Abstract
Lung adenocarcinoma (AC) and squamous cell lung carcinoma (SCC) are two major subtypes of non-small cell lung cancer (NSCLC). Previous studies have demonstrated that fundamental differences exist in the underlying mechanisms of tumor development, growth and invasion between these subtypes. The investigation of differentially-expressed genes (DEGs) between these two NSCLC subtypes is useful for determining and understanding such differences. The present study aimed to identify those DEGs using meta-analysis and the data from four microarray experiments, consisting of 164 AC and 161 SCC samples. Raw gene expression values were converted into the probability of expression (POE) representing the differentially-expressed probability of a gene and expression barcode values representing its expression status. The results indicated that when applying a meta-analysis using barcode values, heterogeneity in genes across studies was less severe than when applying a meta-analysis using POE values. DEGs in each meta-analysis method overlapped substantially (P=1.3×10−4), but the barcode method yielded a lower global false discovery rate. Based on this and several other performance statistics, it was concluded that the barcode approach outperformed the POE method. Finally, using those DEGs, ontology and pathway analyses were conducted. A number of genes and enriched pathways were found to be closely associated with NSCLC.
Keywords: meta-analysis, non-small cell lung cancer, adenocarcinoma, squamous cell carcinoma
Introduction
Adenocarcinoma (AC) and squamous cell carcinoma (SCC) are two major histological types of non-small cell lung cancer (NSCLC), and it has previously been demonstrated that their underlying mechanisms, including tumor development, growth and invasion, are quite different (1). In clinical practice, however, homogeneous treatment strategies have been traditionally implemented for each subtype (2). The poor treatment response of NSCLC may be due to such indifferent treatment strategies for two fundamentally different subtypes. Therefore, a better understanding of their pathogenesis is critical for finding subtype-specific treatment strategies. The investigation of differentially-expressed genes (DEGs) between these two NSCLC subtypes is useful for determining and understanding the biological differences between these two diseases.
A number of studies exist with the objective of identifying DEGs between AC and SCC subtypes. Such investigation enhances our understanding of the cellular and molecular differences between these two subtypes (3–6). However, inconsistencies among those studies, due to small sample sizes and different microarray platforms and analysis techniques used (7), make the integration of results from multiple similar studies difficult. In order to gain more robust, reproducible and accurate results by combining multiple studies with the same objective, meta-analysis methods have been increasingly applied to microarray data.
For instance, previous studies obtained overall summary statistics based on either P-values or effect sizes of each individual study to conduct a meta-analysis (8,9). Moreover, Choi et al (10) proposed a novel method that combined the probability of expression (POE), which is calculated based on the relative expression levels of one phenotype versus the other in individual studies, and then applied this method to identify genes capable of discriminating metastatic and primary tumors. This so-called POE method makes direct comparison of gene expression values from different studies more feasible. In the present study, this method was applied to identify DEGs between early-stage AC and SCC lung cancers.
The Gene Expression Barcode is a novel algorithm in which the absolute expression value of a gene in a specific sample is used to determine its expression status (11). Namely, genes are coded as expressed or unexpressed rather than with relative expression intensity in a specific sample by this algorithm. In this way, genes with certain patterns, such as never/all-expressed or subtype-specific-expressed, may be recognized more easily. Therefore, the present study conducted a meta-analysis using the barcode expression values of a gene, aiming to identify DEGs between AC and SCC. To the best of our knowledge, the present study is the first to apply the barcode method in a meta-analysis.
Previous meta-analyses on NSCLC have mainly focused on either DEGs between NSCLC and normal controls (12–14) or those among different clinical stages of NSCLC (15,16). In the present analysis, however, the objective was to compare the differential expression profile between AC and SCC of NSCLC. Furthermore, the patients in this study were all at early clinical stages (i.e., stage I and II).
Materials and methods
Microarray data
The Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/) was searched using the keywords ‘non-small cell lung cancer’, ‘adenocarcinomas’, ‘squamous cell carcinomas’ and ‘Affymetrix Human Genome U133 Plus 2.0 Array’ between April 7, 2007 and October 31, 2013. Database searches initially yielded 6 studies in total, and 5 studies remained after duplications were removed. The abstracts were examined carefully and studies with original experimental objectives that analyzed the gene expression profiling between the two major subtypes of human lung cancer were included. Additionally, it was known from the SBV Challenge (sbvimprover.com) that partial samples in the GSE2109 dataset met the inclusion criteria (contained the keywords and met the aforementioned abstract criteria). Finally, the study included four experiments whose raw data were deposited in the GEO repository under the accession numbers of GSE10245, GSE18842, GSE2109 and GSE43580. The microarray expression data formed by these experiments contains the expression data of 54,675 probes in 325 specimens, which consist of 164 AC and 161 SCC specimens. It is also worth noting that all these samples were obtained from early-stage NSCLC patients. The details for the four studies are summarized in Table I.
Table I.
Description of data used in the meta-analysis.
| Data source | Platform | Year | Sample | AC, n | SCC, n |
|---|---|---|---|---|---|
| GSE10245 | GPL570 | 2008 | 58 | 40 | 18 |
| GSE18842 | GPL570 | 2009 | 46 | 14 | 32 |
| GSE2109 | GPL570 | 2004 | 71 | 33 | 38 |
| GSE43580 | GPL570 | 2013 | 150 | 77 | 73 |
AC, adenocarcinoma; SCC, squamous cell carcinoma.
Pre-processing procedures
Raw data (CEL files) of all data sets were downloaded from the GEO repository, and expression values were obtained using the frozen robust multiarray analysis algorithm (17). R package hgu133plus2.db was used to annotate the probe-set identifications (IDs) to gene IDs. For those multiple probe-sets that mapped to a same gene, their average values were used. To improve the reproducibility of gene co-expression patterns across studies, only genes that have similar inter-gene correlations across the studies can be used for meta-analysis. The integrative correlation coefficient (ICC) (18,19) is a measure of cross-study reproducibility for gene expression array data. Using the median ICCs (r=0.356) of all genes as a threshold, 9,925 genes with higher ICCs were fed into downstream meta-analysis.
Meta-analysis model
Let Yij represent the measured effect for study j (j=1, … J) for a specific gene i, let ti2 represent the variability between studies and let σ2 represent the within-study variance for the ith study. Yij and σ2 are already known from previous analysis/studies (20). In a meta-analysis setting, the following equation is used:
Here, µi is regarded as the average measure of differential expression across all datasets/studies for this gene, which is the parameter of interest. This is estimated using the following equation:
In this equation, wij equals the inverse of the variance of Yij. The division of this estimate by its estimated standard error, the resultant Z-score, being assumed to follow a standard normal distribution, is used to decide the statistical significance of a gene.
POE model
In the POE model, let xij denote the gene expression measurement for gene i from sample j, and let eij be an indicator of this value being overexpressed, underexpressed or non-differentially expressed in one phenotype relative to the other. xij is then assumed to follow a uniform distribution when it is overexpressed or underexpressed, and a normal distribution when it is non-differentially expressed. Let pij+=P(eij=1|xij) and pij−=P (eij=−1|xij) be the conditional probabilities of eij being 1 and −1 given xij, respectively. Here, pijd=pij+-pij− is the signed conditional probability being differentially expressed and is termed as POE. Details on POE and on its estimation using expectation maximization or Markov chain Monte Carlo algorithms have been described previously (10).
Barcode model
In the barcode algorithm, the expressed genes are coded with ones and the silenced genes are coded with zeros. Briefly, McCall et al (21) used a mixture model to parametrically fit observed log2 transformed intensity values (yig) for each gene. First, they assume that yjg follows a mixture of a silenced normal distribution of N (µg, τg2) and an expressed uniform distribution of U(µg, Sg) from the silenced mean to a saturation value represented by Sg. Next, a parametric distribution is specified for the silenced means and variances for each gene, one coming from a normal distribution and the other from an inverse γ distribution. To identify whether the observed log2 expression value yig is more likely to come from the silenced distribution or the expressed distribution, standardized intensity value, i.e., (yig-µg)/τg, which follows a standard normal distribution under the null hypothesis, was calculated. Using a hard-threshold C, the expression barcode for a gene, a vector of ones and zeros denoting the samples that are expressed and silenced, was coined as follows:
Here Φ is the cumulative density function of a standard normal distribution. The detailed description on how to estimate the parameters has been previously described in the supplementary material of the study by McCall et al (21).
Dichotomization of the actual expression values of a gene into barcoded values may result in numerous genes having the same barcode values in almost all samples. In the present analysis, using barcoded values, there were 6,312 genes expressed in <5% of the total samples and 1,084 genes expressed in >95% of the total samples. Those genes were unlikely to be DEGs and thus were excluded from the barcode meta-analysis.
Statistical analysis
Statistical analysis was performed in the R language, version 3.0 (www.r-project.org), and packages used were from the R Bioconductor project (www.bioconductor.org). Gene function annotation and pathway analysis was conducted by DAVID software (david.ncifcrf.gov). Fisher's exact test was used to decide if a gene was differentially expressed in the barcode meta-analysis method and I2 test was used to determine the heterogeneity level among the five studies. P<0.05 was considered to indicate a statistically significant difference.
Results
Study schema
Two meta-analysis methods were applied to four independent studies, with the aim of identifying DEGs between two major subtypes of NSCLC. The raw gene expression data had been transformed into POE and barcode data. These two methods are referred to as the POE meta-analysis and the barcode meta-analysis correspondingly herein. Next, the results of the two methods were compared and it was found that among the DEGs there were a number of genes that were closely related with the different subtypes of NSCLC, and that certain genes belonged to NSCLC biologically meaningful processes.
Meta-analysis using POE values and barcoded data
To combine gene expression data across multiple studies using a meta-analysis, the expression values of genes were firstly transformed to the POE statistics, as previously described (10). In the POE model, a latent variable eij is introduced, with −1/1 representing underexpressed/overexpressed and 0 representing non-differentially expressed. The POE statistics are then computed to obtain the probability of a gene being overexpressed, underexpressed and non-differentially expressed compared with the baseline.
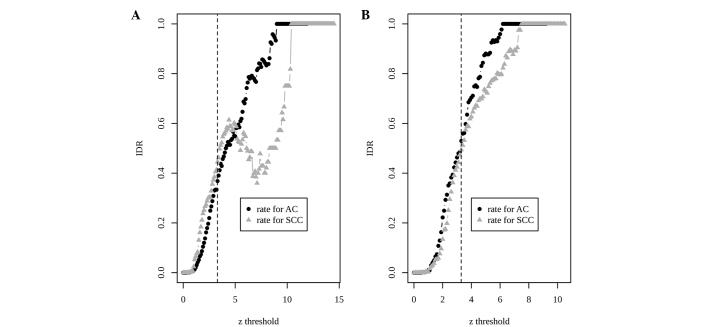
Based on Cochran's Q statistics (22) and quantile-quantile (QQ) plots (data not shown), a random effect model (REM) was chosen and then the z scores of effect sizes were calculated. Using a z score (zth) of 3.29 (P=0.001) as a threshold, there were 1,318 DEGs between the two NSCLC subtypes. An integration-driven discovery (IDD) gene (23) is a gene that can only be found with significantly changed expression level by meta-analysis rather than any single study at the same level of statistical significance. Usually, IDDs are those genes exhibiting weak but consistent signals across studies. Thus, the IDD rate (IDR), the ratio of IDD to total discoveries, may reflect the statistical power of a meta-analysis to gain extra information compared with single-study analysis. In the POE meta-analysis, IDD genes accounted for 36% of the 813 upregulated genes in the AC subtype and for 44% of the 505 upregulated genes in the SCC subtype (Fig. 1A).
Figure 1.
Proportions of IDD genes to total differentially-expressed genes (IDRs) for different threshold values. (A) probability of expression method; (B) barcode method. IDD, integration-driven discovery; IDR, IDD rate; AC, adenocarcinoma; SCC, squamous cell carcinoma.
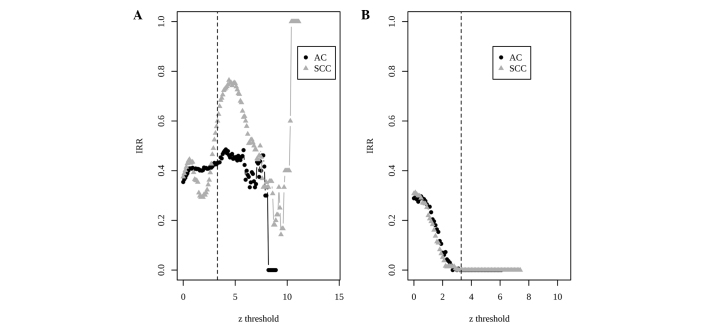
For barcoded data, Cochran's Q statistics were again calculated and a QQ plot was made. Based on the QQ plot, an REM was chosen. Due to the dichotomous feature of barcoded values, the effect size is chosen as the odds ratio on log scale. At the same threshold of z score (zth=3.29), the barcode meta-analysis identified 720 DEGs with 287 overexpressed and 433 underexpressed genes in AC compared with SCC. The IDRs using barcode data increased to 53 and 50% in the AC and SCC upregulated DEGs, respectively (Fig. 1B). The integration-driven revision rate (IRR) was defined in previous studies (23,24) as the percentage of genes that is declared to be differentially expressed in any individual study, but not in meta-analysis. Differing from IDR, which demonstrates the superiority of a meta-analysis, IRR may measure the deficiency of a meta-analysis. In the POE meta-analysis, the IRRs were 43 and 60% for AC and SCC upregulated genes, respectively (Fig. 2A). By contrast, the IRRs decreased to 0% in the two types of DEGs in the barcode meta-analysis (Fig. 2B).
Figure 2.
IRRs for different threshold values. (A) Probability of expression method; (B) barcode method. IRRs, integration-driven revision rates; AC, adenocarcinoma; SCC, squamous cell carcinoma.
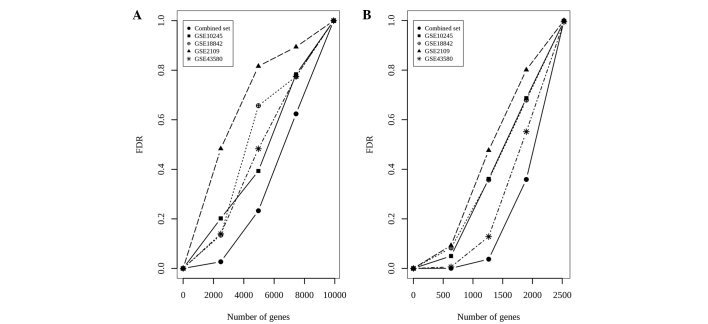
Another metric used to evaluate the performance of a meta-analysis is the false discovery rate (FDR). The calculation of FDRs is described in (25). In general, high IDRs and low FDRs indicate a meta-analysis that outperforms a single-study analysis. In the POE meta-analysis, a corresponding FDR value of 2.5×10−3 was obtained for zth=3.29 (Fig. 3A). The false-positive ratios in the meta-analysis were markedly reduced compared with any individual study.
Figure 3.
FDRs for meta-analysis and single-study analysis. (A) Probability of expression method; (B) barcode method. FDRs, false discovery rates.
In the barcode meta-analysis, the global FDR of 0.001 (Fig. 3B) was non-marginally lower compared with that of the POE meta-analysis. The results indicated that the barcode meta-analysis was more effective than any single-study analysis, and it also outperformed the POE meta-analysis in terms of IDR, IRR and FDR.
Comparison between two methods
The size of overlap between the DEGs in the two meta-analysis methods was 392. Not counting those extra genes excluded from the barcode meta-analysis, this number represented 54.4 and 64.9% of the DEGs in the barcode and POE methods, respectively. The DEGs of these two methods (Fisher's exact test, P=1.3×10−4) were substantially overlapped.
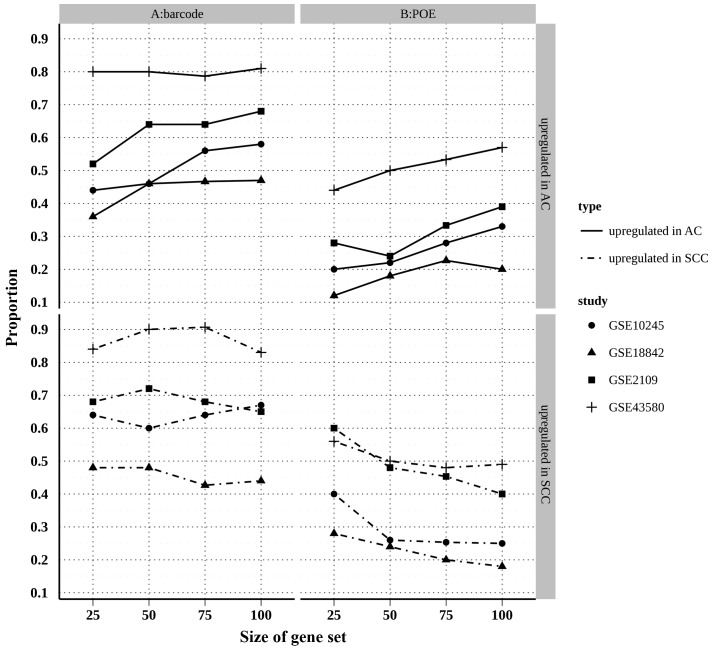
To assess the reliability of the two meta-analysis methods, the consistency of results was compared between the meta-analyses and individual studies. A large proportion of common DEGs between an individual study and a meta-analysis indicates the high reliability of the meta-analysis. In practice, the present study was typically interested in a small subset of genes that appeared to be truly differentially expressed. Therefore, it was more important to assess the consistency of genes that were identified to be most significant in each independent study (26). A correspondence at the top (CAT) plot (27) is a visual means to evaluate the agreement of identified genes between each of two studies. In the present study, CAT plots were created using the top 200 genes (100 upregulated in AC and 100 upregulated in SCC) identified by each individual study and the two meta-analyses (Fig. 4). The results showed that the proportion of overlapped top genes in the barcode method was substantially higher than that of the POE method for the AC and SCC upregulated genes, suggesting that barcode meta-analysis outperformed POE meta-analysis in terms of reliability.
Figure 4.
Correspondence at the top plot for two methods. The proportions of overlapped genes in top 200 differentially expressed genes between meta-analysis and single-study analysis. (A) Barcode method; (B) POE method. AC, adenocarcinoma; SCC, squamous cell carcinoma; POE, probability of expression.
Functional annotation of DEGs
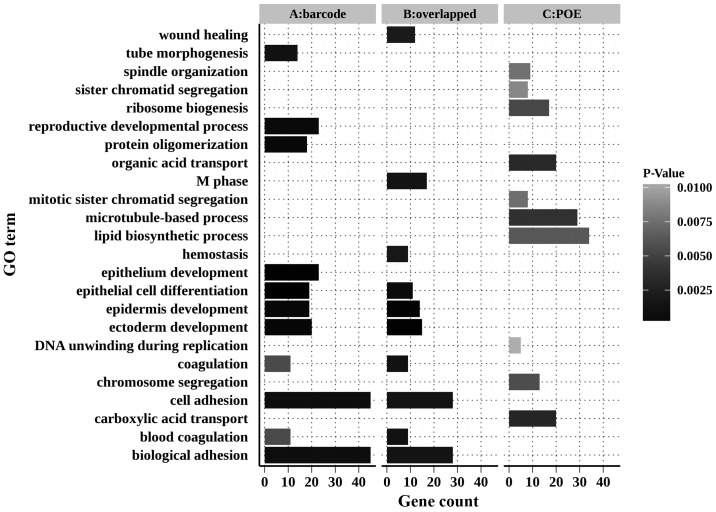
Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes pathway analysis were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (28,29). The top 11 enriched biological processes (please note two processes were tied for 10th place) of those DEGs are illustrated in Fig. 5.
Figure 5.
Enriched GO terms of DEGs identified by meta-analysis methods. GO terms associated with DEGs from different methods with a P-value <0.01 are shown. (A) Barcode method; (B) overlapped DEGs in barcode and POE methods; (C) POE method. GO, Gene Ontology; DEG, differentially-expressed gene; POE, probability of expression.
The DEGs of the barcode meta-analysis and the shared DEGs of these two methods were significantly associated with biological processes such as epithelium development, epidermis development, epithelial cell differentiation, cell adhesion and coagulation. However, the DEGs identified by POE meta-analysis did not present such enrichment.
Additionally, the present study examined the top 50 DEGs from the barcode meta-analysis for their biological relevance and found that a number of the genes have previously been reported to be associated with NSCLC. For example, it has previously been proven that the presence of lung AC depends on the expression of NK2 homeobox 1 (NKX2-1) (30). In the present analysis, NKX2-1 showed a higher expression level in AC compared with that in the SCC subtype. The protein encoded by desmocollin 3 (DSC3) is a member of the desmocollin family that is primarily found in epithelial cells and is required for cell-cell junctions. Desmoglein 3 (DSG3) is a calcium-binding transmembrane glycoprotein component of desmosomes in vertebrate epithelial cells. In the present analysis, DSC3 and DSG3 were significantly upregulated in SCC. Moreover, gap junction protein β5 (GJB5) (4), tumor protein p63 (TP63) (31), tripartite motif containing 29 (32) and keratin 5 (KRT5) (33) have also been reported to be associated with either of the two subtypes and are among the top DEGs identified by meta-analysis.
Using the overlapped DEGs (188 upregulated genes in SCC versus 204 upregulated genes in AC) in the two methods, the enriched GO terms were examined for each subtype, respectively. For AC upregulated DEGs, biological processes, including cell adhesion, biological adhesion and coagulation, were significantly enriched. Elevated expression of the genes associated with blood coagulation in AC is consistent with the claim that patients who have advanced lung AC are prone to thrombophilia (34), even though the present study population consisted of NSCLC patients at early histology stages. In SCC, over-representation of genes in three GO categories, epidermis development, cell division and epithelial cell differentiation, was observed. Furthermore, the high expression of genes involved in the keratinization process was observed, which is consistent with the characteristic of well- and moderately-differentiated SCC (35). Significant GO terms associated with the two subtypes, respectively, are illustrated in Table II.
Table II.
Enriched GO terms of DEGs between AC and SCC.
| 188 genes in SCC vs. AC | 204 genes in AC vs. SCC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GO term | GO ID | Count | % of genes | P-value | FDR | Count | % of genes | P-value | FDR |
| Epidermis development | GO:0008544 | 13 | 7.0 | 3.0×10−7 | 4.8×10−6 | – | – | – | – |
| Ectoderm development | GO:0007398 | 13 | 7.0 | 7.0×10−7 | 1.1×10−5 | – | – | – | – |
| Cell division | GO:0051301 | 14 | 7.5 | 7.8×10−6 | 1.3×10−4 | – | – | – | – |
| M phase | GO:0000279 | 16 | 8.6 | 9.9×10−7 | 1.6×10−5 | – | – | – | – |
| DNA replication | GO:0006260 | 8 | 4.3 | 3.0×10−3 | 4.6×10−2 | – | – | – | – |
| Epithelial cell differentiation | GO:0030855 | 7 | 3.7 | 2.5×10−3 | 3.9×10−2 | – | – | – | – |
| Keratinization | GO:0031424 | 3 | 1.6 | 6.8×10−2 | 8.5×10−3 | – | – | – | – |
| Cell adhesion | GO:0007155 | – | – | – | – | 19 | 9.5 | 4.2×10−4 | 6.7×10−1 |
| Biological adhesion | GO:0022610 | – | – | – | – | 19 | 9.5 | 4.2×10−4 | 6.8×10−1 |
| Blood coagulation | GO:0007596 | – | – | – | – | 7 | 3.5 | 7.4×10−4 | 1.2×10−2 |
| Coagulation | GO:0050817 | – | – | – | – | 7 | 3.5 | 7.4×10−4 | 1.2×10−2 |
GO, Gene Ontology; ID, identification; AC, adenocarcinoma; SCC, squamous cell carcinoma; FDR, false discovery rate.
Analysis based on the POE data only reflects the relativity of expression intensity, namely, genes are expressed at higher/lower levels in AC samples than in SCC samples. Little attention has been devoted to determining which genes are expressed in a specific subtype. By contrast, barcode gene expression values have only binary values and a thorough examination of genes that are silenced in one phenotype but expressed in the other is possible. In the present study, there were 415 such genes, including 219 genes expressed in SCC and 196 genes expressed in AC. Pathway analysis using these subtype specific genes was also conducted. AC-specific genes were significantly involved in the mitogen-activated protein kinase signaling pathway and the O-glycan biosynthesis pathway, while SCC-specific genes were significantly mapped into the Wnt signaling pathway, adherens junctions and the Hedgehog signaling pathway (Table III). Using meta-analysis, the present study further confirmed that different pathological mechanisms are involved in these two NSCLC subtypes.
Table III.
KEGG pathway analysis of subtype-specific genes in barcode meta-analysis.
| 219 genes specific in SCC | 196 genes specific in AC | |||||||
|---|---|---|---|---|---|---|---|---|
| KEGG pathway | Count | % of genes | P-value | FDR | Count | % of genes | P-value | FDR |
| Wnt signaling pathway | 7 | 3.3 | 3.0×10−3 | 0.03 | – | – | – | – |
| Basal cell carcinoma | 4 | 1.9 | 1.6×10−2 | 0.15 | – | – | – | – |
| Hedgehog signaling pathway | 4 | 1.9 | 1.6×10−2 | 0.16 | – | – | – | – |
| Adherens junction | 4 | 1.9 | 3.7×10−2 | 0.33 | – | – | – | – |
| MAPK signaling pathway | – | – | – | – | 8 | 4.2 | 9.4×10−3 | 0.09 |
| O-glycan biosynthesis | – | – | – | – | 3 | 1.6 | 3.0×10−2 | 0.27 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; AC, adenocarcinoma; SCC, squamous cell carcinoma; FDR, false discovery rate; MAPK, mitogen-activated protein kinase.
Discussion
To conduct a meta-analysis, gene expression values were transformed into two different data types, namely, POE and barcode values. To assess the heterogeneity for each gene, I2 statistics for the two approaches were calculated. Overall, 21% of genes out of the POE gene set showed high heterogeneity (I2>50%), and this number decreased to 9% for the barcode gene set. Among all of the common genes with heterogeneity across the studies, 71.74% showed a heterogeneity reduction in barcoded data. This implies that when using the barcode values rather than POE, the heterogeneity of a gene across studies can be reduced.
The transformation of the raw expression value to the gene expression barcode made certain genes present with no difference at all between subtypes. Loosely speaking, exclusion of such genes simplified the analysis. However, even when expressed/silenced in all samples, the expression intensity of a gene may differ between the two NSCLC subtypes. The POE meta-analysis identified 187 and 527 DEGs out of 1,084 expressed and 6,312 silenced genes, respectively. Although these DEGs showed no significant enrichment in any biological processes or pathways, genes involved in the same process showed a similar expression pattern. For example, eukaryotic translation initiation factors such as eukaryotic translation initiation factor 4γ1 (EIF4G1), EIF2A, EIF4H, EIF3J and EIF4D were overexpressed in the majority of the SCC samples. By contrast, major histocompatibility complex genes, including human leukocyte antigen (HLA)-B, HLA-G, HLA-DMB and HLA-DMA, were overexpressed in the AC subtype.
Each meta-analysis method identified 53 common IDD genes (data not shown). Among them, a number are biologically relevant to NSCLC. Namely, claudin 18 (CLDN18), which is commonly expressed in lung AC and in tumors of non-smokers (36), encodes a protein critical for tight junctions. Secretoglobin 3A2 is a downstream target for NKX2-1 in the lungs (37) and is overexpressed in lung AC (38). Aurora-B is a key regulator of mitosis and its overexpression is correlated with NSCLC (39). The inhibition of aurora kinase activity, leading to defective cell division and endoreduplication of NSCLC cells, as well as high aurora B expression levels, were significantly associated with squamous cell carcinoma histology (39).
For those subtype-specific genes identified by barcode meta-analysis, a consistent expression pattern was observed. For instance, TP63, which was silenced in the AC samples but was expressed in the majority of SCC samples, plays a critical role in the development and maintenance of stratified epithelial tissues. KRT5, −14, −13, −16 all belongs to KRT gene family and showed a similar expression pattern of being silenced in almost all the AC subtype tissues but expressed in the majority of the SCC subtype tissues. The proteins encoded by genes in this family are usually tough, fibrous proteins that form the structural framework of epithelial cells (40). In the present study, the two tumor subtypes showed deregulation in the cell junction process through different mechanisms. Tight junction genes, such as CLDN2, and cell adhesion genes, such a hyaluronan binding protein 2, were exclusively overexpressed in the AC tumors, while gap junction genes GJB3 and GJB5 were overexpressed in the SCC tumors.
Previous genome-wide association studies have also made contributions to determining the susceptibility genes associated with a specific subtype of lung cancer. For example, a single-nucleotide polymorphism marker located on the CLPTM1-like (CLPTM1L)-telomerase reverse transcriptase gene region at chromosome 5p15 has been proven to be associated with the risk of AC, but not with SCC (41,42). CLPTM1L at this location exhibited overexpression in AC tumors compared with normal or other lung cancer tissues (43). Consistent with this previous conclusion, CLPTM1L was expressed at higher levels in the AC subtype than in the SCC subtype in the present analysis. By contrast, the polymorphism in the solute carrier family 17 member 8-nuclear receptor subfamily 1 group H member 4 (NR1H4) gene region at 12q23.1 was only significantly associated with the risk of the SCC subtype (44). Loss of NR1H4 will promote Wnt signaling pathway and increase tumor progression (45), and in the present analysis, NR1H4 was expressed at a lower level in the SCC subtype than in the AC subtype.
In conclusion, DEGs identified by POE and barcode meta-analysis substantially overlap. The functional analysis based on DEGs confirmed that there are biological differences between AC and SCC. Thus, these DEGs are useful for diagnosis and personalized treatments in these two subtypes of NSCLC, and future studies are warranted.
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- AC
adenocarcinoma
- SCC
squamous cell carcinoma
- DEG
differentially-expressed gene
- POE
probability of expression
- IDD
integration-driven discovery
- IDR
IDD rate
- IRR
integration-driven revision rate
- ICC
integrative correlation coefficient
- REM
random effect model
- CAT
correspondence at the top
References
- 1.Kikuchi T, Daigo Y, Katagiri T, Tsunoda T, Okada K, Kakiuchi S, Zembutsu H, Furukawa Y, Kawamura M, Kobayashi K, et al. Expression profiles of non-small cell lung cancers on cDNA microarrays: Identification of genes for prediction of lymph-node metastasis and sensitivity to anti-cancer drugs. Oncogene. 2003;22:2192–2205. doi: 10.1038/sj.onc.1206288. [DOI] [PubMed] [Google Scholar]
- 2.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 2015;1856:189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Yang XY, Shi WJ. Identifying differentially expressed genes and pathways in two types of non-small cell lung cancer: Adenocarcinoma and squamous cell carcinoma. Genet Mol Res. 2014;13:95–102. doi: 10.4238/2014.January.8.8. [DOI] [PubMed] [Google Scholar]
- 4.Kuner R, Muley T, Meister M, Ruschhaupt M, Buness A, Xu EC, Schnabel P, Warth A, Poustka A, Sültmann H, Hoffmann H. Global gene expression analysis reveals specific patterns of cell junctions in non-small cell lung cancer subtypes. Lung Cancer. 2009;63:32–38. doi: 10.1016/j.lungcan.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Zhu J, Guo SX, Deng Y. Bicluster and regulatory network analysis of differentially expressed genes in adenocarcinoma and squamous cell carcinoma. Genet Mol Res. 2013;12:1710–1719. doi: 10.4238/2013.May.21.2. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Li D, Wei X, Su Y. In silico comparative genomic analysis of two non-small cell lung cancer subtypes and their potentials for cancer classification. Cancer Genomics Proteomics. 2014;11:303–310. [PubMed] [Google Scholar]
- 7.Siddiqui AS, Delaney AD, Schnerch A, Griffith OL, Jones SJ, Marra MA. Sequence biases in large scale gene expression profiling data. Nucleic Acids Res. 2006;34:e83. doi: 10.1093/nar/gkl404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JK, Yu U, Kim S, Yoo OJ. Combining multiple microarray studies and modeling interstudy variation. Bioinformatics. 2003;19:i84–i90. doi: 10.1093/bioinformatics/btg1010. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 9.Rhodes DR, Barrette TR, Rubin MA, Ghosh D, Chinnaiyan AM. Meta-analysis of microarrays: Interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 2002;62:4427–4433. [PubMed] [Google Scholar]
- 10.Choi H, Shen R, Chinnaiyan AM, Ghosh D. A latent variable approach for meta-analysis of gene expression data from multiple microarray experiments. BMC Bioinformatics. 2007;8:364. doi: 10.1186/1471-2105-8-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zilliox MJ, Irizarry RA. A gene expression bar code for microarray data. Nat Methods. 2007;4:911–913. doi: 10.1038/nmeth1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amelung JT, Bührens R, Beshay M, Reymond MA. Key genes in lung cancer translational research: A meta-analysis. Pathobiology. 2010;77:53–63. doi: 10.1159/000278292. [DOI] [PubMed] [Google Scholar]
- 13.Kim B, Lee HJ, Choi HY, Shin Y, Nam S, Seo G, Son DS, Jo J, Kim J, Lee J, et al. Clinical validity of the lung cancer biomarkers identified by bioinformatics analysis of public expression data. Cancer Res. 2007;67:7431–7438. doi: 10.1158/0008-5472.CAN-07-0003. [DOI] [PubMed] [Google Scholar]
- 14.Tian ZQ, Li ZH, Wen SW, Zhang YF, Li Y, Cheng JG, Wang GY. Identification of commonly dysregulated genes in non-small-cell lung cancer by integrated analysis of microarray data and qRT-PCR validation. Lung. 2015;193:583–592. doi: 10.1007/s00408-015-9726-6. [DOI] [PubMed] [Google Scholar]
- 15.Tan X, Chen M. MYLK and MYL9 expression in non-small cell lung cancer identified by bioinformatics analysis of public expression data. Tumour Biol. 2014;35:12189–12200. doi: 10.1007/s13277-014-2527-3. [DOI] [PubMed] [Google Scholar]
- 16.Huang CH, Chang PM, Lin YJ, Wang CH, Huang CY, Ng KL. Drug repositioning discovery for early- and late-stage non-small-cell lung cancer. Biomed Res Int. 2014;2014:193817. doi: 10.1155/2014/193817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCall MN, Bolstad BM, Irizarry RA. Frozen robust multiarray analysis (fRMA) Biostatistics. 2010;11:242–253. doi: 10.1093/biostatistics/kxp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JK, Bussey KJ, Gwadry FG, Reinhold W, Riddick G, Pelletier SL, Nishizuka S, Szakacs G, Annereau JP, Shankavaram U, et al. Comparing cDNA and oligonucleotide array data: Concordance of gene expression across platforms for the NCI-60 cancer cells. Genome Biol. 2003;4:R82. doi: 10.1186/gb-2003-4-12-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmigiani G, Garrett-Mayer ES, Anbazhagan R, Gabrielson E. A cross-study comparison of gene expression studies for the molecular classification of lung cancer. Clin Cancer Res. 2004;10:2922–2927. doi: 10.1158/1078-0432.CCR-03-0490. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, editors. Introduction to Meta-Analysis. 1st. John Wiley and Sons, Ltd.; Chichester, UK: 2009. [DOI] [Google Scholar]
- 21.McCall MN, Uppal K, Jaffee HA, Zilliox MJ, Irizarry RA. The gene expression barcode: Leveraging public data repositories to begin cataloging the human and murine transcriptomes. Nucleic Acids Res. 2011;39:D1011–D1015. doi: 10.1093/nar/gkq1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–266. doi: 10.2307/2332378. [DOI] [PubMed] [Google Scholar]
- 23.Stevens JR, Doerge RW. Combining Affymetrix microarray results. BMC Bioinformatics. 2005;6:57. doi: 10.1186/1471-2105-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conlon EM, Song JJ, Liu A. Bayesian meta-analysis models for microarray data: A comparative study. BMC Bioinformatics. 2007;8:80. doi: 10.1186/1471-2105-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong F, Breitling R. A comparison of meta-analysis methods for detecting differentially expressed genes in microarray experiments. Bioinformatics. 2008;24:374–382. doi: 10.1093/bioinformatics/btm620. [DOI] [PubMed] [Google Scholar]
- 27.Irizarry RA, Warren D, Spencer F, Kim IF, Biswal S, Frank BC, Gabrielson E, Garcia JG, Geoghegan J, Germino G, et al. Multiple-laboratory comparison of microarray platforms. Nat Methods. 2005;2:345–350. doi: 10.1038/nmeth0605-477b. [DOI] [PubMed] [Google Scholar]
- 28.da Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Kwei KA, Kim YH, Girard L, Kao J, Pacyna-Gengelbach M, Salari K, Lee J, Choi YL, Sato M, Wang P, et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27:3635–3640. doi: 10.1038/sj.onc.1211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massion PP, Taflan PM, Rahman Jamshedur SM, Yildiz P, Shyr Y, Edgerton ME, Westfall MD, Roberts JR, Pietenpol JA, Carbone DP, Gonzalez AL. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63:7113–7121. [PubMed] [Google Scholar]
- 32.Zhou ZY, Yang GY, Zhou J, Yu MH. Significance of TRIM29 and β-catenin expression in non-small-cell lung cancer. J Chin Med Assoc. 2012;75:269–274. doi: 10.1016/j.jcma.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Angulo B, Suarez-Gauthier A, Lopez-Rios F, Medina PP, Conde E, Tang M, Soler G, Lopez-Encuentra A, Cigudosa JC, Sanchez-Cespedes M. Expression signatures in lung cancer reveal a profile for EGFR-mutant tumours and identify selective PIK3CA overexpression by gene amplification. J Pathol. 2008;214:347–356. doi: 10.1002/path.2267. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Wei S, Wang J, Hong L, Cui L, Wang C. Analysis of the factors associated with abnormal coagulation and prognosis in patients with non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2014;17:789–796. doi: 10.3779/j.issn.1009-3419.2014.11.04. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kogan EA, Ugriumov DA, Jaques G. Morphologic and molecular-genetic characteristics of keratinization and apoptosis in squamous cell lung carcinoma. Arkh Patol. 2000;62:16–20. [PubMed] [Google Scholar]
- 36.Merikallio H, Pääkkö P, Harju T, Soini Y. Claudins 10 and 18 are predominantly expressed in lung adenocarcinomas and in tumors of nonsmokers. Int J Clin Exp Pathol. 2011;4:667–673. [PMC free article] [PubMed] [Google Scholar]
- 37.Kurotani R, Tomita T, Yang Q, Carlson BA, Chen C, Kimura S. Role of secretoglobin 3A2 in lung development. Am J Respir Crit Care Med. 2008;178:389–398. doi: 10.1164/rccm.200707-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson B, Stavnes HT, Risberg B, Nesland JM, Wohlschlaeger J, Yang Y, Shih IeM, Wang TL. Gene expression signatures differentiate adenocarcinoma of lung and breast origin in effusions. Hum Pathol. 2012;43:684–694. doi: 10.1016/j.humpath.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Vischioni B, Oudejans JJ, Vos W, Rodriguez JA, Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5:2905–2913. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- 40.Lane EB, McLean WH. Keratins and skin disorders. J Pathol. 2004;204:355–366. doi: 10.1002/path.1643. [DOI] [PubMed] [Google Scholar]
- 41.Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, Mirabello L, Jacobs K, Wheeler W, Yeager M, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85:679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsiung CA, Lan Q, Hong YC, Chen CJ, Hosgood HD, Chang IS, Chatterjee N, Brennan P, Wu C, Zheng W, et al. The 5p15.33 locus is associated with risk of lung adenocarcinoma in never-smoking females in Asia. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001051. pii: e1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni Z, Tao K, Chen G, Chen Q, Tang J, Luo X, Yin P, Tang J, Wang X. CLPTM1L is overexpressed in lung cancer and associated with apoptosis. PLoS One. 2012;7:e52598. doi: 10.1371/journal.pone.0052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong J, Jin G, Wu C, Guo H, Zhou B, Lv J, Lu D, Shi Y, Shu Y, Xu L, et al. Genome-wide association study identifies a novel susceptibility locus at 12q23.1 for lung squamous cell carcinoma in han Chinese. PLoS Genet. 2013;9:e1003190. doi: 10.1371/journal.pgen.1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]