We appreciate the comment by Mold et al. in their Letter to the Editor [1].
Previously our group reported that the adjuvant activity of aluminum hydroxide nanoparticles (AH-NPs, ~110 nm) is significantly stronger than that of aluminum hydroxide microparticles (AH-MPs, ~9 micrometers) [2]. The paper commented by Mold et al. was designed to understand the potential mechanism underlying the stronger vaccine adjuvant activity of AH-NPs, relative to AH-MPs, and to test whether aluminum phosphate nanoparticles also have a stronger adjuvant activity than aluminum phosphate microparticles [3]. We prepared aluminum phosphate nanoparticles (AP-NPs, 122–255 nm) and aluminum phosphate microparticles (AP-MPs, X50, 4.87 μm), and showed that the AP-NPs are more potent than the AP-MPs in activating NLRP3 inflammasome [3]. Similarly, we also showed that the AH-NPs (majority below 100 nm) are more potent than the AH-MPs (X50, 5.36 μm) in activating NLRP3 inflammasome [3]. Based on the findings, we concluded that the mechanism underlying the stronger adjuvant activity of aluminum salt nanoparticles is likely related to their stronger ability to activate the NLRP3 inflammasome than aluminum salt microparticles [3].
To understand why the aluminum salt nanoparticles are more potent in activating the NLRP3 inflammasome than the aluminum salt microparticles, we compared their abilities in inducing reactive oxygen species production and lysosomal rupture, but did not detect any significant difference [3]. We then suspected that the potential difference in the uptakes of the aluminum salt nanoparticles and microparticles by the THP-1 cells may be relevant. We designed an experiment to study the uptake of the particles by THP-1 cells, and reported the data in Figure 3C and Figure 4C in the paper [3]. After the publication of the paper, and a few days before we saw the Letter to the Editor by Mold et al, we noticed that the data in Figure 3C and Figure 4C in our article are not exactly showing intracellular aluminum levels after THP-1 cells were incubated with different aluminum salt particles; they represent instead the levels of aluminum “associated” with THP-1 cells after the incubation, including intracellular and cell surface-bound.
Previously, when we studied the uptake or internalization of particulates by macrophages in culture, we incubated the particulates with macrophages in culture at 37° C as well as 4° C [2, 4]. At 4° C, cell uptake of nanoparticles and microparticles are inhibited due to inhibition of endocytosis. Therefore, a comparison of the amounts of particles associated with macrophages after incubation at 37° C versus at 4° C and then washing allows us to understand the extent to which the particles are internalized (or taken up) by macrophages [2, 4]. We regret that we did not co-incubate the cells and particles at 4° C when conducting the uptake study and were not more careful before publishing the paper [3].
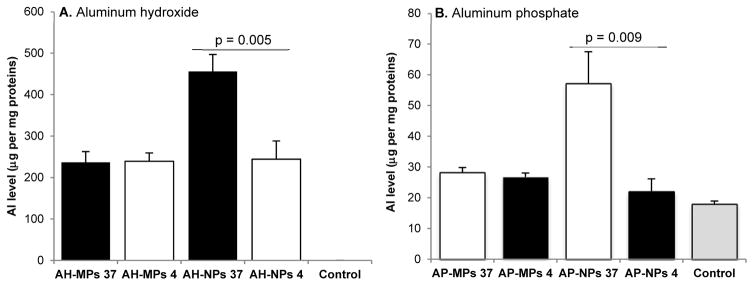
To address this issue, we re-performed the in vitro particle uptake study by co-incubating THP-1 cells (American Type Culture Collection, Manassas, VA) with the particles at 37° C or 4° C. THP-1 cells (6 × 105/well) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (v/v, Sigma-Aldrich, St. Louis, MO), 100 U/ml-100 μg/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA), and 50 mM of β-mercaptoethanol (Sigma-Aldrich) in the presence of 1 μg/ml of phorbol 12-myristate 13-acetate (PMA, InvivoGen, San Diego, CA) for 16 h to differentiate them into macrophages [3]. The medium was replaced with serum-free fresh medium, and the cells were incubated with aluminum salt nanoparticles (i.e. AH-NPs, AP-NPs) or microparticles (i.e. AH-MPs, AP-MPs) in the presence of lipopolysaccharide (10 ng/ml, InvivoGen) at 37° C or 4° C for 3 h. The final concentration of aluminum (Al) was 50 μg/ml [3]. All aluminum salt particles were prepared as previously described [3]. The cell culture medium was discarded, and the cells were washed three times with cold phosphate-buffered saline (PBS, pH 7.4, 10 mM, 3 ml each wash). Cells were trypsinized and re-suspended with 1 ml of sterile PBS; 0.1 ml of the cell suspension were used to measure total protein content using a Pierce BCA protein assay kit (Pierce Biotechnology, Rockford, IL) after the cells were lysed with a Triton X-100 solution. The remaining 0.9 ml of cell suspension were used to measure aluminum content using a Varian 710-ES Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) available in the Civil Architectural and Environmental Engineering Department at The University of Texas at Austin, after the cells were lysed with nitric acid (6.6 N, 60° C for 16 h). As shown in Figure 1A, the level of aluminum obtained from THP-1 cells incubated with AH-NPs at 37° C is significantly higher than that at 4° C (i.e. 87% higher, p = 0.005). However, the level of aluminum obtained from THP-1 cells incubated with AH-MPs at 37° C is not significantly different from that at 4° C (p = 0.9). Similar results were also obtained when cells were incubated with AP-NPs or AP-MPs (Figure 1B). The lack of significant difference in the amounts of aluminum obtained from cells incubated with the aluminum salt microparticles at 37°C vs. 4°C is probably related to the large particle size of the microparticles [3] and/or the method of aluminum quantification. Nonetheless, the results indirectly show that when incubated with differentiated THP-1 cells in culture, more aluminum salt nanoparticles are internalized (or taken up) by the cells than aluminum salt microparticles. We are aware of a previously reported method of directly identifying intracellular aluminum using lumogallion as a fluorescent probe [5], but believe that the method of comparing the amounts of aluminum associated with cells after co-incubation at 37°C versus at 4°C allows us to quantitatively estimate the internalization (or uptake of) the aluminum salt particles.
Figure 1.
Evaluation of the uptake of aluminum salt nanoparticles and microparticles by THP-1 cells in culture. THP-1 cells (6 × 105/well) were differentiated and incubated with aluminum hydroxide particles (i.e. AH-MPs or AH-NPs) (A) or aluminum phosphate particles (i.e. AP-MPs or AP-NPs) (B) at 37°C or 4°C for 3 h. Cells were then washed with cold PBS three times, and the amounts of aluminum obtained from the cells were determined and normalized to total protein content. The numbers of 37 and 4 in the X-axis are the temperature (°C) at which the cells and particles were co-incubated. Data are mean S.D. (n = 3).
In conclusion, our original statement that the mechanism underlying the stronger adjuvant activity of aluminum salt nanoparticles is likely related to their stronger ability to activate the NLRP3 inflammasome is valid. Our new data presented in Figure 1 now support that the difference in the aluminum salt nanoparticles’ and microparticles’ abilities to activate NLRP3 inflammasome in THP-1 cells is likely related to the difference in their uptake by the THP-1 cells.
Acknowledgments
This work is supported in part by a grant from the U.S. National Institute of Allergy and Infectious Diseases (AI105789 to Z.C.).
References
- 1.Mold M, Shardlow E, Exley C. Letter to the Editor: Toward understanding the mechanisms underlying the strong adjuvant activity of aluminum salt nanoparticles. Vaccine. 2017;35(8):1101. doi: 10.1016/j.vaccine.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Aldayel AM, Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 2014;173:148–57. doi: 10.1016/j.jconrel.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruwona TB, Xu H, Li X, Taylor AN, Shi YC, Cui Z. Toward understanding the mechanism underlying the strong adjuvant activity of aluminum salt nanoparticles. Vaccine. 2016;34:3059–67. doi: 10.1016/j.vaccine.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui Z, Hsu CH, Mumper RJ. Physical characterization and macrophage cell uptake of mannan-coated nanoparticles. Drug development and industrial pharmacy. 2003;29:689–700. doi: 10.1081/ddc-120021318. [DOI] [PubMed] [Google Scholar]
- 5.Mold M, Eriksson H, Siesjo P, Darabi A, Shardlow E, Exley C. Unequivocal identification of intracellular aluminium adjuvant in a monocytic THP-1 cell line. Scientific reports. 2014;4:6287. doi: 10.1038/srep06287. [DOI] [PMC free article] [PubMed] [Google Scholar]