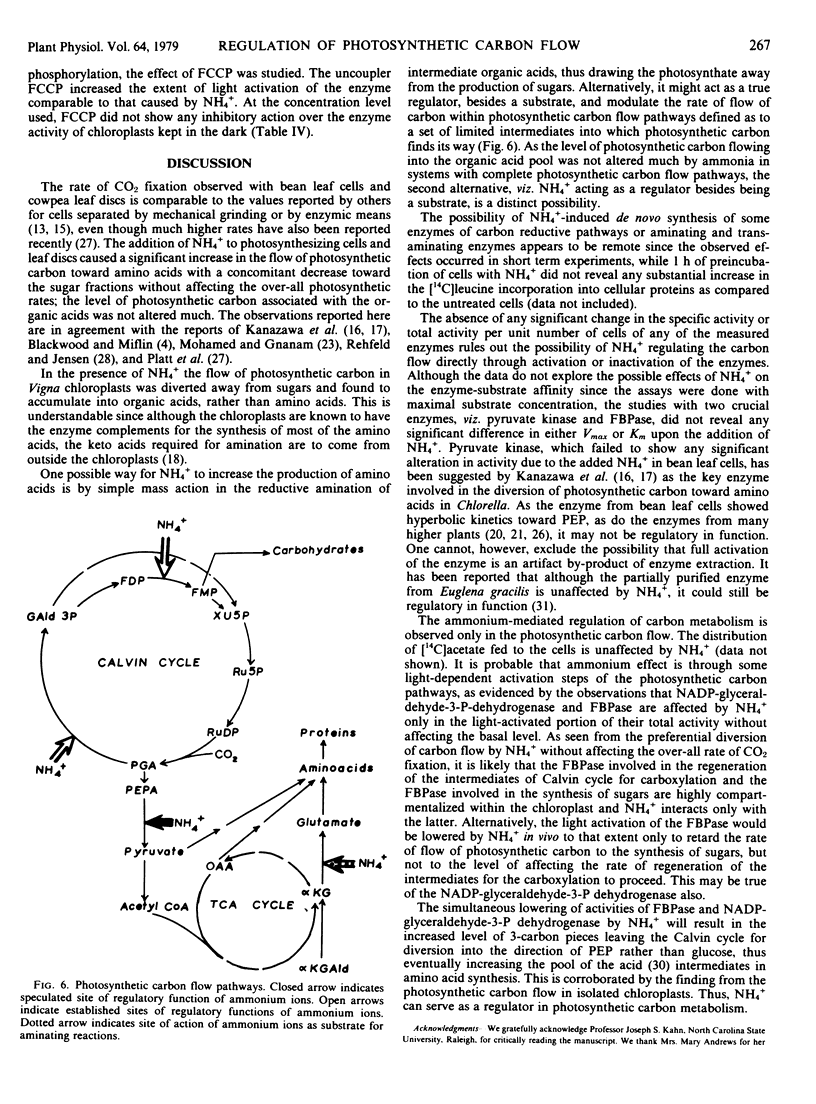
Addition of NH4+ to the photosynthesizing leaf cells of Dolichos lab lab L. var. Lignosis Prain and leaf discs of Vigna sinensis L. savi ex Hassk caused a significant increase in the flow of photosynthetic carbon toward amino acids with a concomitant decrease toward sugars without affecting the over-all photosynthetic rate. Similar diversion of photosynthetic carbon away from sugars was also observed in the photosynthesizing isolated chloroplasts of V. sinensis, but the latter differed in that they accumulated organic acids rather than amino acids. In an effort to understand the mechanism of NH4+-mediated regulation, the specific and total activities of NAD(P)-glutamate dehydrogenase, glutamine synthetase, pyruvate kinase, alkaline fructose 1,6-bisphosphatase, and NAD(P)-glyceraldehyde-3-phosphate dehydrogenase of the cells of D. lab lab were checked but none was affected by the added ammonium salts even after prolonged incubation. At certain concentrations, ammonium ions abolished the light activation of NADP-glyceraldehyde-3-phosphate dehydrogenase and alkaline fructose 1,6-bisphosphatase in isolated chloroplasts from dark-adapted Vigna leaves without interfering with the basal dark activity of these enzymes. Based on these observations, a possible mechanism of action of NH4+ in regulating the photosynthetic carbon flow is postulated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanam A., Kulandaivelu G. Photosynthetic studies with leaf cell suspensions from higher plants. Plant Physiol. 1969 Oct;44(10):1451–1456. doi: 10.1104/pp.44.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Kraayenhof R., Ruuge E. K., Devault D. The site of KCN inhibition in the photosynthetic electron transport pathway. Biochim Biophys Acta. 1973 Sep 26;314(3):328–339. doi: 10.1016/0005-2728(73)90117-5. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Francki R. I., Zaitlin M. Metabolism of separated leaf cells: I. Preparation of photosynthetically active cells from tobacco. Plant Physiol. 1971 Jul;48(1):9–13. doi: 10.1104/pp.48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kanazawa K., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in Chlorella pyrenoidosa during photosynthesis and respiration. Biochim Biophys Acta. 1972 Mar 16;256(3):656–669. doi: 10.1016/0005-2728(72)90201-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Kirk P. R., Leech R. M. Amino Acid Biosynthesis by Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1972 Aug;50(2):228–234. doi: 10.1104/pp.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller G., Evans H. J. The Influence of Salts on Pyruvate Kinase from Tissues of Higher Plants. Plant Physiol. 1957 Jul;32(4):346–354. doi: 10.1104/pp.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B. On the mechanism of the light-induced activation of the NADP-dependent glyceraldehyde phosphate dehydrogenase. Biochim Biophys Acta. 1970 Apr 7;205(1):102–109. doi: 10.1016/0005-2728(70)90066-6. [DOI] [PubMed] [Google Scholar]
- Ohmann E. Die Regulation der Pyruvat-Kinase in Euglena gracilis. Arch Mikrobiol. 1969;67(3):273–292. doi: 10.1007/BF00411262. [DOI] [PubMed] [Google Scholar]
- Platt S. G. Ammonia regulation of carbon metabolism in photosynthesizing leaf discs. Plant Physiol. 1977 Nov;60(5):739–742. doi: 10.1104/pp.60.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Jensen R. G. Metabolism of Separated Leaf Cells: III. Effects of Calcium and Ammonium on Product Distribution During Photosynthesis with Cotton Cells. Plant Physiol. 1973 Jul;52(1):17–22. doi: 10.1104/pp.52.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R., Larson S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem Biophys Res Commun. 1969 Oct 8;37(2):278–282. doi: 10.1016/0006-291x(69)90731-1. [DOI] [PubMed] [Google Scholar]
- Vaccaro D., Zeldin M. H. Some Properties of Partially Purified Pyruvate Kinase from Euglena gracilis Klebs var. bacillaris. Plant Physiol. 1974 Oct;54(4):617–623. doi: 10.1104/pp.54.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]


