Abstract
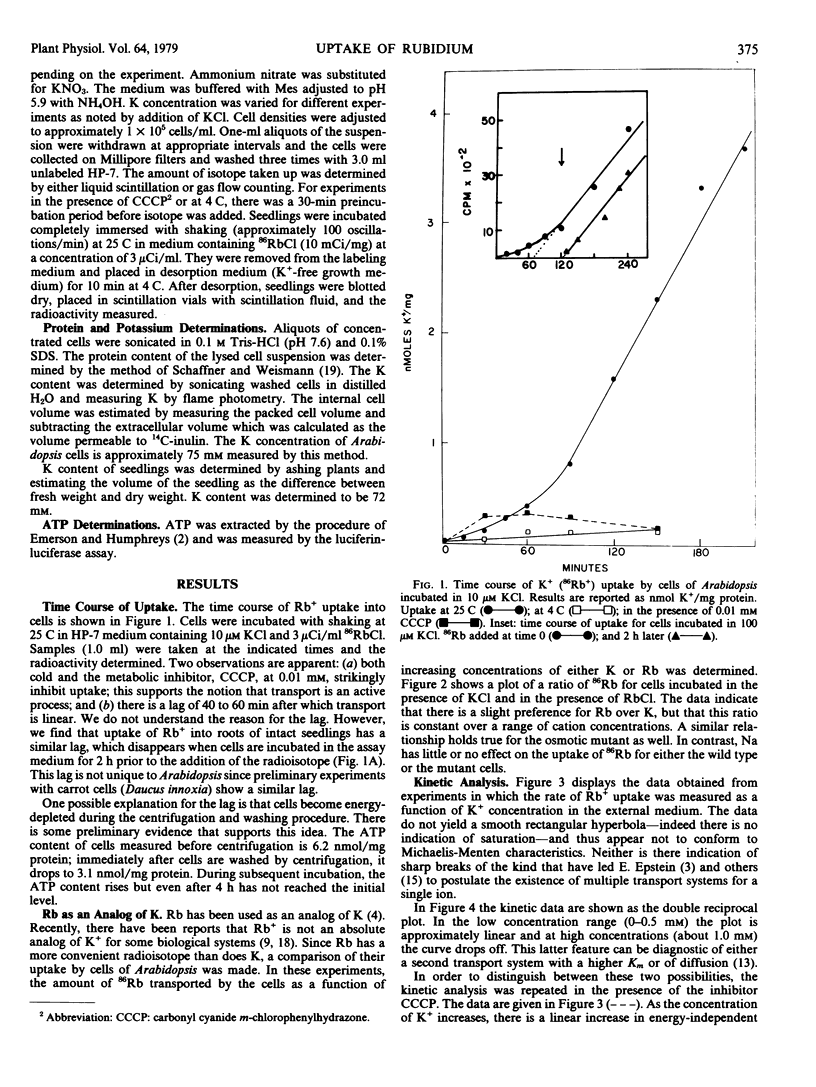
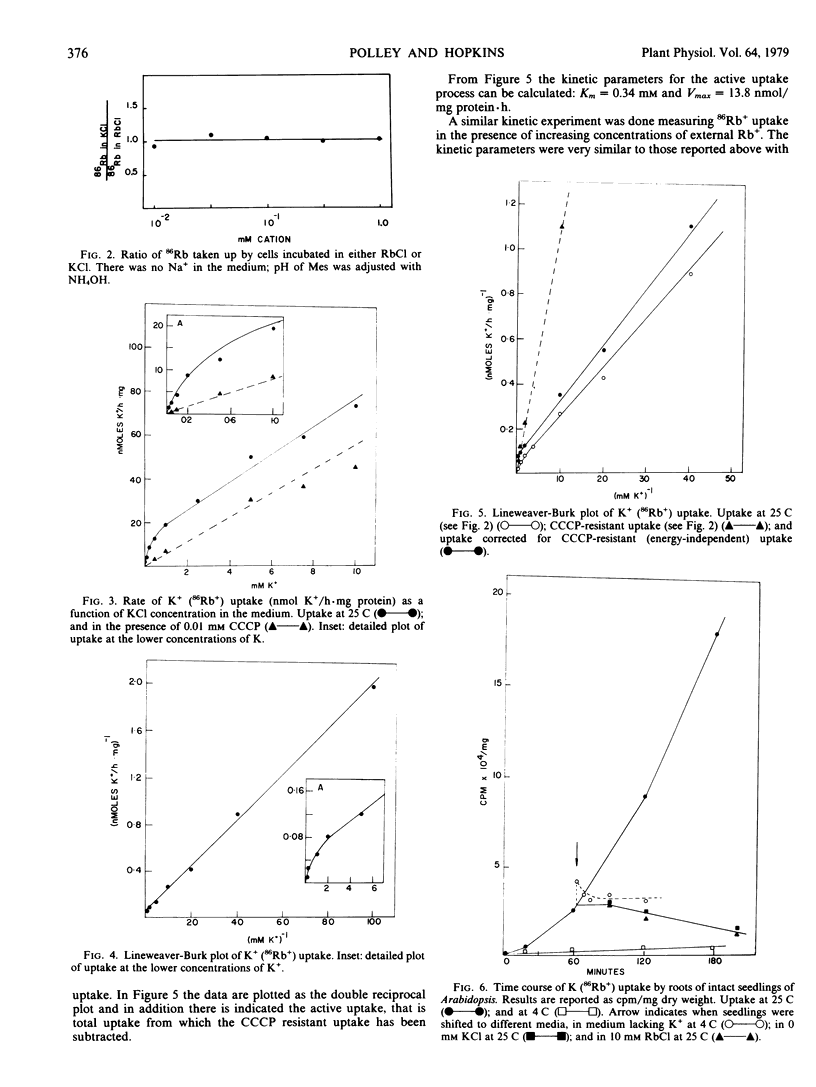
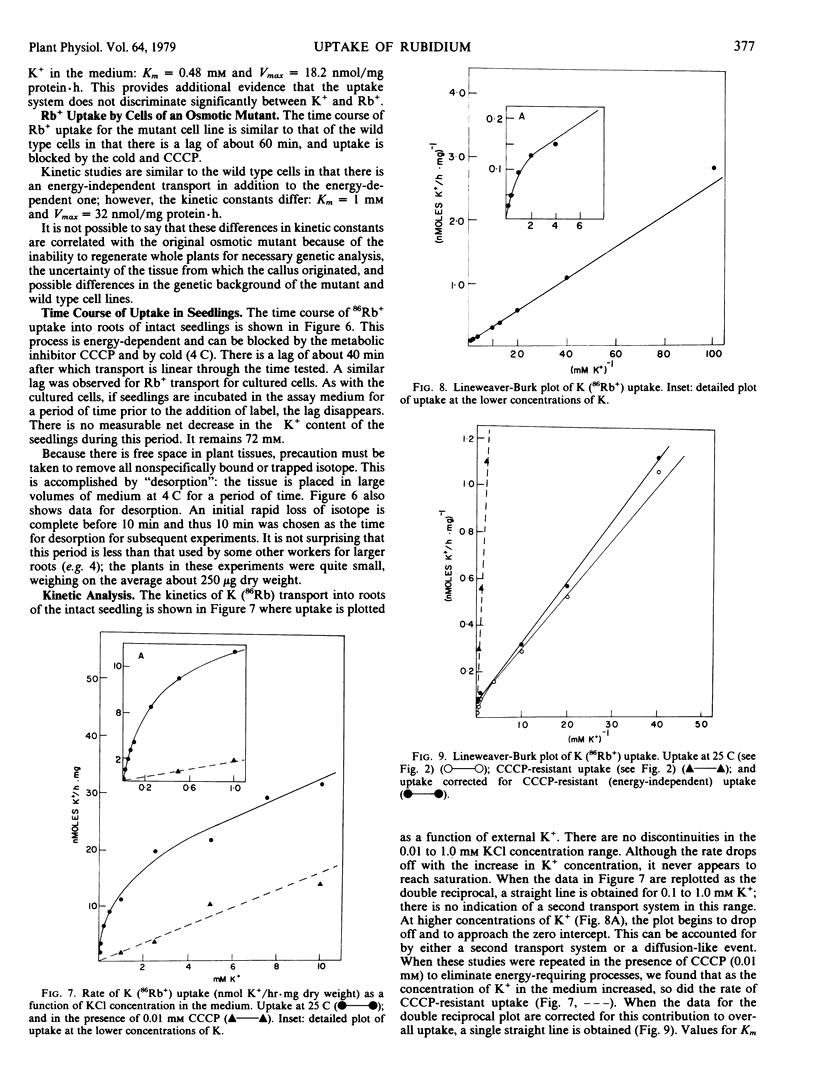
Experiments are reported in which the uptake of 86Rb+, used as an analog of K+, into cultured cells of Arabidopsis thaliana is investigated. A single transport system is found with Km = 0.34 millimolar and Vmax = 14 nmoles per milligram of protein per hour. This system is blocked by the metabolic inhibitor carbonyl cyanide m-chlorophenyl hydrazone (CCCP) and by cold. At high concentrations of external K+ (above 1 millimolar), a significant fraction of total uptake is energy-independent. No evidence is found for more than one energy-dependent uptake system or for concentration-dependent modifications of a carrier as postulated in multiphasic transport models.
Rb+ uptake was also examined in cultured cells derived from an “osmotic mutant” of Arabidopsis. The system closely resembles that found in wild type cells with the exception that the Michaelis-Menten constants are higher: Km = 1 millimolar and Vmax = 32 nanomoles per milligram of protein per hour.
The possibility that these results are artifacts associated with use of cultured cells was checked by examining 86Rb+ uptake by roots of intact seedlings of wild type Arabidopsis. A single energy-dependent transport system is found with Km = 0.42 millimolar which is not significantly different from the Km of cultured cells. There is also energy-independent uptake at high external ion concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emerson C. P., Jr, Humphreys T. A simple and sensitive method for quantitative measurement of cellular RNA synthesis. Anal Biochem. 1971 Apr;40(2):254–266. doi: 10.1016/0003-2697(71)90384-8. [DOI] [PubMed] [Google Scholar]
- Epstein E., Rains D. W., Elzam O. E. RESOLUTION OF DUAL MECHANISMS OF POTASSIUM ABSORPTION BY BARLEY ROOTS. Proc Natl Acad Sci U S A. 1963 May;49(5):684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D. Regulation of potassium absorption in barley roots: an allosteric model. Plant Physiol. 1976 Jul;58(1):33–37. doi: 10.1104/pp.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. The regulation of potassium absorption in barley roots. Plant Physiol. 1975 Sep;56(3):377–380. doi: 10.1104/pp.56.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. B., Woo A., Epstein W. Discrimination between Rb+ and K+ by Escherichia coli. Biochim Biophys Acta. 1977 Aug 15;469(1):45–51. doi: 10.1016/0005-2736(77)90324-8. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]


