Abstract
Data are presented which indicate that dimethylsulfoxide (DMSO) acts selectively on the plasma membrane of cultured tobacco cells, rendering it more permeable to small molecules, while having a far smaller effect on the permeability of the vacuolar membrane. The results which support this conclusion are: (a) DMSO (5 to 10%, by volume) causes complete release of [14C]tryptophan newly synthesized from [14C]indole while causing efflux of only about 20% of the total intracellular tryptophan pool; (b) similar concentrations of DMSO do not cause substantial release from these cells of phenolic compounds or preloaded neutral red, nor of β-cyanin from fresh beet discs; (c) kinetic studies of release of tryptophan and neutral sugars and of efflux of 86Rb+ show that DMSO selectively promotes rapid release of a portion of the total pool, followed by a substantially slower release of the remaining pool; (d) when tobacco cell protoplasts are incubated in the presence of 7.5% (by volume) DMSO, rapid lysis is observed concomitant with the release of intact vacuoles. These data indicate that a procedure involving a brief treatment of intact plant cells or tissues with DMSO may be used to assess the distribution of metabolites between cytoplasmic and vacuolar compartments.
Full text
PDF
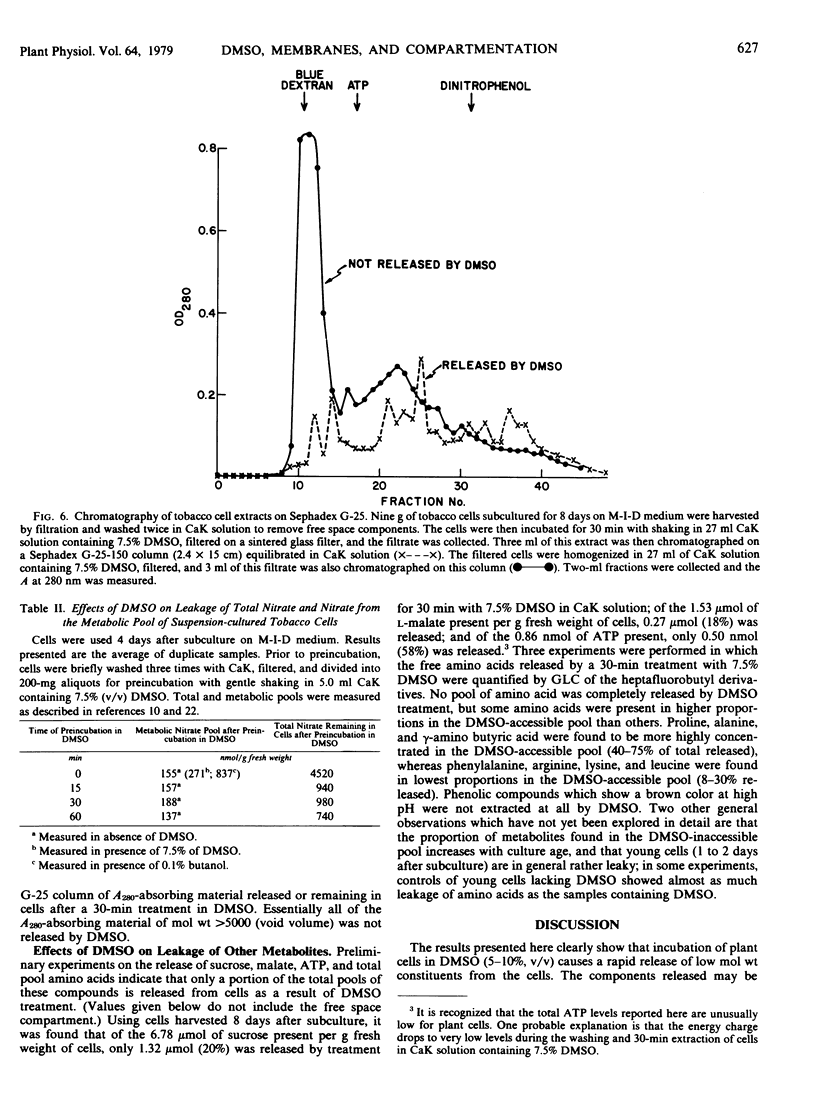


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Oaks A., Huffaker R. C. Effect of light and glucose on the induction of nitrate reductase and on the distribution of nitrate in etiolated barley leaves. Plant Physiol. 1976 Oct;58(4):588–591. doi: 10.1104/pp.58.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser W. L., Murphy J. B., Delmer D. P., Mills S. E. End product control of tryptophan biosynthesis in extracts and intact cells of the higher plant Nicotiana tabacum var. Wisconsin 38. Biochim Biophys Acta. 1971 Apr 20;237(1):1–10. doi: 10.1016/0304-4165(71)90023-7. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Brody S. Circadian rhythms in Neurospora crassa: oscillation in the level of an adenine nucleotide. J Bacteriol. 1975 Feb;121(2):548–553. doi: 10.1128/jb.121.2.548-553.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Mills S. E. A technique for the assay of enzymes in intact plant cells in the presence of dimethylsulfoxide. Plant Physiol. 1969 Jan;44(1):153–155. doi: 10.1104/pp.44.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Mills S. Tryptophan synthase from Nicotiana tabacum. Biochim Biophys Acta. 1968 Oct 8;167(2):431–443. doi: 10.1016/0005-2744(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Ferrari T. E., Yoder O. C., Filner P. Anaerobic nitrite production by plant cells and tissues: evidence for two nitrate pools. Plant Physiol. 1973 Mar;51(3):423–431. doi: 10.1104/pp.51.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P. Semi-conservative replication of DNA in a higher plant cell. Exp Cell Res. 1965 Aug;39(1):33–39. doi: 10.1016/0014-4827(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner H. R., Ben-Bassat D., Reinhold L., Poljakoff-Mayber A. Induction of "pore" formation in plant cell membranes by toluene. Plant Physiol. 1978 Feb;61(2):213–217. doi: 10.1104/pp.61.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan D. H., Beevers H., Harley J. L. 'Compartmentation' of acids in plant tissues. Biochem J. 1963 Nov;89(2):316–327. doi: 10.1042/bj0890316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A., Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. III. Physical & Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963 May;38(3):348–354. doi: 10.1104/pp.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]