Abstract
Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and platelet count (PC) were shown to be prognostic in several solid malignancies. We analysed 603 R0 resected patients to assess whether NLR, PLR and PC correlate with other well-known prognostic factors and survival of patients with colorectal cancer (CRC). Receiver operating characteristic (ROC) curve analysis was performed to define cut-off values for high and low ratios of these indices. Univariate and multivariate analysis were used to determine the prognostic value of NLR, PLR and PC for overall and cancer-related survival. The distribution of NLR, PLR and PC in CRC patients was compared with 5270 healthy blood donors. The distribution of NLR, PLR and PC was significantly different between CRC patients and controls (all p < 0.05). A significant but heterogeneous association was found between the main CRC prognostic factors and high values of NLR, PLR and PC. Survival appeared to be worse in patients with high NLR with cancers in AJCC/UICC TNM Stages I-IV; nonetheless its prognostic value was not confirmed for cancer-related survival in multivariate analysis. After stratification of patients according to AJCC/UICC TNM stages, high PC value was significantly correlated with overall and cancer-related survival in TNM stage IV patients.
Introduction
Despite substantial improvement in early diagnosis, surgical techniques and adjuvant therapies, colorectal cancer (CRC) remains the third most commonly diagnosed cancer and the third leading cause of cancer-related mortality worldwide1.
The most appropriate management of CRC entails a deep knowledge of the pivotal role played by molecular factors involved in the pathogenesis of this condition. Such knowledge can also help identifying prognostic biomarkers that can predicting the outcome. The prognostic value of many putative biomarkers has been investigated so far2–4.
Amongst these, peripheral blood neutrophil-to lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), as well as platelet count (PC) have recently emerged as potentially useful tests, since their value may mirror a shift of the immune response in patients with colorectal malignancies.
Inflammation plays a crucial role in the pathogenesis and progression of many types of cancer. Some recent studies demonstrated that systemic inflammatory response correlates with postoperative survival in different cancer patients5, 6.
Moreover, systemic inflammatory response to tumours is associated with abnormalities of several blood components, especially neutrophils and lymphocytes. Several hypotheses have been proposed to explain the relationship between cancer and increased values of both PC and plasma fibrinogen7, 8. More specifically, platelets release angiogenic and putative tumour growth factors such as platelet factor 4 (PF4), transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF), all of which promote cancer progression and endotelial cell growth9–11.
The aim of this retrospective study was to evaluate the prognostic value of preoperative neutrophils to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and PC in patients undergoing potentially curative (R0) resection for colorectal cancer.
Results
Distribution of NLR, PLR and PC in study group and controls
Overall, 603 out of the 1075 patients with CRC observed were finally included in our study according to our inclusion criteria. The distribution of NLR, PLR and PC in cases and controls are shown in Fig. 1. The mean (±SD) preoperative values of the CRC patients were significantly higher than those of the control population (NLR 3.1 ± 1.8 vs. 1.8 ± 1; p < 0.001, PLR 194 ± 98 vs. 126 ± 38; p < 0.001; PC 298 ± 104 × 109/L vs. 241 ± 51 × 109/L; p < 0.001) (Table 1). The following optimal cut-off values were identified: 3.5 for NLR (i.e. low [L]-NLR ≤ 3.5 and high [H]-NLR > 3.5), 350 for PLR (i.e. low [L]-PLR ≤ 350 and high [H]-PLR > 350) and 350 × 109/L for PC (i.e. low [L]-PC ≤ 350 × 109/L and high [H]-PC > 350 × 109/L), respectively (see Supplementary Fig. S1). According to these thresholds, increased values were observed in 26.2% of CRC cases versus 3.2% of the controls for NLR, 6.8% of cases versus 0.0004% of controls for PLR, and 24.9% of cases versus 2.7% of controls for PC (all p < 0.001).
Figure 1.
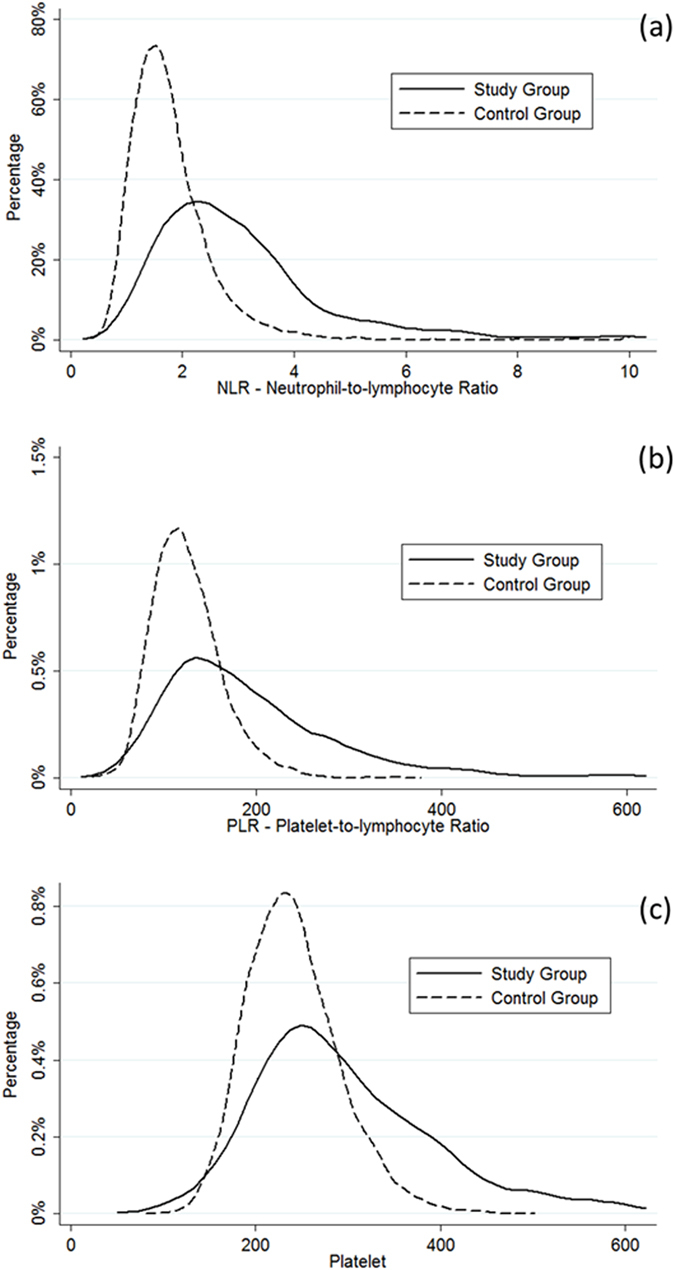
Distribution of (a) NLR, (b) PLR, and (c) PC stratified by study and control groups.
Table 1.
NLR, PLR and PC of the 603 patients under study and of the 5270 blood donors cases of the control group.
| Patients | Controls | p value | |
|---|---|---|---|
| Mean (±SD) age (years) | 68.2 (±12.9) | 41.2 (±12) | <0.001 |
| Male gender | 347 (57.5%) | 3946 (74.8%) | <0.001 |
| Mean (±SD) neutrophil count | 4.7 (±1.8) | 3.6 (±2.9) | <0.001 |
| Mean (±SD) lymphocyte count | 1.7 (±0.7) | 2 (±0.6) | <0.001 |
| Mean (±SD) PC | 298 (±104) | 240 (±51) | <0.001 |
| Mean (±SD) NLR | 3.1 (±1.8) | 1.8 (±1) | <0.001 |
| Mean (±SD) PLR | 194 (±98) | 126 (±38) | <0.001 |
Correlation between NLR, PLR and PC and clinicopathological variables
The correlations of NLR, PLR and PC with clinicopathological variables are shown in Table 2. H-NLR was more frequently observed in patients with increased age (p = 0.026), advanced pT (p < 0.001), TNM stage tumours (p < 0.001), metastatic disease (p < 0.001) and CEA positive cancers (p = 0.017). H-PLR was observed more frequently in advanced pT stage tumours (p = 0.023), although the percentage of patients with H-PLR did not exceeded 12%. H-PC was observed more frequently in the female gender (p = 0.013), colon tumour location (p = 0.006), advanced pT (p < 0.001), TNM stage tumours (p = 0.008) and CEA positive cancer (p = 0.005).
Table 2.
Correlations between NLR, PLR and PC and main clinicopathological variables for the 603 patients under study.
| No. of patients | H-NLR | H-PLR | H-PC | |
|---|---|---|---|---|
| Age | p = 0.026 | p = 0.747 | p = 0.398 | |
| ≤68.9 years | 299 | 66 (22.1%) | 19 (6.4%) | 79 (26.4%) |
| >68.9 years | 304 | 92 (30.3%) | 22 (7.2%) | 71 (23.4%) |
| Gender | p = 0.263 | p = 0.417 | p = 0.013 | |
| Male | 347 | 97 (28%) | 21 (6.1%) | 73 (21%) |
| Female | 256 | 61 (23.8%) | 20 (7.8%) | 77 (30.1%) |
| Tumour location | p = 1.000 | p = 0.280 | p = 0.006 | |
| Colon | 438 | 115 (26.3%) | 33 (7.5%) | 122 (27.9%) |
| Rectum | 165 | 43 (26.1%) | 8 (4.8%) | 28 (17%) |
| Depth of invasion (pT) | p < 0.001 | p = 0.023 | p < 0.001 | |
| pT1 | 77 | 7 (9.1%) | 0% | 8 (10.4%) |
| pT2 | 76 | 22 (28.9%) | 5 (6.6%) | 12 (15.8%) |
| pT3 | 270 | 63 (23.3%) | 16 (5.9%) | 70 (25.9%) |
| pT4a | 122 | 42 (34.4%) | 13 (10.7%) | 37 (30.3%) |
| pT4b | 58 | 24 (41.4%) | 7 (12.1%) | 23 (39.7%) |
| Node involvement (pN) | p = 0.679 | p = 0.374 | p = 0.172 | |
| pN0 | 380 | 95 (25%) | 26 (6.9%) | 88 (23.2%) |
| pN1 | 155 | 44 (28.4%) | 8 (5.2%) | 39 (25.2%) |
| pN2 | 68 | 19 (27.9%) | 7 (10.3%) | 23 (33.8%) |
| Systemic metastasis (M) | p < 0.001 | p = 0.059 | p = 0.101 | |
| M0 | 563 | 137 (24.3%) | 36 (6.4%) | 136 (24.2%) |
| M1a | 33 | 15 (45.5%) | 3 (9.1%) | 10 (30.3%) |
| M1b | 7 | 6 (85.7%) | 2 (28.1%) | 4 (57.1%) |
| AJCC/UICC TNM Stage | p < 0.001 | p = 0.106 | p = 0.008 | |
| Stage I | 130 | 27 (20.8%) | 5 (3.8%) | 18 (13.8%) |
| Stage II | 237 | 64 (27%) | 21 (8.9%) | 65 (27.4%) |
| Stage III | 196 | 46 (23.5%) | 10 (5.1%) | 53 (27%) |
| Stage IV | 40 | 21 (52.5%) | 5 (12.5%) | 14 (35%) |
| CEA serum levelb | p = 0.017 | p = 0.818 | p = 0.005 | |
| ≤5 ng/mL | 303 | 75 (24.8%) | 21 (6.9%) | 69 (22.8%) |
| >5 ng/mL | 92 | 35 (38%) | 7 (7.6%) | 35 (38%) |
No differences in NLR and PLR were observed according to tumour grading, lymphatic, vascular and perineural invasion. Conversely, H-PC was found to be significantly associated with poor cancer differentiation (G3 tumours: 23.3% vs. 8.8%; p < 0.001), vascular invasion (VI + tumours: 32.2% vs. 20.7%; p = 0.013), perineural invasion (NI + tumours: 32.5% vs. 23.3%; p = 0.041) and mucinous histotype (mucinous tumours: 34.5% vs. 22.9%; p = 0.046). No difference in PC values was observed in relation to lymphatic invasion (p = 0.834) and inflammatory reaction (p = 0.986).
NLR, PLR and PC and survival analysis
Overall and cancer-related survival rates in relationship to the main clinicopathological variables, NLR, PLR and PC are shown in Table 3.
Table 3.
Five-year overall and cancer-related survival rates according to the main clinicopathological characteristics, NLR, PLR and PC for the 603 patients under study.
| No. of patients | 5-year survival rate | ||
|---|---|---|---|
| Overall | Cancer-related | ||
| Age | p < 0.001 | p = 0.022 | |
| ≤68.9 years | 299 | 85.9 | 89.4 |
| >68.9 years | 304 | 64.6 | 79.3 |
| Gender | p = 0.055 | p = 0.052 | |
| Male | 347 | 73.7 | 82.6 |
| Female | 256 | 77.2 | 87.7 |
| Tumour location | p = 0.699 | p = 0.818 | |
| Colon | 438 | 74.8 | 84.1 |
| Rectum | 165 | 76.1 | 86.4 |
| Depth of invasion (pT) | p < 0.001 | p < 0.001 | |
| pT1 | 77 | 89.9 | 100 |
| pT2 | 76 | 86.5 | 90.9 |
| pT3 | 270 | 80.8 | 90.8 |
| pT4a | 122 | 54.5 | 65.2 |
| pT4b | 58 | 55.2 | 61.5 |
| Node involvement (pN) | p < 0.001 | p < 0.001 | |
| pN0 | 380 | 81.6 | 92.5 |
| pN1 | 155 | 71.1 | 78.6 |
| pN2 | 68 | 44.7 | 46.9 |
| Systemic metastasis (M) | p < 0.001 | p < 0.001 | |
| M0 | 563 | 77.8 | 87.8 |
| M1a | 33 | 46.5 | 50.8 |
| M1b | 7 | 0 | 0 |
| AJCC/UICC TNM Stage | p < 0.001 | p < 0.001 | |
| Stage I | 130 | 89.2 | 97 |
| Stage II | 237 | 79.1 | 91.6 |
| Stage III | 196 | 68 | 75.5 |
| Stage IV | 40 | 37.9 | 45.8 |
| CEA serum levela | p < 0.001 | p < 0.001 | |
| ≤5 ng/mL | 303 | 78.6 | 86.1 |
| >5 ng/mL | 92 | 58.3 | 73.2 |
| Neutrophil-to-lymphocyte ratio | p < 0.001 | p = 0.022 | |
| L-NLR | 445 | 79.8 | 86.6 |
| H-NLR | 158 | 62.5 | 79.4 |
| Platelet-to-lymphocyte ratio | p = 0.070 | p = 0.239 | |
| L-PLR | 562 | 75.9 | 86.6 |
| H-PLR | 41 | 65.9 | 79.4 |
| Platelet count | p = 0.122 | p = 0.151 | |
| L-PC | 453 | 76.3 | 85.2 |
| H-PC | 150 | 71.8 | 83.5 |
aCEA serum level was available for 401 patients.
Age, depth of tumour invasion (pT), node involvement (pN), metastatic disease (M), TNM stage and CEA positivity were confirmed to be significant predictors of overall and cancer-related survival. Patients with H-NLR had lower 5-year overall survival (p < 0.001) and worse cancer-related survival rate (p = 0.020) (Fig. 2). No differences in overall and cancer-related survival were observed between high and low values of both PLR and PC (Table 3) (see Supplementary Figs S2 and S3).
Figure 2.
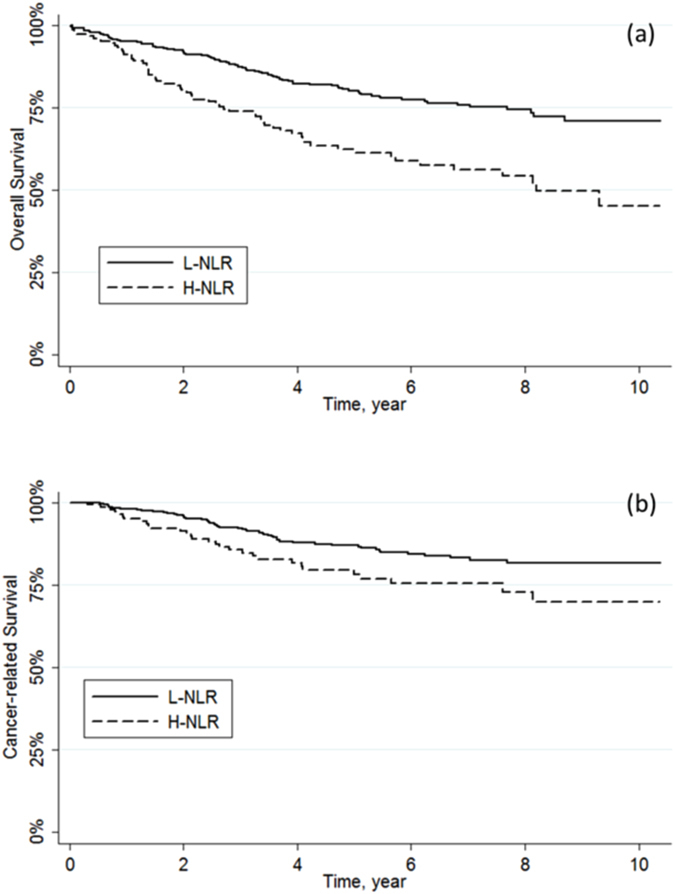
Kaplan-Meier curves for (a) overall survival (p < 0.001) and (b) cancer-related survival (p = 0.022) stratified by NLR.
In the Cox regression model, H-NLR, H-PLR and H-PC were found to be independent predictors of 5-year overall survival but not cancer-related survival after multiple adjustments (Table 4) (see Supplementary Tables S1 and S2).
Table 4.
Multivariable survival analysis including neutrophil-to-lymphocyte ratio (NLR) for the 603 patients under study.
| Overall Survival | Cancer-related Survival | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (years) | <0.001 | 0.017 | ||
| ≤68.9 years | — | — | ||
| >68.9 years | 2.98 (2.07–4.29) | 1.69 (1.09–2.61) | ||
| Gender | 0.10 | 0.08 | ||
| Female | — | — | ||
| Male | 1.33 (0.95–1.87) | 1.50 (0.96–2.36) | ||
| Histological Type | 0.46 | 0.10 | ||
| Adenocarcinoma | — | — | ||
| Mucinous | 0.85 (0.56–1.30) | 0.61 (0.33–1.01) | ||
| TNM Stage | <0.001 | <0.001 | ||
| Stage I | — | — | ||
| Stage II | 1.59 (0.90–2.82) | 2.73 (0.92–8.13) | ||
| Stage III | 2.92 (1.66–5.13) | 9.65 (3.43–27.14) | ||
| Stage IV | 6.49 (3.45–12.21) | 29.78 (10.22–86.75) | ||
| Neutrophil-to-lymphocyte ratio | 0.003 | 0.40 | ||
| L-NLR | — | — | ||
| H-NLR | 1.15 (0.86–1.54) | 1.22 (0.77–1.93) | ||
aValues in parentheses are 95% confidence intervals. Hazard ratio and p values were derived from Cox regression analysis, controlling for all other variables.
To further investigate the prognostic role of NLR, PLR and PC, the survival rates were stratified according to AJCC/UICC TNM stages. No differences in 5-year overall and cancer-related survival were observed between high and low values of both NLR and PLR across TNM stages I to IV (all p > 0.05). Similarly, no differences in 5-year overall and cancer-related survival were observed between high and low values of PC among TNM stages I to III (all p > 0.05). Notably, H-PC was found to be a negative predictor of overall and cancer-related survival compared to L-PC in patients with AJCC/UICC TNM stage IV tumours (overall survival, p = 0.002; cancer-related survival, p = 0.041) (Fig. 3).
Figure 3.
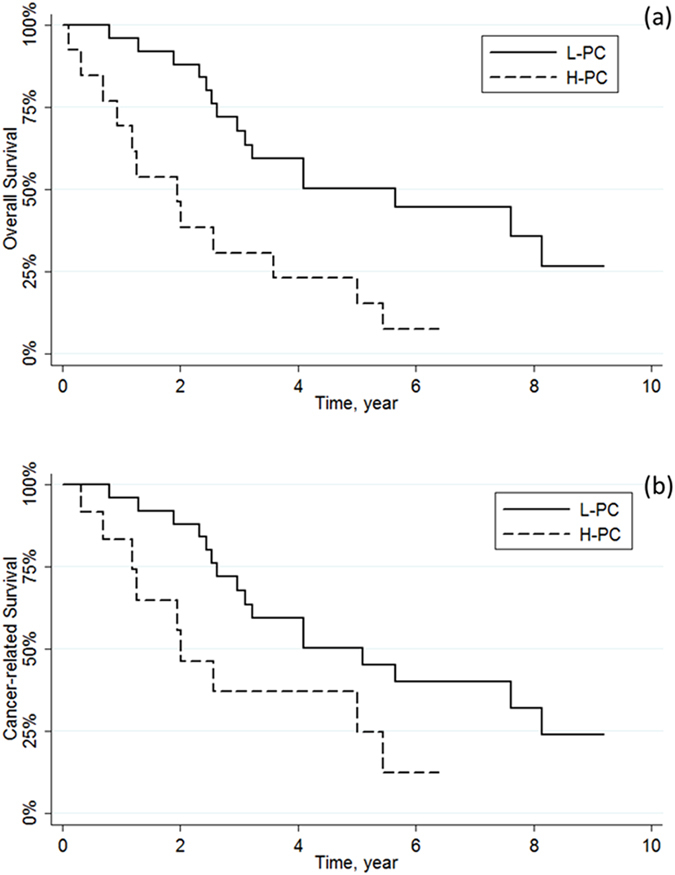
Kaplan-Meier curves for (a) overall survival (p = 0.002) and (b) cancer-related survival (p = 0.041) stratified by PC in AJCC/UICC TNM Stage IV.
Discussion
Increased values of both NLR and PLR were recently found to be negative predictors of survival in several malignancies such as oesophageal, hepato-bilio-pancreatic prostate cancer12–16 as well as CRC6, 17, 18.
The main findings of this study are: (1) the distribution of NLR, PLR and PC was found to be significantly different between CRC patients and controls; (2) a significant but variable association was found between the main known CRC prognostic factors and H-NLR, H-PLR and H-PC; (3) H-NLR was associated with significantly poorer overall survival in univariate as well multivariate analysis, but it had no impact on cancer-related survival; (4) after stratification of patients according to AJCC/UICC TNM stage, H-PC was significantly related to overall and cancer-related survival.
Although the clinical significance of NLR is still unclear, it has been pointed out that this parameter may globally reflect a shift of the immune response towards a pro-inflammatory pattern (i.e. high value of neutrophils) balanced by a depression of cell-mediated immunity (i.e. low value of lymphocytes). Since platelets are active players in inflammatory response, both thrombocytosis and high PLR are probably part of the same pathophysiology process13. Cancer cells produce many pro-inflammatory cytokines and chemokines such as GCSF, IL-1 and IL-6, but they are also actively involved in cytotoxic T-cell and natural killer cell suppression19. Altogether these aspects may contribute to generate a pro-inflammatory and immunosuppressive milieu20.
Interestingly, Turner et al. recently analysed local chronic inflammatory cell infiltrate together with systemic inflammation (i.e. NLR) in stage II CRC and showed that the combination of the two parameters have an increased prognostic value, which is also independent from standard clinical and pathological criteria. In this experience, cases with high intratumoural inflammation and low systemic inflammation showed a significantly better prognosis, whilst cases with low intratumoural inflammation and high systemic inflammatory response had a worse one18.
Beside cancer cells, solid tumours are composed of heterogeneous cell populations, including endothelial cells, fibroblasts and pericytes. Different cells produce several mediators capable of recruiting different leukocytes populations from the circulation to the tumour site. Within solid tumours, the myeloid population is certainly one of the most represented. Amongst these cells, tumour associated monocytes (TAM) and immature myeloid-derived suppressor cells (MDSC) play several important functions such as immunosuppression, angiogenesis tumour growth and cancer dissemination21, 22. It is possible that larger, less differentiated and more advanced tumours, (T stage but also N and M stages), bear more effective chemotactic activity towards the myeloid lineage, which in turn promotes progression of the cancer. It is reasonable to suggest that each individual immune response to cancer cells may contribute to define part of the biological behaviour of a malignancy. In this respect, it is also conceivable that a lower intratumoural inflammation and a higher systemic inflammatory response might translate in a decreased immunological local control, so engendering a systemic pro-inflammatory environment which ultimately facilitates cancer progression. Factors that could drive one or another type of immune response remain unknown so far.
The significant difference of NLR, PLR and PC values between CRC patients and healthy controls is one of the more interesting findings of our study. The sample size of the control population in our investigation is the largest in which NLR and PLR have ever been assessed, and this contributes to reinforce the clinical significance of our data.
Although the mean patient age was different between control and study groups, NLR, PLR and PC did not change in parallel to the increased age of the patients (Supplementary Figs S4 and S5). The higher frequency of H-NLR in patients with an age over the median value is likely due to the higher number of advanced pT and TNM stage tumours in this specific group (P < 0.005, data not shown).
Notably, the cut-off values of NLR, PLR and PC that we have calculated (i.e. 3.5, 350 and 350 × 109/L) partially differ from those reported in other investigations. This is not surprising since this difference may be due to the analysis of a different study population or to the use of a different haematological analyser, highlighting the importance of local calculation of predictive thresholds for these parameters.
In our study, H-NLR, H-PLR and H-PC were found to be significantly associated with some well-known CRC prognostic factors. More specifically, H-NLR correlated with pT, TNM stage, presence of metastatic disease and CEA positivity. H-PLR was associated with advanced pT tumours, whereas H-PC correlated with female gender and colon tumour location. A similar correlation between NLR and tumour burden was previously described. More specifically, in a retrospective study including 504 patients with stage II and III colon cancer, Absenger et al. found that NLR independently predicted the time to recurrence23.
Another study24 described a significant correlation between H-NLR, size (pT) and metastatic disease burden, although the cut-off value was slightly lower than that identified in our investigation (i.e. 3.0 versus 3.5). NLR was also found to be an independent predictor of worse outcome within the same stage cancer (stage IIA), proving to be an important factor in the evaluation of adjuvant therapy25.
H-PC was an independent predictor factor of both overall and cancer-related survival. Interestingly, H-PC was found to be an independent predictor factor of both 5-year overall and cancer-related survival only in patients with stage IV tumours.
The prognostic value of PC in CRC has been previously investigated. Although some results were generated in retrospective or underpowered studies, thrombocytosis at the time of diagnosis of CRC has been convincingly associated with worse disease free and overall survival8. Renal26, gynaecological27 and lung cancers28, 29 have also shown to have an elevated PC at the time of diagnosis. Finally, thrombocytosis could identify a patient subgroup in whom cytokine activation is driving a more aggressive disease course, but which is sensitive to therapeutic intervention. Subgroup analyses of the COIN trial on metastatic CRC suggested that patients with normal baseline platelet counts could gain the benefits of intermittent chemotherapy without detriment in survival, whereas those with raised baseline platelet counts have impaired survival and quality of life with intermittent chemotherapy and should not receive a treatment break30.
Regarding lymph node involvement, we failed to find a significant correlation with NLR, PLR and PC. This finding is not in line with previous studies, wherein Chiang et al. reported that patients with advanced pN stage also had significantly higher NLR values31. This difference cannot be easily explained, especially considering that the lymph node yield in our study was adequate, with a mean number of 21.4 harvested lymph nodes.
A potential explanation for the observed correlation between predictors of CRC and NLR, PLR or PC can be brought back to the putative immunological activities of cancer cells.
In particular, the correlation between H-NLR and the presence of systemic metastases can be explained by the fact that a high neutrophil count was found in our CRC patients, and these blood cells actively release cytokines and circulating vascular endothelial growth factor (VEGF), two molecules deeply involved in angiogenesis, cancer growth and metastasis32. On the other hand, lymphocytes play a pivotal role in tumour suppression, by inducing cytotoxic cell death and cytokine production, which may ultimately contribute to inhibiting the proliferation and metastatic activity of cancer cells33, 34.
The biological background of the observed correlation between lymph node involvement and NLR, PLR or PC remains unexplained. Although Pine et al. previously found a correlation between high NLR and pN stage, this study also included non-curative resection and less than 15 lymph nodes were detected in half of the patients3.
Therefore, we tend to believe that the prognostic significance of these tests may be stronger for certain characteristics of cancer progression such as depth invasion and systemic metastasis rather than for node involvement.
When we considered Stage I-IV tumours, survival appeared to be worse in patients with H-NLR. This intriguing finding may be partly explained by the differences observed in cancer volume between the L-NLR and H-NLR groups, despite the fact that the prognostic significance of NLR for cancer-related survival could not be confirmed in multivariate analysis.
Unlike NLR and PLR, PC was significantly associated with 5-year overall and cancer-related survival after stratification of patients according to TNM stage. This is also reasonable since platelets may contribute to cancer development by promoting neoangiogenesis, increasing both microvessel permeability and extravasation of cancer cells, producing growth factors (e.g. VEGF, PDGF and TGF-β) and facilitating the interaction between cancer cells and endothelia at metastatic sites35, 36.
In conclusion, our study clearly demonstrates that the distribution of NLR, PLR and PC significantly differs between CRC patients and healthy subjects. This difference is more marked in advanced tumours, although it does not unequivocally reflect cancer-related survival. Further studies will be needed to establish the real cost-effectiveness of routinely using these inexpensive, readily available and reproducible biomarkers for establishing the prognosis after R0 resection for CRC.
Methods
Patients and eligibility criteria
The original patient population consisted of all patients undergoing surgery for CRC at the Division of General Surgery A, University of Verona Hospital Trust, between January 2005 and December 2013. Inclusion criteria were: elective surgery for histology-proven CRC and age of 18 years or older.
Patients with evidence of infections or other inflammatory conditions during preoperative evaluation were excluded, as well as those who underwent preoperative chemo or radiotherapy, emergency surgery and an R1 or R2 resection. All methods used in this study were performed in accordance with the relevant ethical guidelines and regulations of the University Hospital of Verona, where the investigation was carried out. Informed consent was obtained from all patients and the study protocol was approved by the local ethical committee (ID number: 42763-CRINF-1034 CESC).
Preoperative work-up and histopathological staging
Prior to surgery, all patients were staged with colonoscopy, computed tomography (CT) of chest-abdomen and pelvis and measurement of cancer biomarkers including carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9). Additional imaging studies including magnetic resonance imaging (MRI) and endoluminal ultrasonography were used for staging rectal cancer. Liver MRI and positron emission tomography were also used to evaluate dubious lesions.
Pathology specimens were analysed in accord with the 7th Edition of the American Joint Committee on Cancer (AJCC) and the Union International Contre Le Cancer (UICC) criteria.
Preoperative assessment of laboratory data
Neutrophil count, lymphocyte count and PC were obtained in venous blood within 2 weeks from the date of surgery. NLR and PLR were calculated by dividing the absolute number of neutrophils or platelets by the absolute number of lymphocytes, respectively.
Blood samples were drawn by an expert phlebotomist in vacuum blood tubes containing K2-EDTA (Terumo Europe NV, Leuven, Belgium). The complete blood cell count (CBC) was performed using Advia 2120 (Siemens Healthcare Diagnostics, Tarrytown NY, USA). The local reference ranges are 150–400 × 109/L for platelets, 4.3–10.0 × 109/L for total white blood cells (WBC), 2.0–7.0 × 109/L for neutrophils and 0.95–4.5 × 109/L for lymphocytes, respectively. The same analyser was used throughout the study period. The quality and comparability of test results was validated by data of both internal quality control (IQC) and external quality assessment (EQA).
Extent of surgery
The complete excision of the tumour burden (R0 resection) was the main outcome of surgery. The extent of surgery was planned according to patient conditions, tumour location and stage. Standard CRC resections (i.e. right hemicolectomy, extended right hemicolectomy, left hemicolectomy, anterior resection, low anterior resection, abdominoperineal resection) with ligation of vessels at their origin were usually performed in order to harvest an adequate number of lymph nodes37. The mean number of lymph nodes analyses was 21.4 (SD, 11.4), with only 13.8% of cases having less than 12 lymph nodes excised. In patients affected by synchronous liver metastases or peritoneal disease, liver resection or partial peritonectomy was needed to achieve R0 resection.
Follow-up and statistical analysis
All clinical and pathological data were retrospectively collected and stored in a digital database. Demographic, clinical, surgical and pathology variables were analysed.
On preliminary analysis, preoperative NLR, PLR and PC were found to be normally distributed. The optimal cut-off values for NLR, PLR and PC as dichotomous predictors of survival was chosen after considering (i) conventional receiver operating characteristics (ROC) curve analysis using death as the outcome (see Supplementary Fig. S1); (ii) Kaplan-Meier curves and proportional hazards regression with cut-off increased in following steps and results recalculated at each step in order to identifying the threshold associated with the greatest separation of curves with the lowest p value; (iii) evaluation of cut-offs proposed by previous literature. The distribution of NLR, PLR and PC in the final study population was also compared with the distribution of these parameters in a total number of 5270 healthy blood donors who had their blood collected during the same period (i.e. control group). The difference was analysed with ANOVA test (Table 1 and Fig. 1); the relationship between study variables (i.e. NLR, PLR and PC) and age was tested using linear regression analysis (see Supplementary Figs S4 and S5).
The significance of differences was evaluated with chi square test or Fisher’s exact test for categorical data (using NLR, PLR and PC as dichotomous variables), and Student’s t-test for continuous variables.
The association of NLR, PLR and PC with overall survival and cancer-related survival was analysed using Kaplan-Meier curves and compared with the log-rank test. The time of survival was measured between the date of surgery and the date of the most recent follow-up examination or death. Multivariable analysis for overall survival and cancer-related survival was performed using Cox regression model by separately considering NLR, PLR and PC values above or below the relative cut-offs and adjusting for the following risk factors: age (>median vs. ≤median), gender (male vs. female), tumour location (rectum vs. colon), histological type (mucinous vs. non-mucinous type) and AJCC/UICC TNM stage (stage II, stage III and stage IV vs. stage I). A p value < 0.05 was considered to be statistically significant.
Statistical analysis was performed using SPSS software version 21.0 version (IBM Corporation, Armonk, NY) and STATA software (Stata Corporation, 2011, MP-Parallel Edition).
Electronic supplementary material
Author Contributions
C.P., G.M. and A.G. provided study concept and design; G.M., G.L.S., T.C. and E.D. collected data; C.P., G.M. and F.B. performed data analysis and interpretation; C.P., G.M., E.F. and G.L. wrote the main manuscript text; C.P., G.M. and E.F. prepared the tables; F.B., G.L.S. and E.D. prepared the figures; A.R., G.L. and A.G. provided critical revision of the paper. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01652-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2014;59:366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 2.Gu L, et al. Prognostic value of preoperative response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep. 2016;6:23846. doi: 10.1038/srep23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pine JK, et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer. 2015;113:204–211. doi: 10.1038/bjc.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passardi A, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7:33210–33219. doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao W, et al. Pretreatment neutrophil-to-lymphocyte ratio and its dynamic changes are associated with overall survival in advanced cancer patients undergoing palliative care. Sci Rep. 2016;6:31394. doi: 10.1038/srep31394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Templeton AJ, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Canc Inst. 2014;6:dju124–dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 7.Pedrazzani C, et al. Elevated fibrinogen plasma level is not an independent predictor of poor prognosis in a large cohort of Western patients undergoing surgery for colorectal cancer. World J Gastroenterol. 2016;22:9994–10001. doi: 10.3748/wjg.v22.i45.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Y, et al. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer. Oncotarget. 2016;7:81849–81861. doi: 10.18632/oncotarget.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verheul HM, et al. Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin Cancer Res. 2000;6:166–171. [PubMed] [Google Scholar]
- 10.Ikeda M, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9:287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- 11.Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for an alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol. 1997;17:2293–2305. doi: 10.1161/01.ATV.17.10.2293. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Q, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel immune parameter for the prognosis of esophageal basaloid squamous cell carcinoma. PLoS One. 2016;11:0168299. doi: 10.1371/journal.pone.0168299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yodying H, et al. Prognostic significance of neutrophil-to lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review ant meta-analysis. Ann Surg Oncol. 2016;23:646–654. doi: 10.1245/s10434-015-4869-5. [DOI] [PubMed] [Google Scholar]
- 14.McNamara MG, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014;50:1581–1589. doi: 10.1016/j.ejca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Gemenetzis, G. et al. Neutrophil-to-lymphocyte ratio is a predictive marker for invasive malignancy in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. (2016) [Epub ahead of print]. [DOI] [PubMed]
- 16.Sciarra A, et al. Prognostic value of inflammation in prostate cancer progression and response to therapeutic: a critical review. J Inflamm. 2016;13:12950. doi: 10.1186/s12950-016-0143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner N, et al. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin Colorectal Cancer. 2015;14:185–191. doi: 10.1016/j.clcc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Turner N, et al. Analysis of local chronic inflammatory cell infiltrate combined with systemic inflammation improves prognostication in stage II colon cancer independent of standard clinicopathologic criteria. Int J Cancer. 2016;138:671–678. doi: 10.1002/ijc.29805. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, et al. Intratumoral immune reaction: a novel paradigm for cancer. J Clin Oncol. 2011;29(4_suppl):471–471. doi: 10.1200/jco.2011.29.4_suppl.471. [DOI] [Google Scholar]
- 20.Kwon KA, et al. Systemic inflammatory response in predicting survival in patients with operable colorectal cancer. J Clin Oncol. 2011;29(4_suppl):456–456. doi: 10.1200/jco.2011.29.4_suppl.456. [DOI] [Google Scholar]
- 21.Caronni, N., Savino, B. & Bonecchi R. Myelod cells in cancer-related inflammation. Immunobiology220, 249–53 (2015). [DOI] [PubMed]
- 22.Borsig L, et al. Inflammatory chemokines and metastasis-tracing the accessory. Oncogene. 2014;33:3217–32124. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- 23.Absenger G, et al. Preoperative Neutrophil-to Lymphocyte Ratio predicts Clinical Outcome in Patients with Stage II and III Colon Cancer. Anticancer Res. 2013;33:4591–4594. [PubMed] [Google Scholar]
- 24.Malietzis G, et al. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2013;260:287–292. doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 25.Ding ER, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427–1433. doi: 10.1007/s00384-010-1052-0. [DOI] [PubMed] [Google Scholar]
- 26.Bensalah K, et al. Prognostic value of thrombocytosis in renal cell carcinoma. J Urol. 2006;175:859–863. doi: 10.1016/S0022-5347(05)00526-4. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez E, Donohue KA, Anderson LL, Heller PB, Stehman FB. The significance of thrombocytosis in patients with locally advanced cervical carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78:137–142. doi: 10.1006/gyno.2000.5838. [DOI] [PubMed] [Google Scholar]
- 28.Gu X, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep. 2016;6:23893. doi: 10.1038/srep23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoe K, et al. Thrombocytosis as a useful prognostic indicator in patients with lung cancer. Respiration. 2004;71:170–173. doi: 10.1159/000076679. [DOI] [PubMed] [Google Scholar]
- 30.Adams RA, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for fist-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12:642–653. doi: 10.1016/S1470-2045(11)70102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang SF, et al. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27:1347–1357. doi: 10.1007/s00384-012-1459-x. [DOI] [PubMed] [Google Scholar]
- 32.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 33.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2010;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 35.Egan K, et al. Platelet adhesion and degranulation induce pro-suvival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011;6:26125. doi: 10.1371/journal.pone.0026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. 2004;29:132–140. doi: 10.1097/00006676-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Pedrazzani C, et al. Management of nodal disease from colon cancer in the laparoscopic era. Int J Colorectal Dis. 2015;30:303–314. doi: 10.1007/s00384-014-2075-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


