Abstract
Analytical methodologies to comprehensively evaluate beef quality are increasingly needed to accelerate improvement in both breeding and post-mortem processing. Consumer palatability towards beef is generally attributed to tenderness, flavor, and/or juiciness. These primary qualities are modified by post-mortem aging and the crude content and fatty acid composition of intramuscular fat. In this study, we report a nuclear magnetic resonance (NMR)-based metabolic profiles of Japanese Black cattle to evaluate the compositional attributes of intramuscular fat and the long-term post-mortem aging. The unsaturation degree of triacylglycerol was estimated by the 1H NMR spectra and was correlated with the content ratio of unsaturated fatty acids (R 2 = 0.944) and the melting point of intramuscular fat (R 2 = 0.871). NMR-detected profiles of water-soluble metabolites revealed overall metabolic change (R 2 = 0.951) and several metabolites (R 2 > 0.818) linearly correlated with long-term aging duration, which can be used to evaluate the aging rate and aging duration of beef. This approach also provided the pH profile during aging, which is related to the water-holding capacity of beef. Thus, NMR-based metabolomics has the potential to evaluate multiple parameters related to the beef qualities of Japanese Black cattle.
Introduction
Beef is among the edible meats with high demand in many countries. Consumer palatability toward beef is generally attributed to tenderness, flavor, and/or juiciness1. The primary beef qualities are improved by post-mortem aging due to proteolysis within the muscle tissue structures, such as myofibrillar proteins, over time1–4. Beef metabolites are changed during aging, e.g., amino acid production by proteolytic degradation. Water-soluble metabolites, such as sugars and amino acids, contribute to the production of beef flavor with heat treatment through the Maillard reaction5. In Japanese Black cattle, beef quality has been improved, based on the bovine marbling score (BMS), through genetic selection. In addition to post-mortem aging, the crude fat and the fatty acid compositions attribute to the tenderness, flavor, and/or juiciness, which affect the palatability of Japanese Black cattle6–8. Among the fatty acids composing the intramuscular fat in beef, the content of unsaturated fatty acids is most strongly related to beef quality parameters, such as the strength of the aroma and flavor9.
There is an increasing need for holistic analytical techniques capable of rapidly assessing beef quality parameters. Impedance measurement is an analytical technique used for a broad range of beef quality parameters, such as fat content, tenderness, ultimate pH value and aging10. Spectroscopic techniques, such as near infrared reflectance spectroscopy (NIRS) and Raman spectroscopy, have potential use for the evaluation of tenderness and water-holding capacity10. These techniques also provide structural information on the changes of myofibrillar proteins, water and lipids. As non-destructive techniques, ultrasound and color images were applied to measure the texture and the content of intramuscular fat11. Several analytical techniques, such as gas-chromatography or liquid-chromatography coupled with mass spectrometry (GC-MS or LC-MS) and nuclear magnetic resonance (NMR) spectroscopy, are required to measure diverse types of low molecular weight metabolites, such as sugars, amino acids, nucleotides, lipids and aromatic compounds12. These techniques are often combined with chemometric analysis to evaluate the beef quality parameters13. NMR is a high-throughput analytical technique that enables simultaneous and reproducible detection of a large number of metabolites and systematic metabolic changes in biological samples14–18. NMR spectroscopy is used to obtain metabolic profiles of food samples without complicated manipulation19–23. Several NMR studies have been reported on the metabolic profiles of beef, which are related to conservation24, post-mortem aging25, 26, irradiation doses27, and geographic origins28.
In the present study, we aimed to identify NMR-detected metabolite indicators to simultaneously evaluate multiple determinants of primary beef quality, including the composition of intramuscular fat and the progression of the aging process, in advanced marbling meat from Japanese Black cattle. Although long-term aged beef (over 4 weeks) is often marketed as quality-modified meat of heifers and delivered cows, the metabolic profiles remain unknown in the long-term aging process. We analyzed the long-term changes in metabolites in wet-aging beef samples, which were strictly controlled in a laboratory. In addition, our approach evaluated several indicators related to beef quality, such as the composition of intramuscular fat.
Results and Discussion
Compositional analysis of sirloin lean tissues using 1H NMR spectra
1H NMR spectra were obtained from the D2O extracts of sirloin samples that are standard edible part in the experiments. Figure 1 shows a representative spectrum of a sirloin sample. Detected signals were derived from various water-soluble compounds, which were mainly extracted from sirloin lean tissues. Based on the 2D NMR analyses and the spiking experiments, 25 compounds were identified in the D2O extracts of the sirloin samples (Supplementary Table S1).
Figure 1.
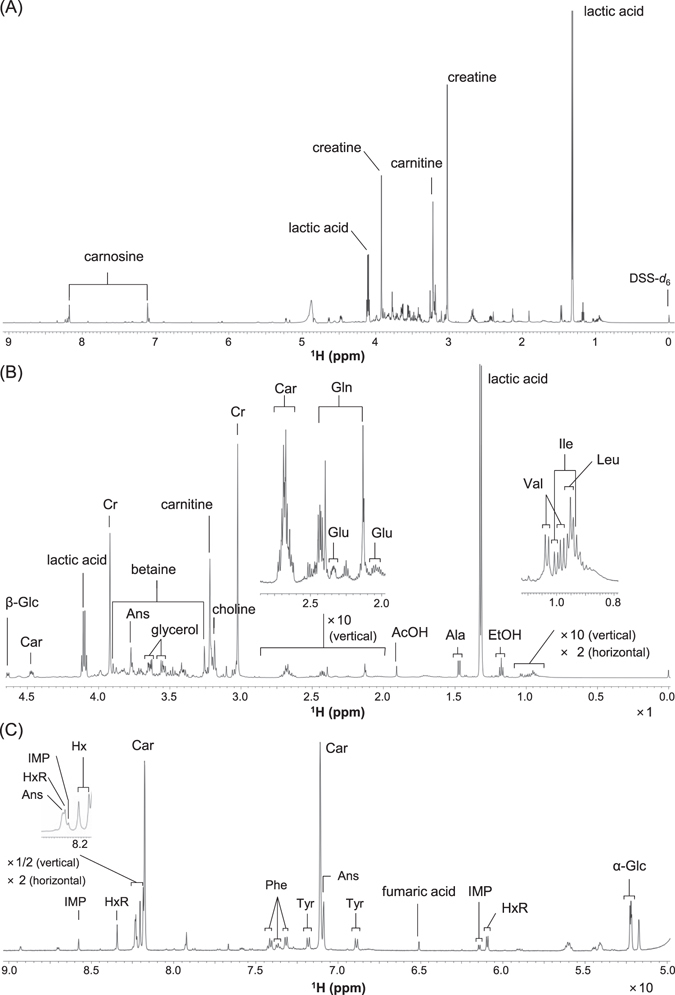
1H NMR spectra of the D2O extract of a sirloin sample from a brand A Black heifer (2-week aging). Assigned signals are labeled in (A) full spectrum, (B) high-field region, and (C) low-field region. AcOH, acetic acid; Ala, alanine; Ans, anserine; Car, carnosine; Cr, creatine; EtOH, ethanol; α-Glc, α-D-glucose; β-Glc, β-D-glucose; Gln, glutamine; Glu, glutamic acid; Hx, hypoxanthine; HxR, inosine; Ile, isoleucine; IMP, inosine monophosphate; Leu, leucine; Phe, phenylalanine; Tyr, tyrosine; and Val, valine.
Among the identified compounds, 13 compounds were amino acids (alanine, anserine, carnitine, carnosine, creatine, glutamic acid, glutamine, glycine, isoleucine, leucine, phenylalanine, tyrosine, and valine). Generally, amino acids contribute to various gustatory sensations29. For example, alanine, glutamine and glycine contribute to sweetness; isoleucine, leucine, phenylalanine, tyrosine and valine are associated with bitterness; and a glutamate is typically associated with umami29. Creatine is stored in muscle as creatine phosphate, which is used to resynthesize adenosine 5′-triphosphate (ATP) from adenosine 5′-diphosphate (ADP). Creatine is also converted to a brothy taste modifier, N-(4-methyl-5-oxo-1-imidazolin-2-yl)sarcosine, through an aminocarbonyl reaction with methylglyoxal during heat treatment30. Anserine and carnosine are classified as imidazole dipeptides. They are contained in large amounts in vertebrate muscles and prevent a decrease in pH by lactic acid accumulation during exercise31. Carnitine is a characteristic compound in beef; its content is higher in beef than in the meat of chicken and pig32.
α-Glucose and β-glucose were detected in a molar ratio of 8:9 in the D2O extracts of sirloin samples using 1H NMR spectroscopy. Although molar ratio of α-glucose to β-glucose is generally known to be 37:63 at pH 7, it is affected by solution conditions such as pH value33. Glucose is generally produced from glycogen stored in muscle. Inosine (HxR), inosine 5′-monophosphate (IMP) and hypoxanthine (Hx) were identified as purine derivatives, which are often produced by ATP hydrolysis during the aging process of beef samples12. IMP is a typical compound with an umami taste34. Acetic acid, fumaric acid and lactic acid were detectable organic acids in the D2O extracts of sirloin samples. Acetic acid is a volatile fatty acid (VFA) that is produced through cellulose degradation mediated by rumen bacteria. Fumaric acid is often used as a supplement to elevate the utilization efficiency of forage nutrition and the VFA concentration of fattening cattle35. Lactic acid showed the highest signal intensity among the signals of other compounds. Ethanol, glycerol, betaine and choline were also identified in the D2O extracts of sirloin samples. Betaine is biologically synthesized as a metabolite of choline and is also used as a food additive because of its taste modification effects.
Among the compounds identified in the D2O extracts, 21 compounds were quantified by comparing the integral values of well-separated signals (asterisks in Supplementary Table S1) with that of the DSS-d 6 signal (0 ppm). Table 1 summarizes the quantitative value of each compound. In accordance with the signal intensities, lactic acid was the most abundant compound in the D2O extracts. The amount was 23- and 63-fold those of acetic acid and fumaric acid, respectively, indicating that lactic acid is a major source of sour taste in beef. After slaughtering, lactic acid is dramatically increased through glycogen degradation and the growth of lactic acid bacteria under the anaerobic conditions of wet-aging36, affecting the ultimate pH, which contributes to meat tenderness and water-holding capacity37.
Table 1.
Contents of the Compounds Detected in the D2O Extracts.
| Compounds | Content (μmol/g meata) |
|---|---|
| Amino acids | |
| alanine | 0.203 ± 0.036b |
| carnitine | 0.377 ± 0.060 |
| carnosine | 0.567 ± 0.134 |
| creatine | 1.28 ± 0.24 |
| glutamic acid | 0.092 ± 0.012 |
| glutamine | 0.360 ± 0.083 |
| isoleucine | 0.008 ± 0.003 |
| leucine | 0.218 ± 0.038 |
| phenylalanine | 0.042 ± 0.006 |
| tyrosine | 0.036 ± 0.005 |
| valine | 0.070 ± 0.013 |
| Sugars | |
| α-glucose | 0.159 ± 0.019 |
| β-glucose | 0.180 ± 0.030 |
| Purine derivatives | |
| inosine | 0.057 ± 0.013 |
| inosine monophosphate | 0.045 ± 0.014 |
| Organic acids | |
| acetic acid | 0.115 ± 0.018 |
| fumaric acid | 0.043 ± 0.006 |
| lactic acid | 2.70 ± 0.47 |
| Alcohol | |
| ethanol | 0.056 ± 0.013 |
| glycerol | 0.460 ± 0.068 |
| Other compounds | |
| betaine | 0.089 ± 0.022 |
aSirloin from brand A Black heifers (2-week aging).
bMean ± standard deviation (n = 5).
NMR-based compositional analysis of sirloin intramuscular fat
Figure 2 shows a representative spectrum of the CDCl3 extract of a sirloin sample. The spectrum was consistent with the typical signal pattern of triacylglycerol (TAG), in which a glycerol backbone forms ester bonds with three fatty acid moieties. The signal pattern characterizing TAG was observed as triplicate signals of the glycerol backbone at 4.09 and 4.22 ppm (-CH 2-OCO-R) and 5.20 ppm (-CH-OCO-R). There was no signal from mono- and diacylglycerol in the 1H NMR spectra of the CDCl3 extract.
Figure 2.
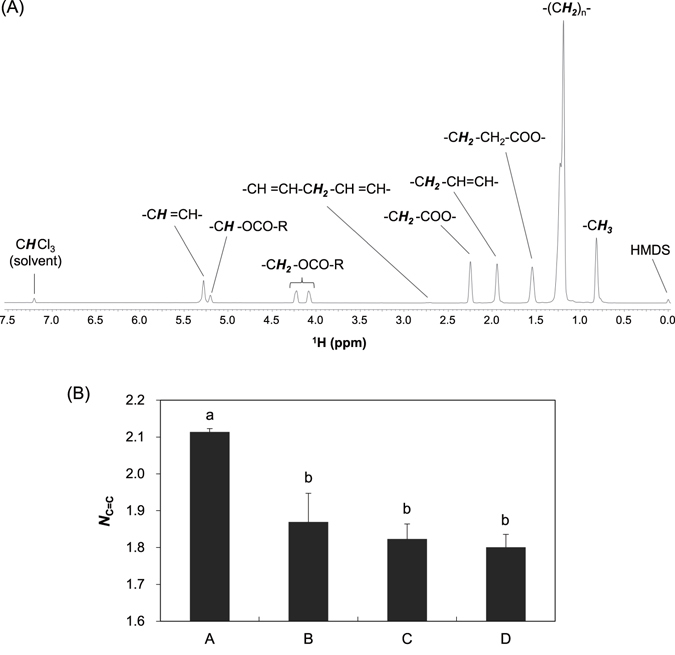
Triacylglycerol (TAG) and its unsaturation degree (N C=C) in the sirloin of Japanese Black cattle. (A) 1H NMR spectra of the CDCl3 extract of a sirloin sample after 2-week aging. Each signal is assigned to the proton represented by the italic and bold style (H) in the partial structural formula of TAG. (B) The N C=C values of the sirloin samples from four different brands. The data are presented as the mean ± standard error (SE; n = 5). Means with different letters (a and b) are significantly different at p < 0.05.
Although TAG can be composed of various types of fatty acids, only 7 major signals derived from fatty acid moieties were observed in the 1H NMR spectra (Fig. 2A). These signals were shifted in the spectra based on the relative position of double bond(s) and/or ester linkage. The signal at 5.28 ppm was derived from the protons directly attached to the carbon forming a double bond, which provided the double bond content in TAG as its integral value. The TAG content in the CDCl3 extract was represented as the integral of the signal at 4.09 or 4.22 ppm. Therefore, we estimated the unsaturation degree of TAG (N C=C) using the following equation (1) and the integral values of the signals at 5.28 ([CH=CH]) and 4.22 ppm (or 4.09 ppm, [TAG]).
| 1 |
The N C=C values of 20 sirloin samples (5 samples from each of the four brands) were calculated in the range from 1.69 to 2.14. Figure 2B shows the N C=C values of sirloin samples derived from different brands of Black heifers. The averaged N C=C values were 2.11, 1.87, 1.82, and 1.80 in the sirloin samples of brands A, B, C and D, respectively. Brand A had a significantly higher N C=C than the other brands, suggesting that brand A may be characterized by a high ratio of unsaturated fatty acids although the fatty acid compositions are influenced by the dietary backgrounds/production systems of the animals.
Correlation of NMR-detected NC=C values with intramuscular fat quality
The fatty acids in the intramuscular fat of beef were measured using GC-FID. We analyzed 7 major fatty acids in the intramuscular fat of sirloin using GC-FID and compared the total ratio of unsaturated fatty acids with the NMR-detected N C=C value. The ratio of each fatty acid was summarized as a weight percent in Table 2. Oleic acid (C18:1, cis-9) showed the highest ratio (>50 wt%) among the observed fatty acids. The ratios of other fatty acids decreased in the following order: palmitic acid (C16:0) > stearic acid (C18:0) > palmitoleic acid (C16:1, cis-9) > linoleic acid (C18:2, cis-9,12) > myristic acid (C14:0) > myristoleic acid (C14:1, cis-9). However, palmitoleic acid (C16:1, cis-9) was found at a higher content than stearic acid (C18:0) only in the sirloin samples of brand A, which may be the cause of the higher N C=C value of brand A. The total ratio of unsaturated fatty acids ranged from 60.2 wt% to 73.1 wt%, which was calculated by summation of the ratio of myristoleic acid (C14:1, cis-9), palmitoleic acid (C16:1, cis-9), oleic acid (C18:1, cis-9), and linoleic acid (C18:2, cis-9,12). The NMR-detected N C=C values were plotted against the total ratio of unsaturated fatty acids (Fig. 3A), which showed a good correlation between these two evaluated values (R 2 = 0.944). The results suggest that the total ratio of unsaturated fatty acids, which attribute to the tenderness, flavor, and/or juiciness6–8, can be estimated using the NMR-detected N C=C value.
Table 2.
Fatty Acid Composition of the Sirloin Derived from Japanese Black Cattle.
| Brand | Ratio (wt%) | MWb | N C=C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C14:1 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | UFAa | |||
| A | 2.5 | 2.0 | 21.8 | 8.1 | 5.5 | 56.2 | 3.9 | 70.1 | 851.1 | 2.10 |
| 2.0 | 1.6 | 18.7 | 7.0 | 6.3 | 60.3 | 4.2 | 73.1 | 856.3 | 2.13 | |
| 2.1 | 1.6 | 20.5 | 6.8 | 5.9 | 58.3 | 4.7 | 71.4 | 854.6 | 2.13 | |
| 2.0 | 1.8 | 19.7 | 7.8 | 5.6 | 59.0 | 4.0 | 72.7 | 854.2 | 2.13 | |
| 1.8 | 1.1 | 21.0 | 6.0 | 7.5 | 59.4 | 3.1 | 69.7 | 856.6 | 2.09 | |
| B | 2.9 | 0.9 | 26.8 | 5.5 | 9.2 | 51.8 | 2.8 | 61.1 | 850.9 | 1.75 |
| 1.9 | 1.2 | 19.2 | 5.5 | 7.4 | 60.4 | 4.4 | 71.5 | 858.2 | 2.12 | |
| 2.3 | 1.4 | 21.3 | 6.3 | 7.6 | 57.5 | 3.6 | 68.8 | 854.7 | 1.98 | |
| 2.9 | 0.9 | 26.6 | 4.9 | 10.1 | 52.2 | 2.4 | 60.4 | 851.8 | 1.73 | |
| 3.1 | 1.5 | 27.4 | 6.2 | 8.2 | 50.9 | 2.6 | 61.3 | 848.3 | 1.77 | |
| C | 3.6 | 1.1 | 27.6 | 6.5 | 7.8 | 50.2 | 3.3 | 61.1 | 847.8 | 1.73 |
| 2.8 | 1.5 | 25.4 | 6.1 | 8.3 | 52.9 | 3.0 | 63.5 | 850.5 | 1.81 | |
| 3.3 | 1.7 | 26.6 | 6.4 | 8.9 | 51.1 | 2.0 | 61.2 | 848.1 | 1.75 | |
| 2.2 | 1.1 | 22.4 | 5.5 | 9.3 | 56.5 | 3.0 | 66.1 | 855.3 | 1.93 | |
| 2.3 | 1.0 | 23.9 | 5.8 | 8.4 | 55.4 | 3.1 | 65.4 | 853.8 | 1.91 | |
| D | 1.5 | 0.7 | 23.6 | 4.9 | 8.9 | 57.8 | 2.6 | 66.0 | 857.2 | 1.82 |
| 1.9 | 0.9 | 21.2 | 4.9 | 10.3 | 57.5 | 3.2 | 66.6 | 857.8 | 1.83 | |
| 2.4 | 1.1 | 27.4 | 5.2 | 10.1 | 51.4 | 2.4 | 60.2 | 851.4 | 1.69 | |
| 2.5 | 1.2 | 25.3 | 5.1 | 10.2 | 52.8 | 3.1 | 62.1 | 852.9 | 1.77 | |
| 2.2 | 1.1 | 23.5 | 5.5 | 9.2 | 55.4 | 2.9 | 65.1 | 854.4 | 1.90 | |
aTotal ratio of unsaturated fatty acids.
bAveraged molecular weight of triacylglycerol (TAG).
Figure 3.
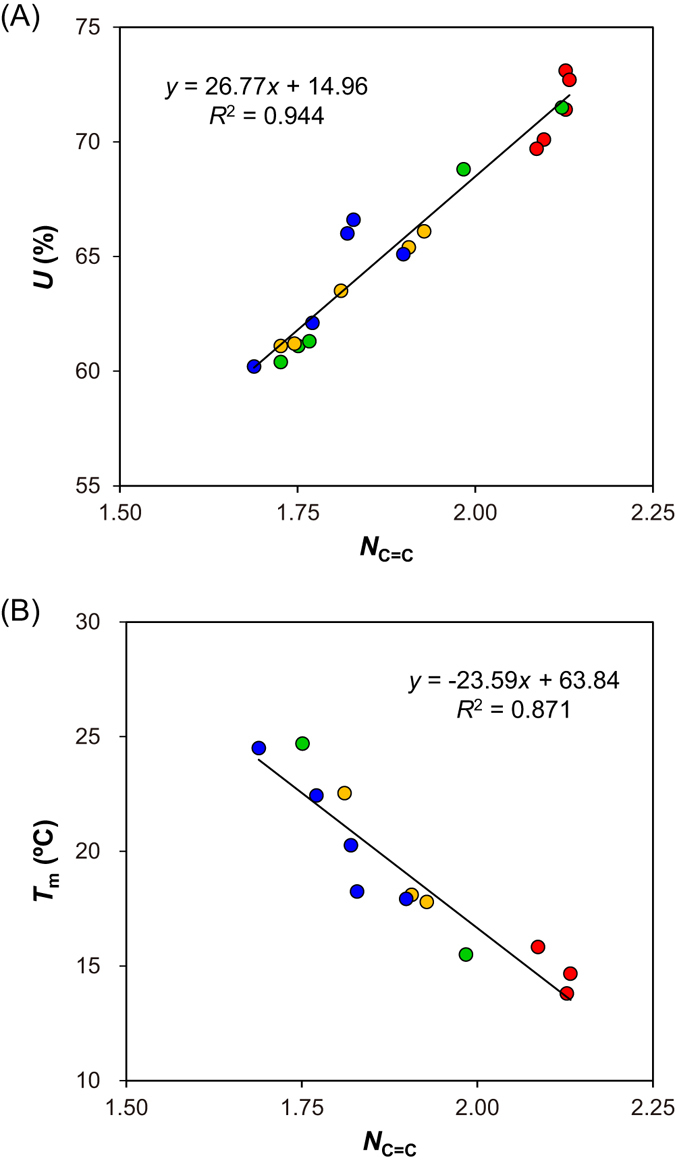
Correlation of the unsaturation degree of TAG (N C=C) with (A) the ratio of unsaturated fatty acids (U) and (B) the melting point (T m) of the sirloin samples. The plots are color-coded according to brand: A, red; B, green; C, yellow; and D, blue.
In general, the melting point of each fatty acid is related to its structure, including the numbers of double bonds and carbon atoms. We further analyzed the correlation between the NMR-detected N C=C and melting point. Thirteen sirloin samples were chosen to cover the broad range of the N C=C value: 3 brand A, 2 brand B, 3 brand C, and 5 brand D. In Fig. 3B, the NMR-detected N C=C values were plotted against the melting points of 13 sirloin samples. The plots showed a good correlation between the N C=C value and the melting point (R 2 = 0.871). The melting point was decreased in association with the increased N C=C value. The melting point is considered to be one of indicators of beef quality in Japanese Black cattle because it is related to tenderness and juiciness through the solubility of intramuscular fat in the mouth. The NMR-detected N C=C value provides a useful and simple method to evaluate the melting point of TAG in various food materials, such as beef, regardless of the melting point range.
Among the major indicators of edible fat and oil, the iodine value is widely used to evaluate the chemical and physicochemical properties, such as oxidative stability and fat firmness38. In a pure lipid compound, the iodine value (IV) is calculated based on its molecular weight (MW) and unsaturation degree (N C=C) according to the following equation (2) 39.
| 2 |
MWlipid and MWI represent the molecular weight of the lipid and iodine (253.8), respectively. Although the intramuscular fat of beef is composed of various types of lipids, the NMR-detected N C=C of TAG was used to calculate the iodine value of the sirloin samples in the present study. In addition, the MW of TAG in the samples was estimated based on the ratio and average MW of each fatty acid (Table 2) and was in the range from 847.9 to 858.2. The standard deviation was approximately 1.2% of the averaged MW of all samples (853.3), which is much lower than the standard deviation of N C=C (~23%). Therefore, it is possible to use the averaged MW to calculate the iodine value based on the NMR-detected N C=C value.
NMR-based evaluation of the long-term aging process
1H NMR spectra were obtained from the D2O and CDCl3 extracts of ribeye samples after different aging duration. Beef ribeye is a major edible part that is generally subjected to post-mortem aging. We chose both heifers and delivered cows to generalize the evaluation model of long-term post-mortem aging. The data set of spectral buckets was processed by multivariate data analysis to determine the overall compositional changes during aging. Figure 4A shows the PCA score plot of the D2O extracts of ribeye samples from 5 heifers and 4 delivered cows. All samples were distributed in the 95% confidence interval of the score plot with high statistical values of R 2 x (58.6% for PC1 and 15.3% for PC2). As aging proceeded, the plots shifted from positive to negative along the PC1 axis, suggesting that the compounds contributing to PC1 characterize the aging process. The loading plot of the PC1 axis (Fig. 4B) indicated that after longer aging duration, the ribeye samples contained higher amounts of acetic acid, alanine, glutamic acid, isoleucine, leucine, phenylalanine, tyrosine and valine, and lower amounts of carnitine, carnosine, creatine, IMP and lactic acid. PC2 reflected the differences between heifers and delivered cows, including the age-related influence. Based on the loading plot of the PC2 axis, the amount of alanine, anserine, betaine, fumaric acid and glutamine in heifers is higher than that in delivered cows (Supplementary Fig. S1).
Figure 4.
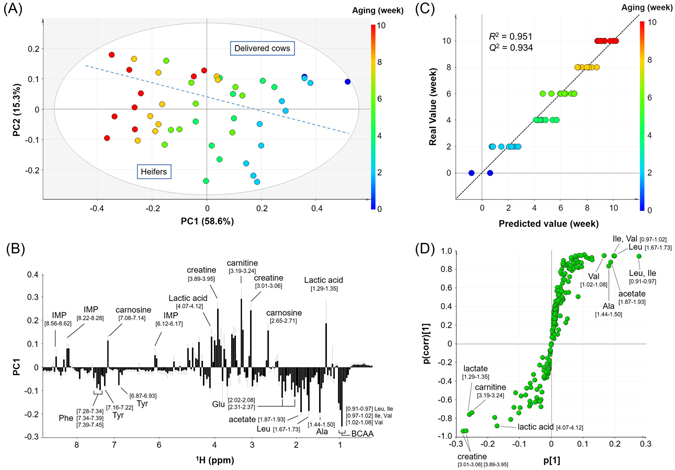
Multivariate data analysis of the 1H NMR spectra of the D2O extracts of ribeye samples with different aging duration. Ribeye samples are derived from heifers and delivered cows. (A) PCA score plot of PC1 versus PC2. The plots are color-coded according to aging duration (week): 0, blue; 2, cyan; 4, turquoise; 6, light green; 8, yellow; and 10, red. The heifer samples are positioned separately from the delivered cow on the score plot, and the individual regions are divided by a dashed line. (B) Loading plot for PC1 of the score plot shown in panel A. Buckets with high loading values are labeled by the assigned compound names. The square brackets represent the chemical shift range of each spectral bucket. (C) OPLS regression model for the prediction of aging duration. Predicted values are derived from the OPLS model of the 1H NMR spectra. The plots are color-coded according to aging duration (week): 0, blue; 2, cyan; 4, turquoise; 6, light green; 8, yellow; and 10, red. (D) S plot for the OPLS regression model shown in panel C. Buckets with high absolute values of p[1] and p(corr)[1] are labeled with the assigned compound names. The square brackets represent the chemical shift range of each spectral bucket.
The PCA score plot was also investigated for the CDCl3 extracts of ribeye samples (Supplementary Fig. S2). The R 2 x values were 56.4% for PC1 and 33.2% for PC2. In contrast to the D2O extracts, the distribution of samples did not provide information related to differences in aging duration. All heifer samples were separated from the delivered cow samples in the region of negative PC1 and positive PC2. The total fat contents were 38.6 ± 5.9 wt% and 17.3 ± 3.6 wt% in the ribeye samples from heifers and delivered cows, respectively. These results suggest that the TAG composition may be strongly influenced by the fattening process rather than the aging process.
According to the PCA results, we built an OPLS regression model to predict aging duration using the 1H NMR spectra of the D2O extracts. As shown in Fig. 4C, the actual values of aging duration were well correlated with the aging duration predicted by the NMR data. The goodness of fit (R 2) and the predictability (Q 2) were 0.951 and 0.934, respectively. The S plot for the OPLS regression model was used to evaluate the compounds related to aging (Fig. 4D). As suggested by the loading plot (Fig. 4B), aging duration was positively correlated with acetic acid, alanine, isoleucine, leucine and valine. Carnitine, creatine, and lactic acid showed a negative correlation with aging duration. To evaluate whether the contents of these compounds were linearly changed by an increased aging duration, the integral value of each bucket was further plotted against aging duration. As shown in Fig. 5, acetic acid, alanine, isoleucine, leucine and valine increased with aging. Leucine (a spectral bucket of 1.67‒1.73 ppm) showed the strongest positive correlation with aging duration (R 2 = 0.856). In contrast, creatine (a spectral bucket of 3.89‒3.95 ppm) had the strongest negative correlation with aging duration (R 2 = 0.828) among the other compounds, including carnitine and lactic acid.
Figure 5.
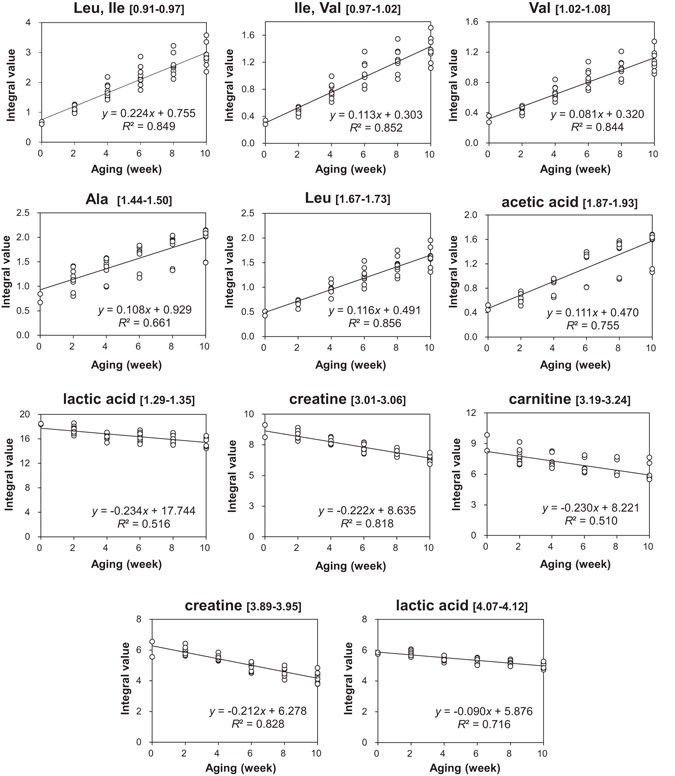
Changes in the contents of aging-related compounds, which are given as the integral values of individual buckets. The square brackets represent the chemical shift range of each spectral bucket, which is labeled by the assigned compound name.
The K value is a robust indicator of freshness and aging and is defined by the following equation (3) according to the content of nucleotides ([ATP], [ADP], [AMP] and [IMP]), inosine ([HxR]) and hypoxanthine ([Hx])40.
| 3 |
The 1H NMR spectra provided the total content of ATP, ADP, AMP and IMP as an integral of the signal at approximately 6.14 ppm (Supplementary Fig. S3). The contents of HxR and Hx were obtained from the signals at 6.10 and 8.20 ppm, respectively. Therefore, it was possible to calculate K using the 1H NMR spectra. Figure 6A shows the K values of ribeye samples after different aging durations. K reached 1.0 within 4 weeks after starting aging and was unchanged until 10 weeks. Therefore, the long-term aging (over 4 weeks) of ribeye could not be evaluated using K. Since the contents of leucine ([Leu]) and creatine ([Cr]) were correlated with long-term aging, the [Leu]/[Cr] ratio is expected to be useful evaluate long-term aging. The [Leu]/[Cr] ratio for aging evaluation was defined as “L” in the present study. The muscle tissue structures, such as myofibrillar proteins, are degraded to amino acids including Leu by post-mortem aging24. The 1H NMR spectra provided the content of leucine and creatine as the integral of the signals at 1.70 and 3.92 ppm, respectively. These signals were derived from two protons of the methylene group (-CH 2); therefore, the [Leu]/[Cr] ratio was calculated by dividing the integral value of the signal at 1.70 ppm by that at 3.29 ppm. The NMR-detected [Leu]/[Cr] ratios were plotted against aging duration (Fig. 6B) and showed a strong correlation (R 2 = 0.844). Larger variance in L was observed in the ribeye samples after longer aging duration, which may reflect the different aging rates among samples.
Figure 6.
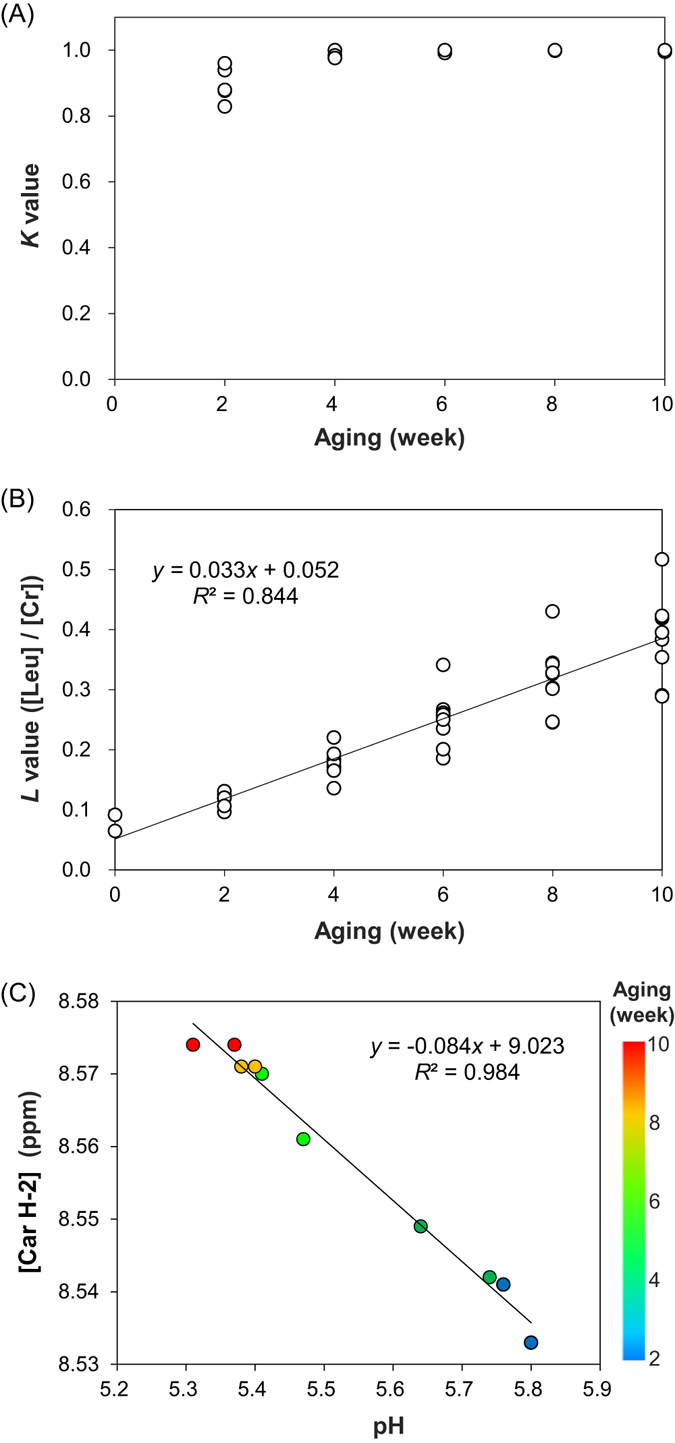
Indicators of aging progression. Correlation of aging duration with (A) K value and (B) L value, represented by the ratio of leucine ([Leu]) to creatine ([Cr]). (C) Correlation between pH and [Car H-2], represented by the chemical shift of the 1H signal at C-2 of the imidazole ring of carnosine (Car). The [Car H-2] value in the observed pH range shows a linear correlation with pH, although overall pH profile of [Car H-2] is sigmoidal42. The plots are color-coded according to aging duration (week): 2, blue; 4, green; 6, light green; 8, yellow; and 10, red.
After slaughtering, the pH of beef markedly decreases and reaches its ultimate pH by the production of lactic acid accompanying glycogen degradation41. pH changes have been reported during short-term post-mortem aging (within 3 weeks)42, 43. We observed the pH profile of long-term aging (over 4 weeks), which may be used as an indicator of aging progression. Since the D2O extract of ribeye contained carnosine, whose imidazole ring is affected by pH43, 44, we measured the chemical shift of the 1H signal at the C-2 position of the imidazole ring of carnosine [Car H-2] and the pH of ribeye samples with different aging durations. Figure 6C shows a strong correlation between [Car H-2] and pH (R 2 = 0.984). In addition, the pH decreased with aging progression. These results suggest that the D2O extract of ribeye provides the pH profile during aging. However, this pH profile was not consistent with the age-related decrease in lactic acid and may be affected by the buffering power of other components, such as creatine phosphate45. The content of acetic acid increased during long-term aging (Fig. 5), which may contribute to the decreased pH profile. pH is related to water-holding capacity, both in raw and heat-treated meats, and tenderness37. Therefore, the NMR-detected pH profile may be useful to evaluate meat quality during aging. As compared with regular muscle pH measurements, NMR is useful to measure the pH value under stable conditions such as temperature easily and reproducibly.
Conclusions
1H NMR spectra were applied to evaluate the quality of the lean tissues and intramuscular fat of Japanese Black cattle based on the D2O and CDCl3 extracts, respectively (Fig. 7). This approach revealed that the NMR-detected unsaturation degree of TAG (N C=C) was related to the compositional attributes of beef, such as the total ratio of unsaturated fatty acids, melting point, and iodine value (IV). The present study also shows NMR-based metabolic profiles of both polar and non-polar metabolites at longer-term post-mortem aging within 10 weeks. We found that leucine and creatine were available as biomarkers and were, respectively, positively and negatively correlated with aging duration. The L value (the ratio of leucine to creatine) was more appropriate to evaluate meat quality during long-term aging than the K value. Aging is a common processing technique used to improve meat quality. The 1H NMR spectra of the D2O extracts may be valuable to monitor the aging process through the detection of the L value with other metabolites and the pH profile, which also provides information on meat quality, such as the water-holding capacity and tenderness. This NMR-based metabolomics has potential to evaluate multiple beef qualities detected using different analytical techniques and can be applied to assess other edible meats.
Figure 7.
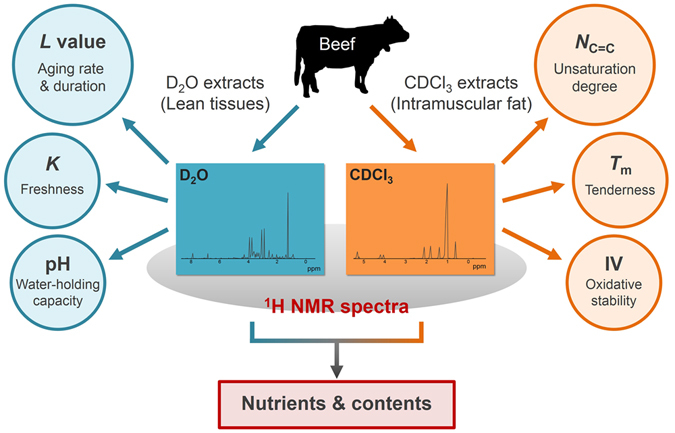
Multiple determinants of primary beef quality that are evaluated by the 1H NMR spectra of metabolite extracts from lean tissues and intramuscular fat.
Materials and Methods
Beef samples
We obtained beef samples from 29 Japanese Black cattle, which are composed of 25 heifers and 4 delivered cows of four brands in Japan (‘Yamagata beef’, ‘Hida beef’, ‘Sendai beef’, and ‘Kobe beef’) at the ages of 27.7‒39.6 and 103.1‒149.7 months, respectively. All samples were randomly chosen from commercially available dressed carcass at a local meat supplier; therefore, diet and production system were not controlled in the experiments. However, the samples from only a brand are considered to tend to show a similar fatty acid composition. To expand the variation of fatty acid composition, we chose four available brands, though the chosen samples may not be representative of each brand. All Japanese Black cattle are graded using the beef marbling standard (BMS), beef fat standard (BFS), and beef color standard (BCS) by the Japan Meat Grading Association (JMGA). The quality grades of all heifers were higher than Grade 4 (4 or 5). All cattle have been registered in the individual identification information database of the National Livestock Breeding Center, Japan.
Chemicals
Deuterated water (D2O, 99.9%) was purchased from Sigma-Aldrich Co. (St. Louis, Mo). All other regents were supplied from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Sample collection and preparation
Sirloin samples were collected from 5 Japanese Black heifers of 4 brands (total 20 samples). After the storage of slaughtered heifers at 2 °C for 3 days, sirloins were cut and vacuum-sealed. Wet-aging was performed by storing each sample at 2 °C for 2 weeks. Ribeye samples from 5 heifers and 4 delivered cows (‘Yamagata beef’) were treated using the same method as the sirloin samples and were then aged at 2 °C for 2, 4, 6, 8 or 10 weeks (total 42 samples including 2 non-aging samples from delivered cows). Each minced sample (250 mg) was disrupted using a Multi-beads Shocker (Yasui Kikai, Osaka, Japan; metal corn; 2500 rpm for 30 s) with 1.5 ml each of D2O and deuterated chloroform (CDCl3, 99.8%). The homogenate was centrifuged at 800 g for 10 min. The D2O and CDCl3 phases (each 1 ml) were separately transferred to microtubes and stored at −80 °C until use. The part of CDCl3 extracts was dried and weighed for lipid measurement.
NMR samples
The D2O extract (510 μl) was mixed with 130 μl of deuterated sodium phosphate (pH 7.0) and 10 μl of sodium 3-(trimethylsilyl)-1-propane- 1,1,2,2,3,3-d 6-sulfonate (DSS-d 6) at final concentrations of 0.1 M and 34.2 μM, respectively. For the pH evaluation, deuterated sodium phosphate was not added to the D2O samples. The CDCl3 extract (600 μl) was mixed with 10 μl of hexamethyldisiloxane (HMDS) at a final concentration of 0.45 mM. After centrifugation, the supernatants (600 μL) were transferred to 5-mm NMR tubes.
NMR spectroscopy
All NMR spectra were measured at 20 °C using a Varian Unity INOVA-600 spectrometer (599.78 MHz for 1H and 125.65 MHz for 13C) equipped with a cryogenic probe (Agilent Technologies, Ltd., Santa Clara, CA). 1H NMR spectra were measured with the following parameters: number of data points, 64 k; spectral width, 7,200 Hz; acquisition time, 4.55 s; delay time, 70.0 s (for D2O extracts) or 30.0 s (for CDCl3 extracts); and number of scans, 32. The delay time for quantification was determined by the spin-lattice relaxation time (T 1) measurement and was set to >5 × T 1. A ninety degree (90°) pulse was used as the RF pulse to acquire free-induction decay (FID). The water signal was suppressed by the presaturation method.
The parameters for 1H-1H gradient correlation spectroscopy (gCOSY) were as follows: number of data points, 2,048 (F1) and 512 (F2); spectral width, 7,200 Hz; acquisition time, 0.155 s; delay time, 2.0 s; and number of scans, 64. The 1H-13C heteronuclear single quantum coherence (HSQC) was obtained in the phase-sensitive mode with the following parameters: number of data points, 2,048 for 1H and 512 for 13C; spectral width, 7,200 Hz for 1H and 24,128 Hz for 13C; acquisition time, 0.155 s; delay time, 7.5 s; and number of scans, 64. The 1H-13C heteronuclear multiple-bond correlation (HMBC) was measured in absolute mode, and the acquisition parameters were as follows: number of data points, 2,048 for 1H and 512 for 13C; spectral width, 7,200 Hz for 1H and 35,445 Hz for 13C; acquisition time, 0.162 s; delay time, 3.5 s; and number of scans, 128.
Data processing and NMR signal assignment
NMR data were processed using ACD/NMR Workbook Suite V2012 (Advanced Chemistry Development, Inc., Toronto, Canada). Phase and baseline corrections were manually adjusted on the software. The signal assignments were performed by comparison with the published data for beef samples13, 24. For the assignment of unreported signals, we predicted candidate compounds using the Biological Magnetic Resonance Bank (BMRB)46. Finally, we confirmed that the signals were correctly assigned to the candidate compounds using 2D NMR spectra and spiking experiments21. The integral value of each signal was calculated using ACD/NMR Workbook Suite V2012. The integral value of the DSS-d 6 signal at 0 ppm (the trimethylsilyl protons) was used as an internal standard for the quantification of D2O extracts.
GC-FID analysis of the fatty acid composition
Gas chromatography-flame ionization detection (GC-FID) was applied to determine the fatty acid composition of beef tallow. Total lipids were extracted using the Folch method47 and were dissolved in benzene. After heating at 80 °C for 20 min with sodium methoxide methanol, methyl-esterified fatty acids were analyzed using a Shimadzu GC-FID 2010 system (Shimadzu Corporation, Kyoto, Japan). Separation was achieved using an InertCap Pure WAX capillary column (30 m × 0.250 mm × 0.25 μm; GL Sciences Inc., Tokyo, Japan) and helium as the carrier gas. The injector and detector temperatures were 240 and 250 °C, respectively. The oven temperature was held at 210 °C for 8 min and was then increased to 230 °C at a rate of 20 °C/min. Finally, the temperature was held at 230 °C for 2 min. The total fatty acid content was calculated by summing the contents of 7 major fatty acids: myristic acid (C14:0), myristoleic acid (C14:1, cis-9), palmitic acid (C16:0), palmitoleic acid (C16:1, cis-9), stearic acid (C18:0), oleic acid (C18:1, cis-9), and linoleic acid (C18:2, cis-9,12).
Melting point measurement of intramuscular fat
The melting point was measured using the ascending heat method employing a glass capillary48. Heat-fused intramuscular fat was filled into the glass capillary to a height of 1 cm and was then stored at −30 °C until use. The frozen capillary was placed in a water bath that was initially set to 5 °C. The water temperature was gradually increased using a hot stirrer. When the fused beef tallow rose 1 cm, the temperature was recorded as the melting point.
pH measurement
The pH of the D2O extracts of the beef samples was measured at room temperature before NMR measurements using pH electrodes (HORIBA, Ltd., Kyoto, Japan).
Statistical data analysis
For the multivariate data analysis, the 1H NMR spectra of D2O extracts (0.0‒9.0 ppm) were reduced into 0.04 ppm spectral buckets using the ACD/NMR Workbook Suite V2012. The region of the water signal (4.70‒5.15 ppm) was removed, and the sum of spectral buckets was set to 100. Similarly, the 1H NMR spectra of the CDCl3 extracts (0.5‒5.5 ppm) were reduced into 0.04 ppm spectral buckets and normalized.
The resulting data sets were imported into SIMCA-P version 13 (Umetrics, Umeå, Sweden) as X variables, and Pareto was chosen as the scaling method. Principle component analysis (PCA) was performed as an unsupervised classification method to examine the variation in the data sets. Discriminant analyses based on orthogonal projection to latent structures (OPLS-DA) was used to assess the chemical composition of the ribeye samples with different aging durations, which were imported as Y variables and were scaled with unit variance (UV). The modeled variation in the score plot was defined with a 95% confidence interval. The goodness of fit (R 2) and the predictability (Q 2) described the quality of the OPLS regression model.
Mean values, standard errors and regression lines were calculated using Microsoft Excel 2016 (Microsoft Corp., San Leonardo, CA). Significant differences were analyzed by one-way analysis of variance (ANOVA) using EZR, an interface program of R49.
Electronic supplementary material
Acknowledgements
We are grateful to Prof. Keiichi Suzuki (Tohoku University, Japan), who provided all the Japanese Black cattle beef samples. This study was supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry from the Ministry of Agriculture, Forestry and Fisheries, Japan.
Author Contributions
M.T., T.K. plan the project, Y.K., T.M., T.K. performed the experiments and data analyses, T.M., M.T. wrote the paper, M.T. edited the manuscript. All authors discussed the results.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yoshinori Kodani and Takuya Miyakawa contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01272-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Filho LA, et al. Hanging the beef carcass by the forequarter to improve tenderness of the Longissimus Dorsi and Biceps Femoris muscles. Sci. Agric. 2005;62:483–486. doi: 10.1590/S0103-90162005000500013. [DOI] [Google Scholar]
- 2.Campbell RE, Hunt MC, Levis P, Chambers E. Dry-aging effects on palatability of beef longissimus muscle. J. Food Sci. 2001;66:196–199. doi: 10.1111/j.1365-2621.2001.tb11315.x. [DOI] [Google Scholar]
- 3.Tornberg E. Biophysical aspects of meat tenderness. Meat Sci. 1996;43:175–191. doi: 10.1016/0309-1740(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 4.Kemp CM, Sensky PL, Bardsley RG, Buttery PJ, Parr T. Tenderness — An enzymatic view. Meat Sci. 2010;84:248–256. doi: 10.1016/j.meatsci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Maillard LC. Action des acides aminés sur les sucres; formation des mélanoïdines par voie méthodique. C. R. Acad. Sci. 1912;154:66–68. [Google Scholar]
- 6.Busboom JR, et al. Effects of biological source on cooking and palatability attributes of beef produced for the Japanese market. Meat Sci. 1993;35:241–258. doi: 10.1016/0309-1740(93)90054-L. [DOI] [PubMed] [Google Scholar]
- 7.Iida F, Saitou K, Kawamura T, Yamaguchi S, Nishimura T. Effect of fat content on sensory characteristics of marbled beef from Japanese Black steers. Anim. Sci. J. 2015;86:707–715. doi: 10.1111/asj.12342. [DOI] [PubMed] [Google Scholar]
- 8.Iida F, et al. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese Black cattle. Meat Sci. 2016;112:46–51. doi: 10.1016/j.meatsci.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki, K. et al. Search for an index for the taste of Japanese Black cattle beef by panel testing and chemical composition analysis. Anim. Sci. J. 88, 421–432, doi: 10.1111/asj.12663 (2017). [DOI] [PubMed]
- 10.Damez JL, Clerjon S. Meat quality assessment using biophysical methods related to meat structure. Meat Sci. 2008;80:132–149. doi: 10.1016/j.meatsci.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Nunes JL, et al. Beef quality parameters estimation using ultrasound and color images. MBC Bioinformatics. 2015;16:S6. doi: 10.1186/1471-2105-16-S4-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koutsidis G, et al. Water-soluble precursors of beef flavour. Part II: effect of postmortem conditioning. Meat Sci. 2008;79:124–130. doi: 10.1016/j.meatsci.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Graham SF, et al. Comparing GC-MS, HPLC and ¹H NMR analysis of beef longissimus dorsi tissue extracts to determine the effect of suspension technique and ageing. Food Chem. 2012;134:1633–1639. doi: 10.1016/j.foodchem.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Beckonert O, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 15.Bertram HC, Eggers N, Eller N. Potential of human saliva for nuclear magnetic resonance-based metabolomics and for health-related biomarker identification. Anal. Chem. 2009;81:9188–9193. doi: 10.1021/ac9020598. [DOI] [PubMed] [Google Scholar]
- 16.Lamichhane S, et al. Strategy for Nuclear-Magnetic-Resonance-Based Metabolomics of Human Feces. Anal. Chem. 2015;87:5930–5937. doi: 10.1021/acs.analchem.5b00977. [DOI] [PubMed] [Google Scholar]
- 17.Hu F, Furihata K, Ito-Ishida M, Kaminogawa S, Tanokura M. Nondestructive observation of bovine milk by NMR spectroscopy: analysis of existing States of compounds and detection of new compounds. J. Agric. Food Chem. 2004;52:4969–4974. doi: 10.1021/jf049616o. [DOI] [PubMed] [Google Scholar]
- 18.Wei F, Furihata K, Hu F, Miyakawa T, Tanokura M. Two-dimensional 1H-13C nuclear magnetic resonance (NMR)-based comprehensive analysis of roasted coffee bean extract. J. Agric. Food Chem. 2011;59:9065–9073. doi: 10.1021/jf201716w. [DOI] [PubMed] [Google Scholar]
- 19.Wei F, et al. F2-selective two-dimensional NMR spectroscopy for the analysis of minor components in foods. J. Agric. Food Chem. 2012;60:1005–1012. doi: 10.1021/jf205315r. [DOI] [PubMed] [Google Scholar]
- 20.Koda M, Furihata K, Wei F, Miyakawa T, Tanokura M. Metabolic discrimination of mango juice from various cultivars by band-selective NMR spectroscopy. J. Agric. Food Chem. 2012;60:1158–1166. doi: 10.1021/jf2041438. [DOI] [PubMed] [Google Scholar]
- 21.Koda M, Furihata K, Wei F, Miyakawa T, Tanokura M. NMR-based metabolic profiling of rice wines by F2-selective total correlation spectra. J. Agric. Food Chem. 2012;60:4818–4825. doi: 10.1021/jf3008647. [DOI] [PubMed] [Google Scholar]
- 22.Wei F, Furihata K, Miyakawa T, Tanokura M. A pilot study of NMR-based sensory prediction of roasted coffee bean extracts. Food Chem. 2014;152:363–369. doi: 10.1016/j.foodchem.2013.11.161. [DOI] [PubMed] [Google Scholar]
- 23.Liang T, et al. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015;63:683–691. doi: 10.1021/jf504836d. [DOI] [PubMed] [Google Scholar]
- 24.Graham SF, et al. The application of NMR to study changes in polar metabolite concentrations in beef longissimus dorsi stored for different periods post mortem. Metabolomics. 2010;6:395–404. doi: 10.1007/s11306-010-0206-y. [DOI] [Google Scholar]
- 25.Kim YH, Kemp R, Samuelsson LM. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016;111:168–176. doi: 10.1016/j.meatsci.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Castejón D, García-Segura JM, Escudero R, Herrera A, Cambero MI. Metabolomics of meat exudate: Its potential to evaluate beef meat conservation and aging. Anal. Chim. Acta. 2015;901:1–11. doi: 10.1016/j.aca.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Zanardi E, et al. Metabolic profiling by 1H NMR of ground beef irradiated at different irradiation doses. Meat Sci. 2015;103:83–89. doi: 10.1016/j.meatsci.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Jung Y, et al. Discrimination of the geographical origin of beef by 1H NMR-based metabolomics. J. Agric. Food Chem. 2010;58:10458–10466. doi: 10.1021/jf102194t. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura T, Kato H. Taste of free amino acids and peptides. Food Rev. Int. 2010;4:175–194. doi: 10.1080/87559128809540828. [DOI] [Google Scholar]
- 30.Shima K, Yamada N, Suzuki E, Harada T. Novel brothy taste modifier isolated from beef broth. J. Agric. Food Chem. 1998;46:1465–1468. doi: 10.1021/jf9708709. [DOI] [Google Scholar]
- 31.Aruoma OI, Laughton MJ, Halliwell B. Carnosine, homocarnosine and anserine: could they act as antioxidants in vivo? Biochem. J. 1989;264:863–869. doi: 10.1042/bj2640863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada K, et al. Species and muscle differences in L-carnitine levels in skeletal muscles based on a new simple assay. Meat Sci. 2004;68:357–362. doi: 10.1016/j.meatsci.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Toghill KE, Compton RG. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010;5:1246–1301. [Google Scholar]
- 34.Maga, J. A. Flavor of Meat and Meat Products (ed. Shahide, S.) 98–115 (Blackie Academic and Professional: London, 1994).
- 35.Beauchemin K, McGinn S. Methane emissions from beef cattle: Effects of fumaric acid, essential oil, and canola oil. J. Anim. Sci. 2006;84:1489–1496. doi: 10.2527/2006.8461489x. [DOI] [PubMed] [Google Scholar]
- 36.Huis in’t Veld JHJ. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996;33:1–18. doi: 10.1016/0168-1605(96)01139-7. [DOI] [PubMed] [Google Scholar]
- 37.Gault NF. The relationship between water-holding capacity and cooked meat tenderness in some beef muscles as influenced by acidic conditions below the ultimate pH. Meat Sci. 1985;15:15–30. doi: 10.1016/0309-1740(85)90071-3. [DOI] [PubMed] [Google Scholar]
- 38.Gray JI. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978;55:539–546. doi: 10.1007/BF02668066. [DOI] [Google Scholar]
- 39.Ham B, Shelton R, Butler B, Thionville P. Calculating the iodine value for marine oils from fatty acid profiles. J. Am. Oil Chem. Soc. 1998;75:1445–1446. doi: 10.1007/s11746-998-0197-2. [DOI] [Google Scholar]
- 40.Saito T, Arai K, Matsuyoshi M. A new method for estimating the freshness of fish. Bull. Jap. Soc. Sci. Fish. 1959;24:749–750. doi: 10.2331/suisan.24.749. [DOI] [Google Scholar]
- 41.Mungure TE, Bekhit A-D, Birch EJ, Stewart I. Effect of rigor temperature, ageing and display time on the meat quality and lipid oxidative stability of hot boned beef Semimembranosus muscle. Meat Sci. 2016;114:146–153. doi: 10.1016/j.meatsci.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Gudjónsdóttir M, et al. Effects of electrospun chitosan wrapping for dry-ageing of beef, as studied by microbiological, physicochemical and low-field nuclear magnetic resonance analysis. Food Chem. 2015;184:167–175. doi: 10.1016/j.foodchem.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 43.Tanokura M, Tasumi M, Miyazawa T. 1H nuclear magnetic resonance studies of histidine-containing di- and tripeptides. Estimation of the effects of charged groups on the pKa value of the imidazole ring. Biopolymers. 1976;15:393–401. doi: 10.1002/bip.1976.360150215. [DOI] [PubMed] [Google Scholar]
- 44.Baryshnikova OK, Williams TC, Sykes BD. Internal pH indicators for biomolecular NMR. J. Biomol. NMR. 2008;41:5–7. doi: 10.1007/s10858-008-9234-6. [DOI] [PubMed] [Google Scholar]
- 45.Tanokura M, Yamada K. Changes in intracellular pH and inorganic phosphate concentration during and after muscle contraction as studied by time-resolved 31P-NMR. FEBS Lett. 1984;171:165–168. doi: 10.1016/0014-5793(84)80480-9. [DOI] [PubMed] [Google Scholar]
- 46.Ulrich EL, et al. BioMagResBank. Nucleic Acids Res. 2008;36:D402–D408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folch J, Lees M, Sloane Sanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 48.The Japanese pharmacopoeia 16th edition (JP16) http://jpdb.nihs.go.jp/jp16e/000152908.pdf (2011).
- 49.Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


