Abstract
We have developed a phytochrome immunoaffinity purification procedure that yields undegraded oat (Avena sativa L., cv. Garry) phytochrome of greater than 98% purity within 2 hours when starting with a brushite-purified preparation. Immunoaffinity-purified phytochrome, except for its greater purity, is indistinguishable from conventionally purified phytochrome by gel exclusion chromatography, isoelectric focusing, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. We have also used the immunoaffinity technique to purify phytochrome from crude oat extracts, and from brushite-purified pea (Pisum sativum L., cv. Alaska) and rye (Secale cereale L., cv. Balbo) preparations.
Full text
PDF
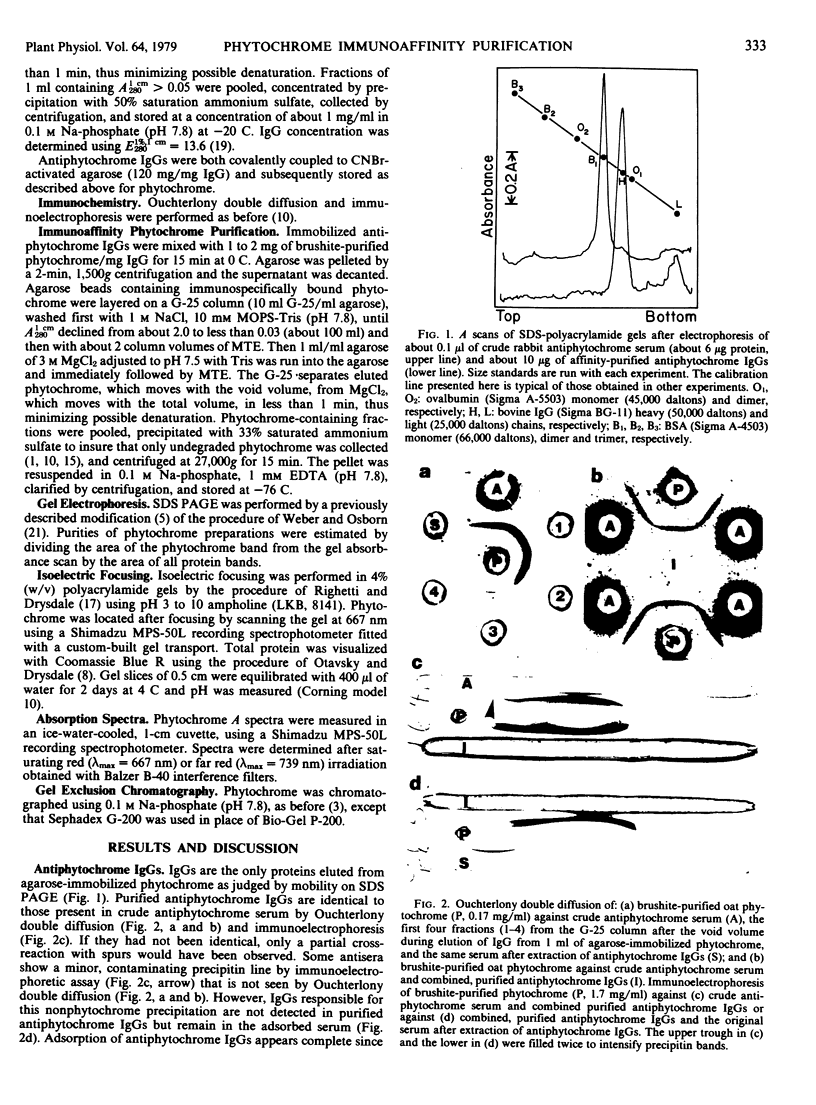
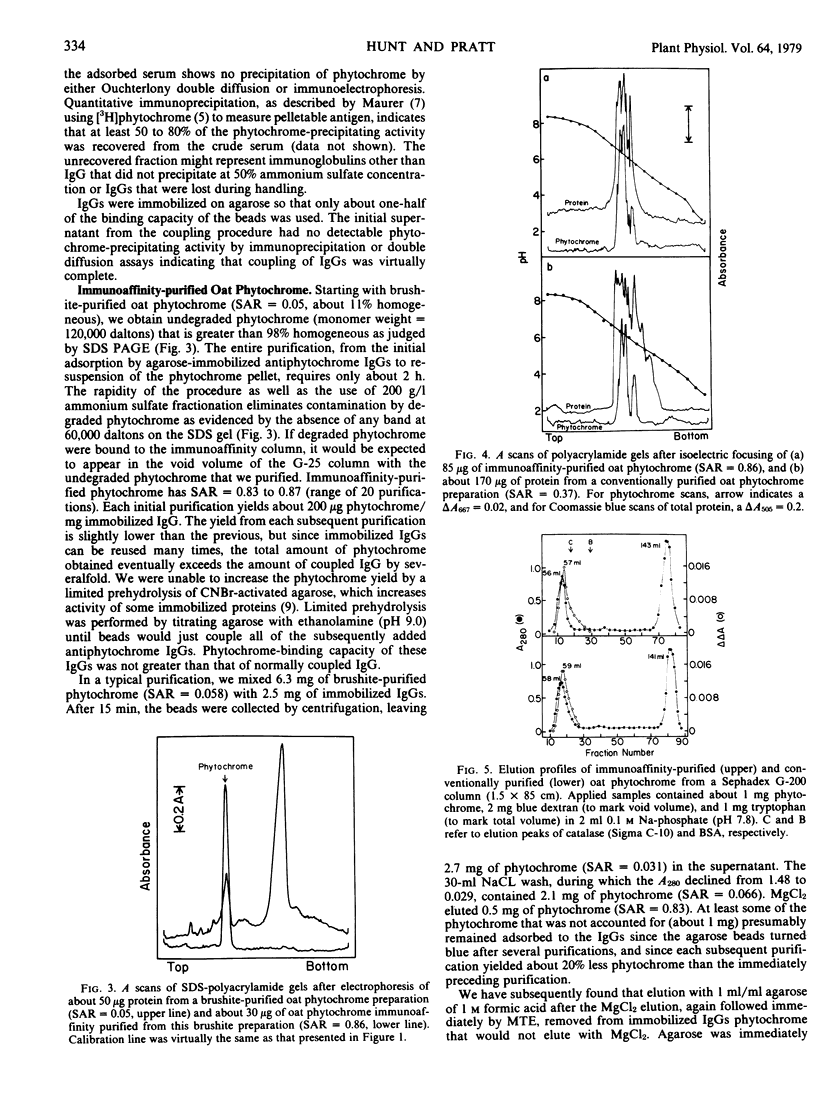
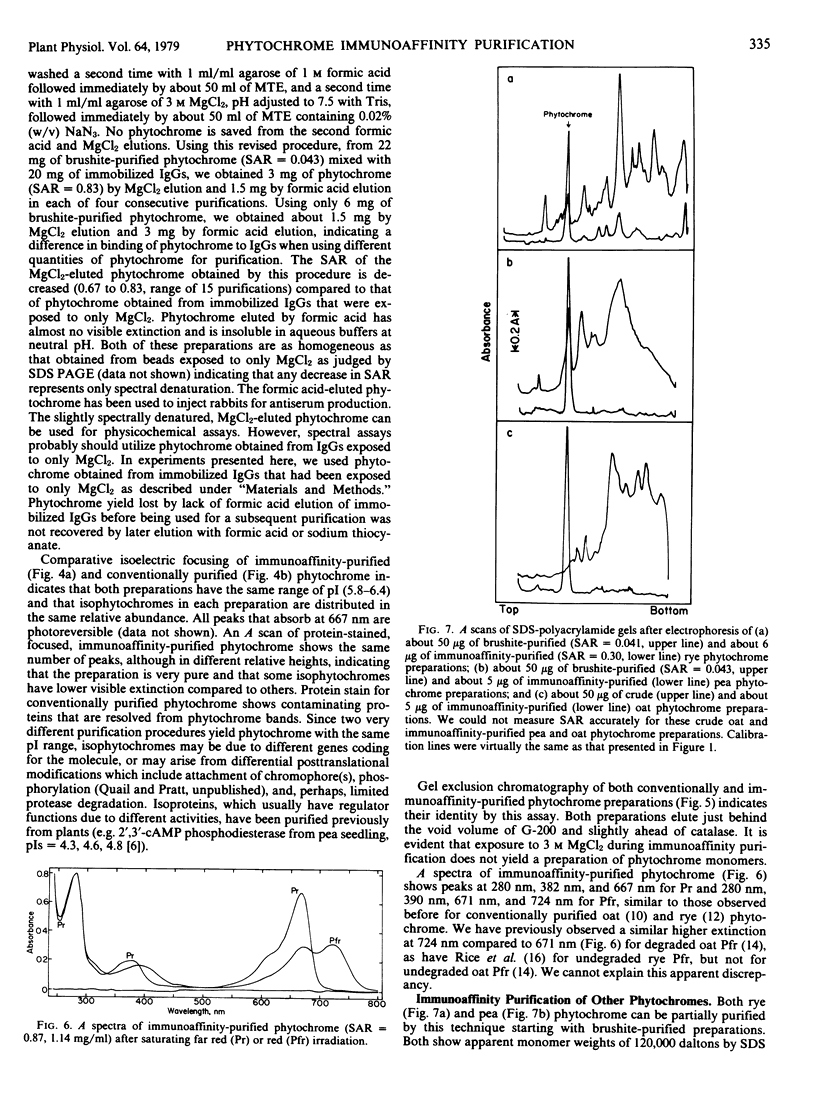

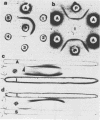
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Correll D. L., Edwards J. L. The aggregation States of phytochrome from etiolated rye and oat seedings. Plant Physiol. 1970 Jan;45(1):81–85. doi: 10.1104/pp.45.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Immunological and physical characterization of the products of phytochrome proteolysis. Plant Physiol. 1975 Feb;55(2):212–217. doi: 10.1104/pp.55.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome radioimmunoassay. Plant Physiol. 1979 Aug;64(2):327–331. doi: 10.1104/pp.64.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. P., Varner J. E. Cyclic nucleotide phosphodiesterase in pea seedlings. Biochim Biophys Acta. 1972 Aug 28;276(2):454–474. doi: 10.1016/0005-2744(72)91007-8. [DOI] [PubMed] [Google Scholar]
- Otavsky W. I., Drysdale J. W. Recent staining artifacts with LKB "Ampholines" on gel isoelectric-focusing. Anal Biochem. 1975 May 12;65(1-2):533–536. doi: 10.1016/0003-2697(75)90538-2. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Coleman R. A. Immunocytochemical localization of phytochrome. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2431–2435. doi: 10.1073/pnas.68.10.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Cundiff S. C. Spectral characterization of high-molecular-weight phytochrome. Photochem Photobiol. 1975 Feb;21(2):91–97. doi: 10.1111/j.1751-1097.1975.tb06634.x. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Kidd G. H., Coleman R. A. An immunochemical characterization of the phytochrome destruction reaction. Biochim Biophys Acta. 1974 Sep 13;365(1):93–107. doi: 10.1016/0005-2795(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R., Jackson-White C. J. Purification of oat and rye phytochrome. Plant Physiol. 1973 May;51(5):917–926. doi: 10.1104/pp.51.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- Tobin E. M., Briggs W. R. Studies on the protein comformation of phytochrome. Photochem Photobiol. 1973 Dec;18(6):487–495. doi: 10.1111/j.1751-1097.1973.tb06454.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]