Abstract
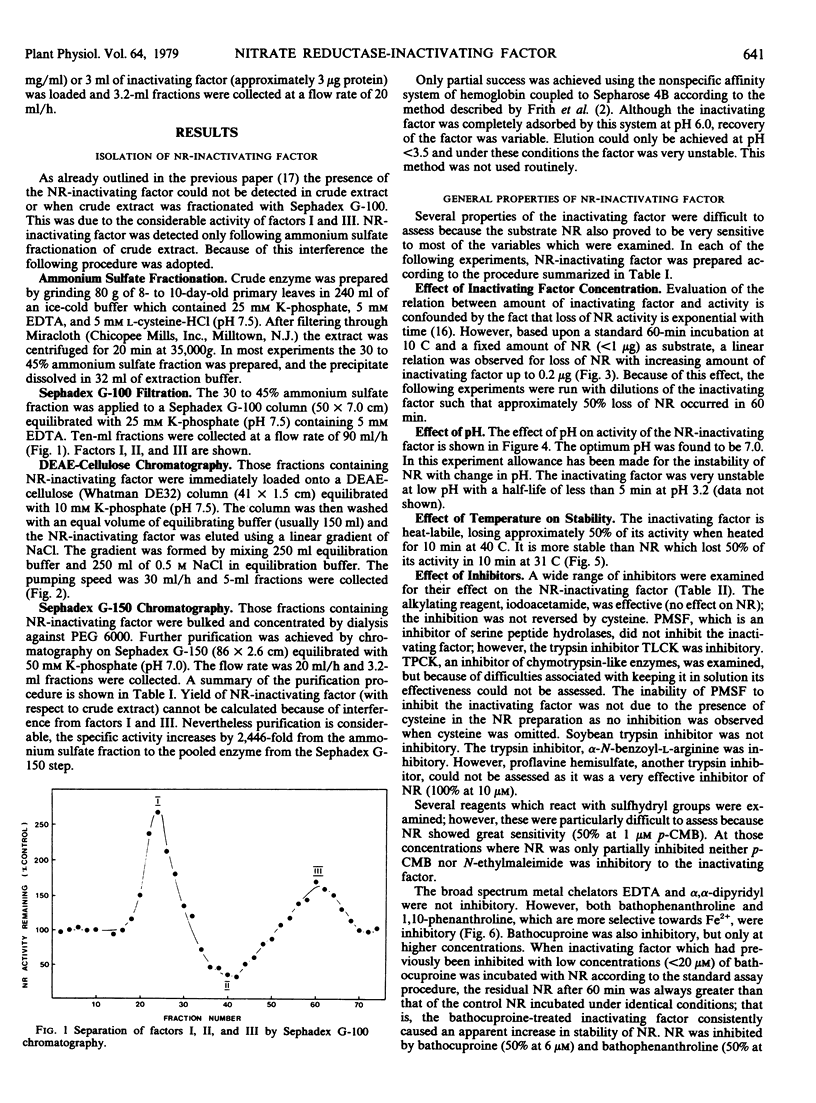
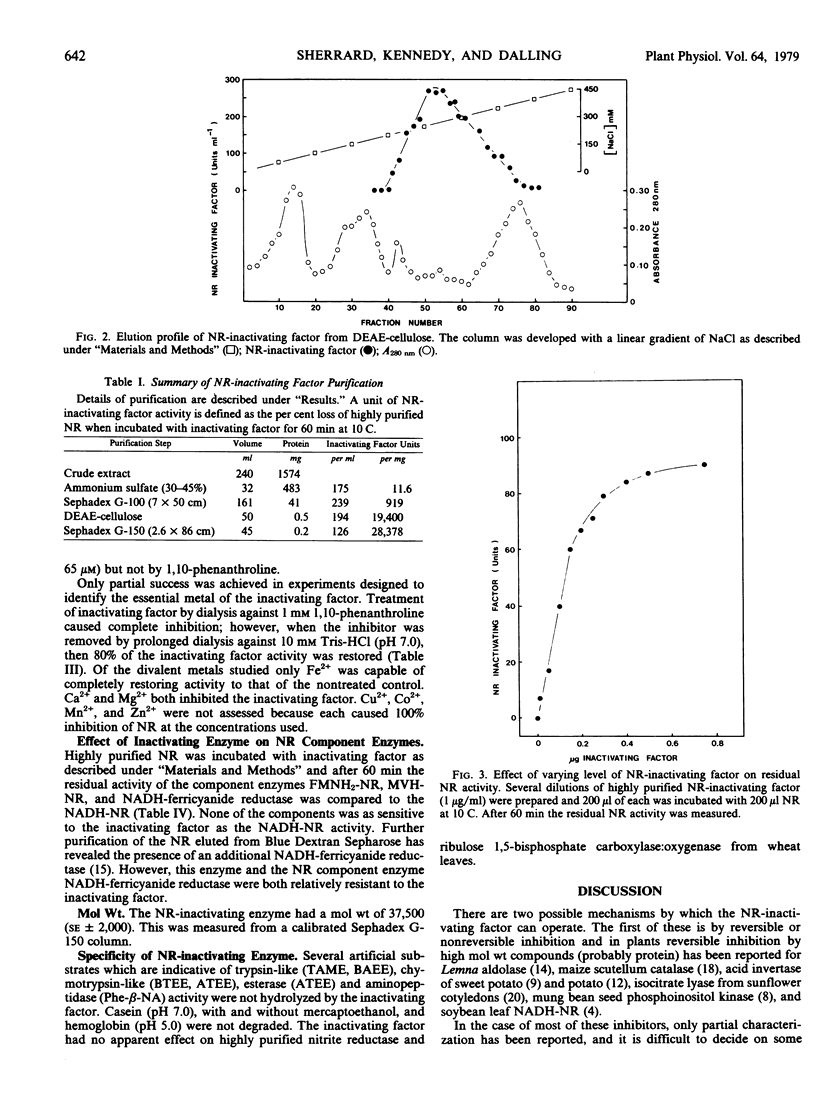
A nitrate reductase (EC 1.6.6.1)-inactivating factor has been isolated from 8-day-old wheat leaves. The purification schedule involved ammonium sulfate precipitation, Sephadex G-100 filtration, DEAE-cellulose chromatography, and Sephadex G-150 filtration. No accurate assessment could be made as to the degree of purification relative to crude extract as the inactivating factor could not be detected in crude extract. However a 2,446-fold purification was achieved from the ammonium sulfate fraction to the pooled enzyme from the Sephadex G-150 step.
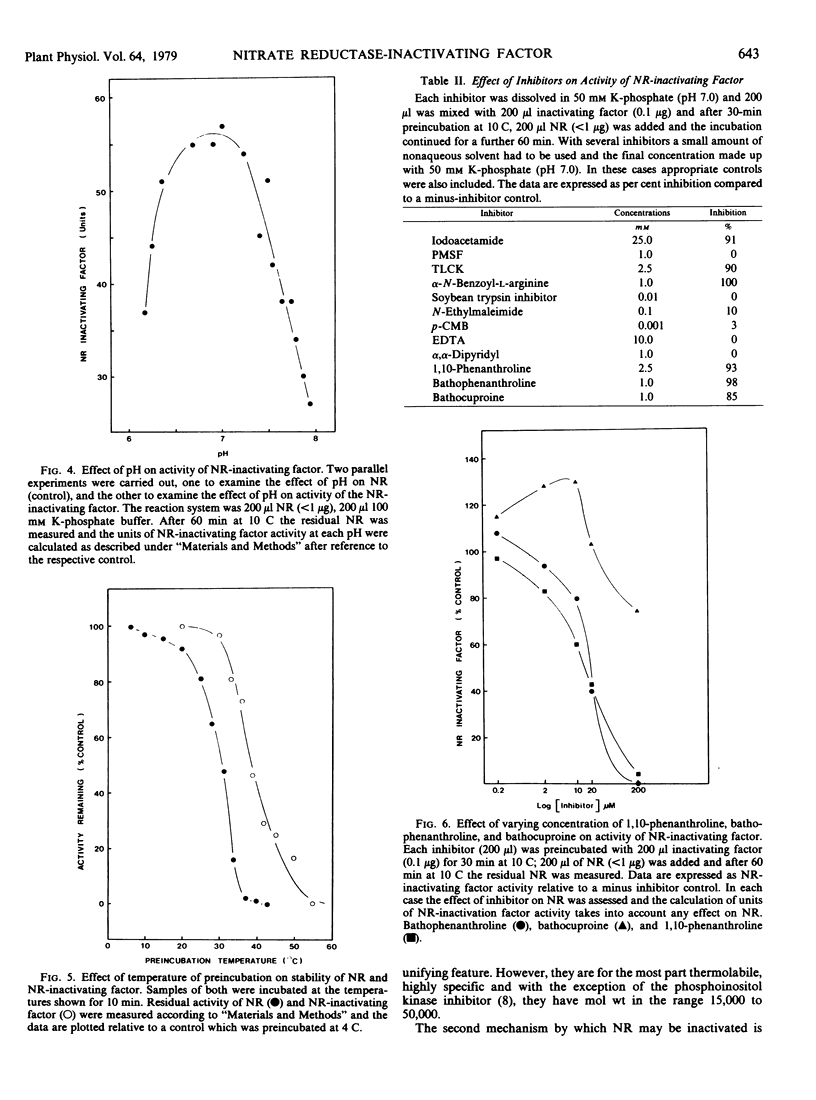
The inactivating factor was heat-labile and had a molecular weight of 37,500. The inactivating factor was particularly sensitive to the divalent metal chelators, 1,10-phenanthroline and bathophenanthroline. Evidence indicated that Fe2+ may be the functional metal. The trypsin inhibitors N-α-p-tosyl-l-lysine chloromethyl ketone and α-N-benzoyl-l-arginine were inhibitory. However, phenylmethyl sulfonyl fluoride, an inhibitor of serine peptide hydrolases, was not inhibitory. Neither casein nor hemoglobin nor a range of artificial substrates were hydrolyzed by the inactivating factor. Highly purified wheat leaf nitrite reductase (EC 1.7.99.3) and ribulose 1,5-bisphosphate carboxylase:oxygenase (EC 4.1.1.39) were not affected by the nitrate reductase-inactivating factor.
The inactivating factor was more active toward the NADH-nitrate reductase compared to either of the component enzymic activities flavin adenine mononucleotide-nitrate reductase and methyl viologen-nitrate reductase. The NADH-ferricyanide reductase (diaphorase) component was the least sensitive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jolly S. O., Tolbert N. E. NADH-Nitrate Reductase Inhibitor from Soybean Leaves. Plant Physiol. 1978 Aug;62(2):197–203. doi: 10.1104/pp.62.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S. S., Gandhi A. P., Sawhney S. K., Naik M. S. Inhibitor of nitrate reductase in the roots of rice seedlings and its effect on the enzyme activity in the presence of NADH. Biochim Biophys Acta. 1974 May 20;350(1):162–170. doi: 10.1016/0005-2744(74)90214-9. [DOI] [PubMed] [Google Scholar]
- Kadam S. S., Sawhney S. K., Naik M. S. Effect of NADH on the activities of nitrate reductase and its inactivating enzyme. Indian J Biochem Biophys. 1975 Jun;12(2):81–85. [PubMed] [Google Scholar]
- Katsunuma T., Schött E., Elsässer S., Holzer H. Purification and properties of tryptophan-synthase-inactivating enzymes from yeast. Eur J Biochem. 1972 Jun 9;27(3):520–526. doi: 10.1111/j.1432-1033.1972.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Uritani I. Isolation and characterization of acid invertase inhibitor from sweet potato. J Biochem. 1976 Mar;79(3):633–639. doi: 10.1093/oxfordjournals.jbchem.a131107. [DOI] [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Sherrard J. H., Dalling M. J. In vitro stability of nitrate reductase from wheat leaves: I. Stability of highly purified enzyme and its component activities. Plant Physiol. 1979 Feb;63(2):346–353. doi: 10.1104/pp.63.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard J. H., Kennedy J. A., Dalling M. J. In Vitro Stability of Nitrate Reductase from Wheat Leaves: II. Isolation of Factors from Crude Extract Which Affect Stability of Highly Purified Nitrate Reductase. Plant Physiol. 1979 Sep;64(3):439–444. doi: 10.1104/pp.64.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson J. C., Scandalios J. G. Purification of a catalase inhibitor from maize by affinity chromatography. Biochem Biophys Res Commun. 1975 Mar 3;63(1):239–246. doi: 10.1016/s0006-291x(75)80035-0. [DOI] [PubMed] [Google Scholar]
- Sorger G. J., Premakumar R., Gooden D. Demonstration in vitro of two intracellular inactivators of nitrate reductase from Neurospora. Biochim Biophys Acta. 1978 Apr 19;540(1):33–47. doi: 10.1016/0304-4165(78)90432-4. [DOI] [PubMed] [Google Scholar]
- Theimer R. R. A specifc inactivator of glyoxysomal isocitrate lyase from sunflower (Helianthus annuus L.) cotyledons. FEBS Lett. 1976 Mar 1;62(3):297–300. doi: 10.1016/0014-5793(76)80079-8. [DOI] [PubMed] [Google Scholar]
- Wallace W. A Re-evaluation of the Nitrate Reductase Content of the Maize Root. Plant Physiol. 1975 Apr;55(4):774–777. doi: 10.1104/pp.55.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Comparison of a nitrate reductase-inactivating enzyme from the maize root with a protease form yeast which inactivates tryptophan synthase. Biochim Biophys Acta. 1978 Jun 9;524(2):418–427. doi: 10.1016/0005-2744(78)90179-1. [DOI] [PubMed] [Google Scholar]
- Wallace W. Effects of a nitrate reductase inactivating enzyme and NAD(P)H on the nitrate reductase from higher plants and Neurospora. Biochim Biophys Acta. 1975 Feb 19;377(2):239–250. doi: 10.1016/0005-2744(75)90306-x. [DOI] [PubMed] [Google Scholar]
- Wallace W., Johnson C. B. Nitrate Reductase and Soluble Cytochrome c Reductase(s) in Higher Plants. Plant Physiol. 1978 May;61(5):748–752. doi: 10.1104/pp.61.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Walls S., Sorger G. J., Gooden D., Klein V. The regulation of the decay of nitrate reductase. Evidence for the existence of at least two mechanisms of decay. Biochim Biophys Acta. 1978 Apr 19;540(1):24–32. doi: 10.1016/0304-4165(78)90431-2. [DOI] [PubMed] [Google Scholar]
- Yu P. H., Kula M. R., Tsai H. Studies on the apparent instability of Neurospora tryptophan synthase. Evidence for protease. Eur J Biochem. 1973 Jan 3;32(1):129–135. doi: 10.1111/j.1432-1033.1973.tb02588.x. [DOI] [PubMed] [Google Scholar]


