Abstract
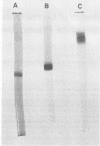
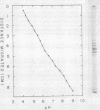
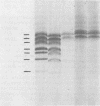
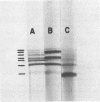
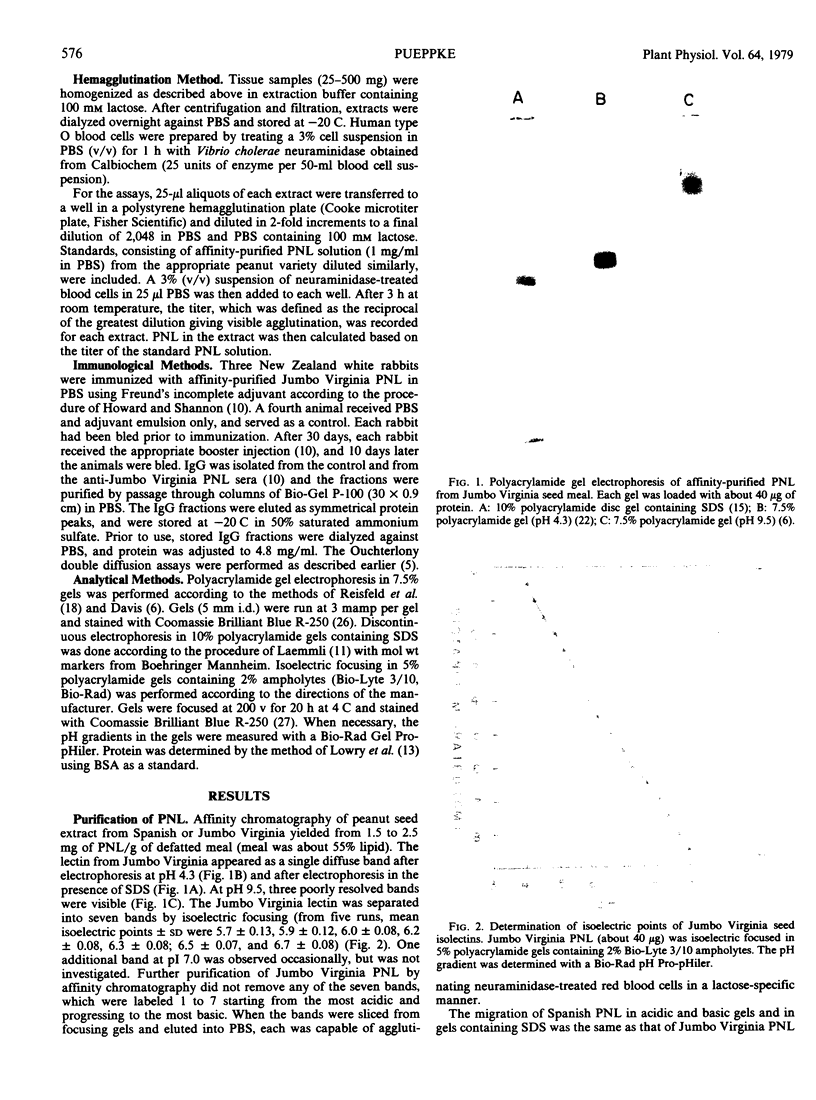
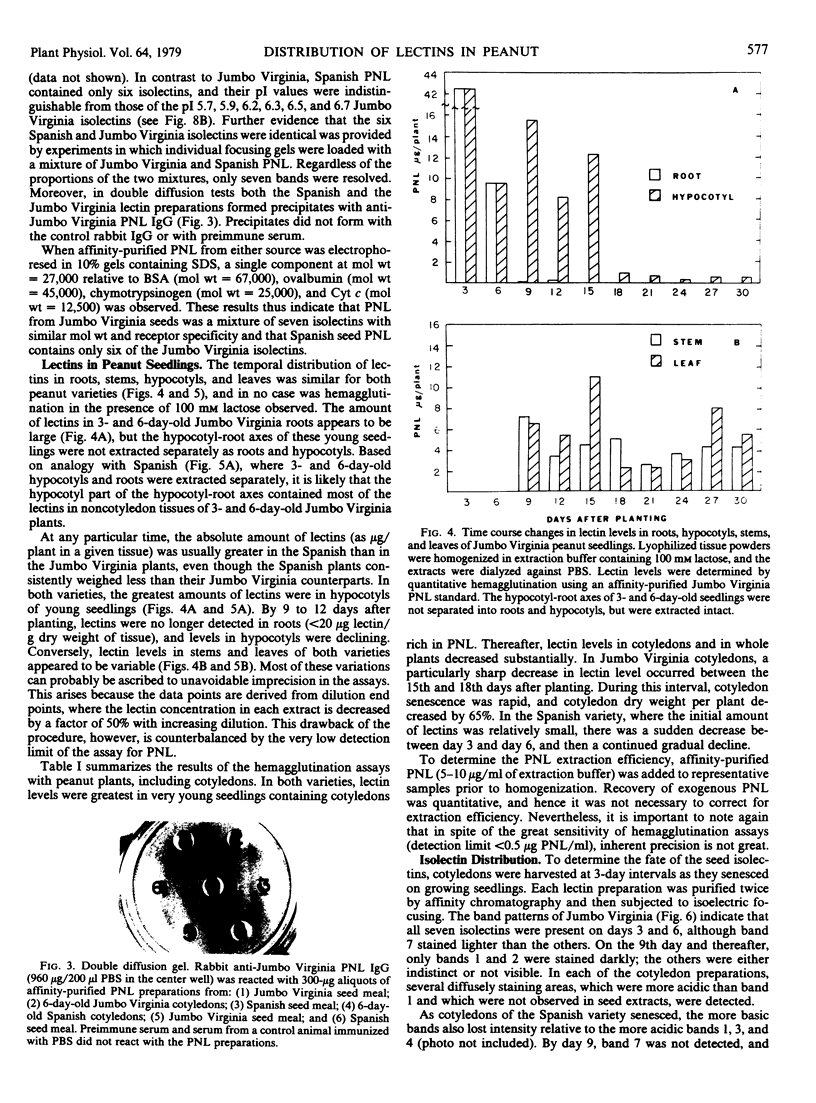
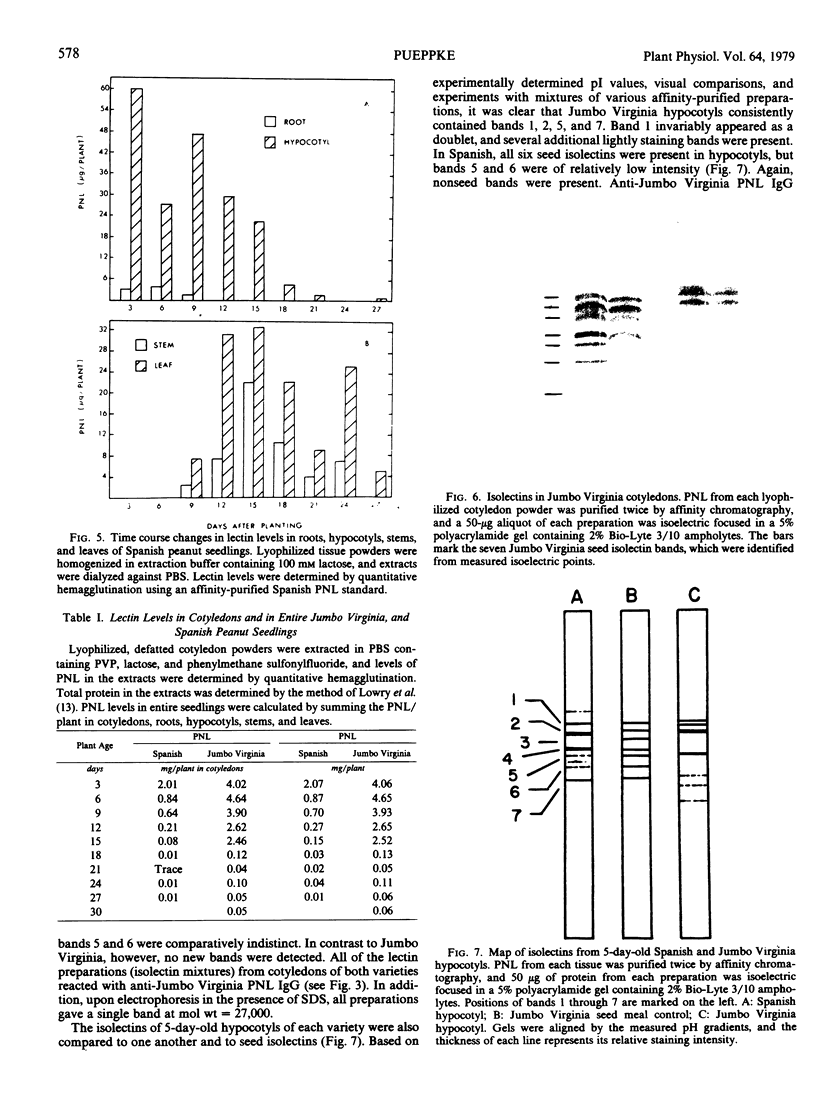
Peanut lectin was purified from seed meal of the Spanish and Jumbo Virginia varieties of peanut (Arachis hypogaea L.) by affinity chromatography on lactose coupled to Sepharose 4B. Polyacrylamide gel isoelectric focusing resolved the lectin preparation from Jumbo Virginia seeds into seven isolectins (pI 5.7, 5.9, 6.0, 6.2, 6.3, 6.5, and 6.7). Seed meal from the Spanish variety contained six isolectins which were indistinguishable from the pI 5.7, 5.9, 6.2, 6.3, 6.5, and 6.7 isolectins from Jumbo Virginia. Quantitative, lactose-specific hemagglutination was used to examine the lectins in tissues of both peanut varieties. In young (3- to 9-day-old) seedlings of each variety, more than 90% of the total amount of lectins detected in the plants was in the cotyledons. Most of the remainder was in hypocotyls, stems, and leaves; young roots contained no more than 4 micrograms of lectin per plant. Lectins were present in all nonroot tissues of 21- to 30-day-old seedlings, except 27-day-old Spanish hypocotyls. As cotyledons of each variety senesced, several of the more basic isolectins decreased to undetectable levels, but the acidic isolectins remained until at least 15 days after planting. Some of the seed isolectins and several apparently new lactose-binding lectins were also identified in affinity-purified extracts of 5-day-old roots and hypocotyls. Rabbit antibodies raised against the Jumbo Virginia seed isolectin preparation reacted with seed, cotyledon, and hypocotyl lectin preparations from both varieties. Analysis of seed lectin preparations from seven varieties of A. hypogaea and of a related species (A. villosulicarpa) indicated that isolectin composition in Arachis may be a characteristic of both the species and the subspecies (botanical type) to which the variety belongs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD W. C., GREEN D. M., FUJINAGA D. M., DRABIK J. S., WASZCZENKO-ZACHARCZENKO E. A blood factor, possibly new, detected by extracts of Arachis hypogaea. Vox Sang. 1959 Dec;4:456–467. doi: 10.1111/j.1423-0410.1959.tb03653.x. [DOI] [PubMed] [Google Scholar]
- BOYD W. C., WASZCZENKO-ZACHARCZENKO E., GOLDWASSER S. M. List of plants tested for hemagglutinating activity. Transfusion. 1961 Nov-Dec;1:374–382. doi: 10.1111/j.1537-2995.1961.tb00078.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fialová D., Tichá M., Kocourek J. Studies on phytohemagglutinins. XXVI. A new type of a lentil hemagglutinin isolated from Lens esculenta Moench., subsp. microsperma (Baumg.) Barulina. Biochim Biophys Acta. 1975 May 30;393(1):170–181. doi: 10.1016/0005-2795(75)90228-7. [DOI] [PubMed] [Google Scholar]
- Foutain D. W., Yang W. K. Isolectins from soybean (Glycine max). Biochim Biophys Acta. 1977 May 27;492(1):176–185. doi: 10.1016/0005-2795(77)90224-0. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Howard J., Shannon L. A rapid, quantitative, and highly specific assay for carbohydrate-binding proteins. Anal Biochem. 1977 May 1;79(1-2):234–239. doi: 10.1016/0003-2697(77)90398-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Newman R. A. Heterogeneity among the anti-TF lectins derived from Arachis hypogaea. Hoppe Seylers Z Physiol Chem. 1977 Nov;358(11):1517–1520. [PubMed] [Google Scholar]
- Pueppke S. G., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: II. Distribution of Soybean Lectin in Tissues of Glycine max (L.) Merr. Plant Physiol. 1978 May;61(5):779–784. doi: 10.1104/pp.61.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull S. P., Pueppke S. G., Hymowitz T., Orf J. H. Soybean lines lacking the 120,000-dalton seed lectin. Science. 1978 Jun 16;200(4347):1277–1279. doi: 10.1126/science.200.4347.1277. [DOI] [PubMed] [Google Scholar]
- Pusztai A., Watt W. B. Isolectins of Phaseolus vulgaris. A comprehensive study of fractionation. Biochim Biophys Acta. 1974 Sep 13;365(1):57–71. doi: 10.1016/0005-2795(74)90250-5. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Rougé P. Devenir des phytohémagglutinines provenant des diverses parties de la graine dans les jeunes germinations du pois. C R Acad Sci Hebd Seances Acad Sci D. 1975 May 12;280(18):2105–2108. [PubMed] [Google Scholar]
- Talbot C. F., Etzler M. E. Development and Distribution of Dolichos biflorus Lectin as Measured by Radioimmunoassay. Plant Physiol. 1978 May;61(5):847–850. doi: 10.1104/pp.61.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao T., Irimura T., Osawa T. Purification and characterization of a hemagglutinin from Arachis hypogeae. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1685–1692. doi: 10.1515/bchm2.1975.356.2.1685. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. 1,4-Butanediol diglycidyl ether coupling of carbohydrates to Sepharose: affinity adsorbents for lectins and glycosidases. Anal Biochem. 1977 Jul;81(1):98–107. doi: 10.1016/0003-2697(77)90602-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wellner D., Hayes M. B. Isoelectric focusing in polyacrylamide gels. Ann N Y Acad Sci. 1973 Jun 15;209:34–43. doi: 10.1111/j.1749-6632.1973.tb47517.x. [DOI] [PubMed] [Google Scholar]